Introduction
Laryngeal squamous cell carcinoma is the second most
common malignant squamous cell carcinoma of the head and neck
(1). Although the 5-year survival
rate has been increased to a high level, numerous patients succumb
to local recurrence, regional recurrence or distant metastasis
following surgery (1). Therefore, it
is important to discover the molecular mechanisms underlying the
development of laryngeal squamous cell carcinomas. Enhancer of
zeste 2 polycomb repressive complex 2 subunit (EZH2) is the
catalytic subunit of polycomb repressive complex 2 (PRC2), which is
a highly-conserved histone methyltransferase that targets lysine-27
of histone H3 (2). EZH2 contains a
signature SET domain, which provides the methyltransferase active
site. The interaction of EZH2 with DNA methyltransferases (DNMTs)
results in the transcriptional repression of target genes (3). EZH2 has been demonstrated importance in
the development of human embryonic stem cells (4). Ezhkova et al (5) reported that EZH2 regulates the
proliferative capacity of epidermal progenitor cells by suppressing
the Ink4A-Ink4B locus, and moderates their differentiation by
preventing the early recruitment of the jun proto-oncogene
transcriptional activator to the structural genes required for
epidermal differentiation. In addition, EZH2 has been shown to be
highly expressed in cancer cells, particularly in stem cell-like
cancer cell lines, in numerous cancer models (6,7). However,
the expression and function of EZH2 have been seldom investigated
in laryngeal squamous cell carcinomas, and the role of EZH2 in
laryngeal carcinoma is currently unknown. The present study
systematically evaluated the expression of EZH2 in laryngeal
carcinomas and investigated the functions of EZH2 in laryngeal
cancer cells in vitro and in vivo.
Materials and methods
Ethics statement
The tumor specimens used in the present study were
obtained with the approval of the ethics committee of the Eye, Ear,
Nose and Throat Hospital of Fudan University, Shanghai, China.
Signed informed consent was obtained from each patient. The animal
care and experimental protocols were approved by the Shanghai
Medical Experimental Animal Care Committee, Shanghai, China.
Animals were fed in laminar flow cabinets at the Department of
Laboratory Animal Science of Fudan University under specific
pathogen-free conditions. All of the surgeries were performed under
ketamine/xylazine-induced anesthesia, and all efforts were made to
minimize suffering.
Patients and tissue specimens
All the surgical tissue specimens were obtained from
patients with laryngeal squamous cell carcinomas, who had
previously received surgical treatment at the Eye, Ear, Nose and
Throat Hospital of Fudan University, between March 2012 and
December 2013. No patient had received chemotherapy or
radiotherapy. A total of 80 primary tumor tissues and the
corresponding paracancerous epithelial tissues were stored in
paraformaldehyde at room temperature. An additional 25 primary
tumor tissues and the corresponding paracancerous epithelial
tissues were stored at −80°C. Tissues were categorised using the
most recent (7th) edition of the tumor, node, metastasis (TNM)
system of classification defined by the International Union Against
Cancer (8)
Tissue microarray construction
Samples of 80 tumor tissues and 80 paracancerous
epithelial tissues were selected for the construction of the tissue
microarray. All samples were fixed with 4% paraformaldehyde (Wuhan
Boster Biological Technology, Ltd., Wuhan, China), embedded in
paraffin (Wuhan Boster Biological Technology, Ltd.), cut to the
desired thickness of 3 µm, and affixed to slides. The slides were
stained with hematoxylin and eosin (Wuhan Boster Biological
Technology, Ltd.) and assessed by two histopathologists. Two
representative tissue cores of each tissue block were selected for
transfer to a master block using a manual tissue microarray
instrument (ATA-27; Beecher Instruments, Inc., Sun Prarie, WI,
USA). The master block was cut to the desired thickness of 4 µm,
and sections were placed on 3-aminopropyltriethoxysilane-coated
slides (Wuhan Boster Biological Technology, Ltd.).
Immunohistochemistry
The tissue microarray section was deparaffinized
with xylene, rehydrated with a graded series of ethanol solutions,
rinsed with phosphate-buffered saline (PBS), and treated with 3%
hydrogen peroxide to inactivate the endogenous peroxidases. The
section was then treated with boiling water containing
ethylenediaminetetraacetic acid (EDTA; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 5 min to retrieve the
epitopes. Subsequent to washing the section 3 times with PBS and
incubating it with the primary antibody (anti-KMT6/EZH2
antibody-ChIP grade; rabbit polyclonal; dilution, 1:100; catalog
no., ab3748; Abcam, Cambridge, MA, USA) for 12 h, the section was
washed 3 times with PBS and incubated with a goat anti-rabbit
secondary antibody (dilution, 1:250; cat no., BA1003; Wuhan Boster
Biological Technology, Ltd, Shanghai, China) for 1 h.
3,3′-diaminobenzidine (Wuhan Boster Biological Technology, Ltd.)
acted as the chromogen. Images were captured with a fluorescence
microscope (DMI4000b; Leica, Wetzlar, Germany) and were assessed by
two pathologists. The standard for evaluation was as follows:
Percentage of positive cells was scored 0, 0% positive cells; 1,
1–10% positive cells; 2, 11–50% positive cells; and 3, >50%
positive cells; the staining intensity was scored 0, negative; 1,
weak; 2, moderate; and 3, high. The final score was the sum of the
two scores. A score of 0–3 was considered negative, and a score of
4–9 was considered positive (9).
Reverse transcription
(RT)-quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from the following samples
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.): Freshly
frozen tissue samples, including 25 tumor tissue and 25
corresponding paracancerous epithelial tissue samples; AMC-HN-8
cells; and EZH2-overexpressing AMC-HN-8 cells. RNA (1 µg) was
reverse-transcribed into complementary DNA (cDNA) using the
PrimeScript RT Master Mix, which included PrimeScript RTase, RNase
inhibitor, Random 6 mers, Oligo dT Primer, dNTP Mixture and buffer
(catalog no., RR036Q; Takara Biotechnology Co., Ltd., Dalian,
China). The protocol included 37°C for 15 min, followed by 85°C for
5 sec and 4°C for a sustained period of time. RT-qPCR was performed
with SYBR Premix Ex Taq™ (Takara Biotechnology Co., Ltd.) and an
ABI7500 instrument (Applied Biosystems; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions (initial
denaturation for 30 sec at 95°C, then 40 cycles of 95°C for 5 sec
and 60°C for 34 sec). Glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) was selected as the control. The following primers were
used for PCR: EZH2 forward primer (5′-3′), GCCAGACTGGGAAGAAATCTG
and EZH2 reverse primer (5′-3′), TGTGCTGGAAAATCCAAGTCA; GAPDH
forward primer, (5′-3′) CGGAGTCAACGGATTTGGTCGTAT and GAPDH reverse
primer, (5′-3′) AGCCTTCTCCATGGTGGTGAAGAC (Sangon Biotech Co., Ltd.,
Shanghai, China). The level of EZH2 messenger RNA (mRNA) expression
was calculated using the 2−ΔΔCq method.
Cell culture
The laryngeal squamous cell cancer AMC-HN-8 cell
line was obtained from the Central Laboratory of the Eye, Ear, Nose
and Throat Hospital of Fudan University. The AMC-HN-8 cell line was
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (FBS; Invitrogen; Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) in a humidified
incubator at 5% CO2 and 37°C. The medium was replaced
every 2 days.
Construction of the EZH2
overexpression lentiviral vector and transfection
Information regarding the lentiviral EZH2
overexpression vector system (Fig. 1)
is described below. The primer sequences were as follows: Human
EZH2-F (Xhol+Flag), CCGCTCGAGGCCACCATGGGCCAGACTGGGAAGAA; and human
EZH2-R (BamHI), CCGGGATCCTCAAGGGATTTCCATTTCTCTT. The primers were
synthesized at Sangon Biotech Co., Ltd.. After the DNA was linked
with the vector using DNA ligase subsequent to double enzyme
digestion (Fig. 1A), the plasmid was
transfected into 293T competent cells. The supernatant was
collected following ultracentrifugation at 4,500 × g. The virus
titer was determined using flow cytometry (BD FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA). Finally, the
EZH2-overexpressing lentiviruses were transfected into AMC-HN-8
cells in the aforementioned conditions of cell culture for 1 day. A
stably transfected cell line overexpressing EZH2 was established
after selection using puromycin. RT-qPCR (using the same protocol
as above) and western blotting were performed to determine the
degree of overexpression.
Western blotting
Total protein was extracted from AMC-HN-8 cells and
EZH2-overexpressing cells with radioimmunoprecipitation assay lysis
and extraction buffer (Beyotime Institute of Biotechnology, Haimen,
China). Western blotting was performed as previously described
(10) using an anti-KMT6/EZH2
antibody (rabbit polyclonal; dilution, 1:1,000; catalog no.,
ab3748; Abcam) and a mouse anti-human β-actin antibody (dilution,
1:1,000; catalog no., A5441; Sigma-Aldrich, St. Louis, MO, USA).
The secondary antibody was a goat anti-rabbit IgG horseradish
peroxidase-conjugated antibody (dilution, 1:2,000; cat no. sc-2030;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The membrane was
developed using an enhanced chemiluminescent substrate (Thermo
Fisher Scientific, Inc.).
Cell proliferation assay
AMC-HN-8 cells and the EZH2-overexpressing AMC-HN-8
cells were cultured in a 96-well plate (Corning Incorporated,
Corning, NY, USA) at a density of 1×103 cells/well in
0.1 ml RPMI-1640 medium, containing 10% FBS in a humidified
incubator at 5% CO2 and 37°C. The medium was changed
every 2 days. The wells containing each cell line were divided into
4 groups, according to the day on which the cell proliferation
assay was performed as follows: Day 1, 3, 5 and 7. Each group
contained 6 replicate wells. cell counting kit-8 reagent (CCK-8; 10
µl; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was
added to the wells, and the plate was incubated for 2.5 h. The
absorbance of each sample at 450 nm was determined using an ELISA
microplate reader (Bio-Rad 680; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Apoptosis assay
AMC-HN-8 cells and EZH2-overexpressing cells were
harvested by adding a trypsin solution without EDTA (Gibco; Thermo
Fisher Scientific, Inc.) followed by the addition of medium
containing FBS to terminate the trypsin activity. The cells were
centrifuged at 800 × g and washed twice with PBS. The cells were
incubated with fluorescein isothiocyanate-conjugated anti-annexin V
(Invitrogen; Thermo Fisher Scientific, Inc.) and propidium iodide
(PI; Invitrogen; Thermo Fisher Scientific, Inc.), and the rates of
apoptosis were determined using flow cytometry (BD FACSCalibur: BD
Biosciences, Inc, Franklin Lakes, NJ, USA). The results were
analyzed using FlowJo 7.6 (FlowJo, LLC, Ashland, OR, USA).
Cell cycle assay
AMC-HN-8 cells and EZH2-overexpressing cells
(1×106) were washed twice with PBS and were centrifuged
at 800 × g. The cells were then suspended in 1 ml of PBS, to which
9 ml of 70% ethanol was slowly added while vortexing. The cells
were maintained at 4°C overnight. Prior to flow cytometric
analysis, the cells were washed twice with PBS, treated with 10
mg/ml RNase A (Sigma-Aldrich) for 30 min, and stained with 50 µg/ml
PI in the dark for 1 h. Each cell type was analyzed in
triplicate.
Chemotherapy sensitivity assay
AMC-HN-8 cells and EZH2-overexpressing cells were
cultured in a 96-well plate (Corning Incorporated) at a density of
1×104/ml. The two types of cells were divided into 4
groups, according to the concentration of cisplatinum applied.
Cisplatinum was applied to the groups at concentrations of 0, 3, 6
and 12 µg/ml. Each group contained 6 replicate wells. Subsequent to
incubation with cisplatinum for 24 h, the number of cells in each
well was determined using a CCK-8 assay, following the
aforementioned method. The growth inhibition rate was calculated
using the following formula: Inhibition rate = 1 - (mean absorbance
of the test well) / (mean absorbance of the control well) ×
100%.
In vivo tumorigenicity test
Six non-obese diabetic (NOD) mice (4-weeks old,
male) were purchased from Shanghai Super-B&K Laboratory Animal
Corp., Ltd. (Shanghai, China). AMC-HN-8 cells and
EZH2-overexpressing cells were trypsinized, resuspended in
RPMI-1640 medium at a density of 1×107/ml, and then
injected into the subcutaneous space of the axillary fossa of mice
under ketamine/xylazine-induced anesthesia (2×106
cells/mouse). After 20 days, the NOD mice were sacrificed by
cervical dislocation, and the tumors were removed surgically. The
tumors were photographed, weighed, fixed with paraformaldehyde,
stained with hematoxylin-eosin and examined.
Statistical analysis
All experiments were performed independently 3
times. All results, with the exception of the immunohistochemical
results, were expressed as the mean values ± standard deviation and
were analyzed with the independent sample Student's t-test. The
immunohistochemical results were analyzed using the χ2
test. IBM SPSS statistics version 20 (IBM SPSS, Armonk, NY, USA)
was used to compare the statistical difference between EZH2
overexpression cells and AMC-HN-8 cells. A P-value of <0.05 was
considered to indicate a statistically significant difference.
Results
EZH2 is expressed more highly in
laryngeal squamous cell cancer tissues compared with paracancerous
epithelial tissues
In the present study, RT-qPCR and
immunohistochemical assays of primary laryngeal squamous cell
cancer tissues and paracancerous epithelial tissues were performed
to detect the expression of EZH2 at the transcriptional level and
protein expression level, respectively. The total mRNA of 25 tumor
tissues and 25 corresponding paracancerous epithelial tissues was
extracted and then reverse transcribed into cDNA, which was
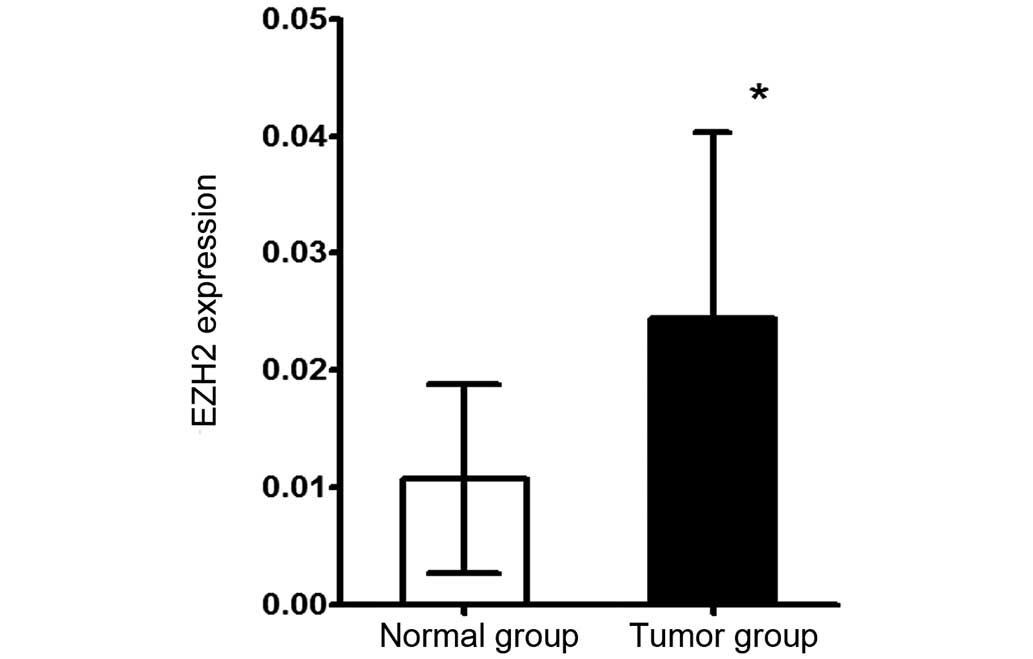
utilized for qPCR. This quantitative analysis showed that the EZH2
mRNA expression level in the tumor tissues was significantly
increased compared with paracancerous epithelial tissues (P=0.0003;
Fig. 2). To determine whether EZH2
was highly expressed at the protein level in tumor tissues, 80
tumor tissue samples and 80 corresponding paracancerous epithelial
tissue samples from 80 patients were used to prepare a tissue
microarray for the immunohistochemical detection of EZH2 protein.
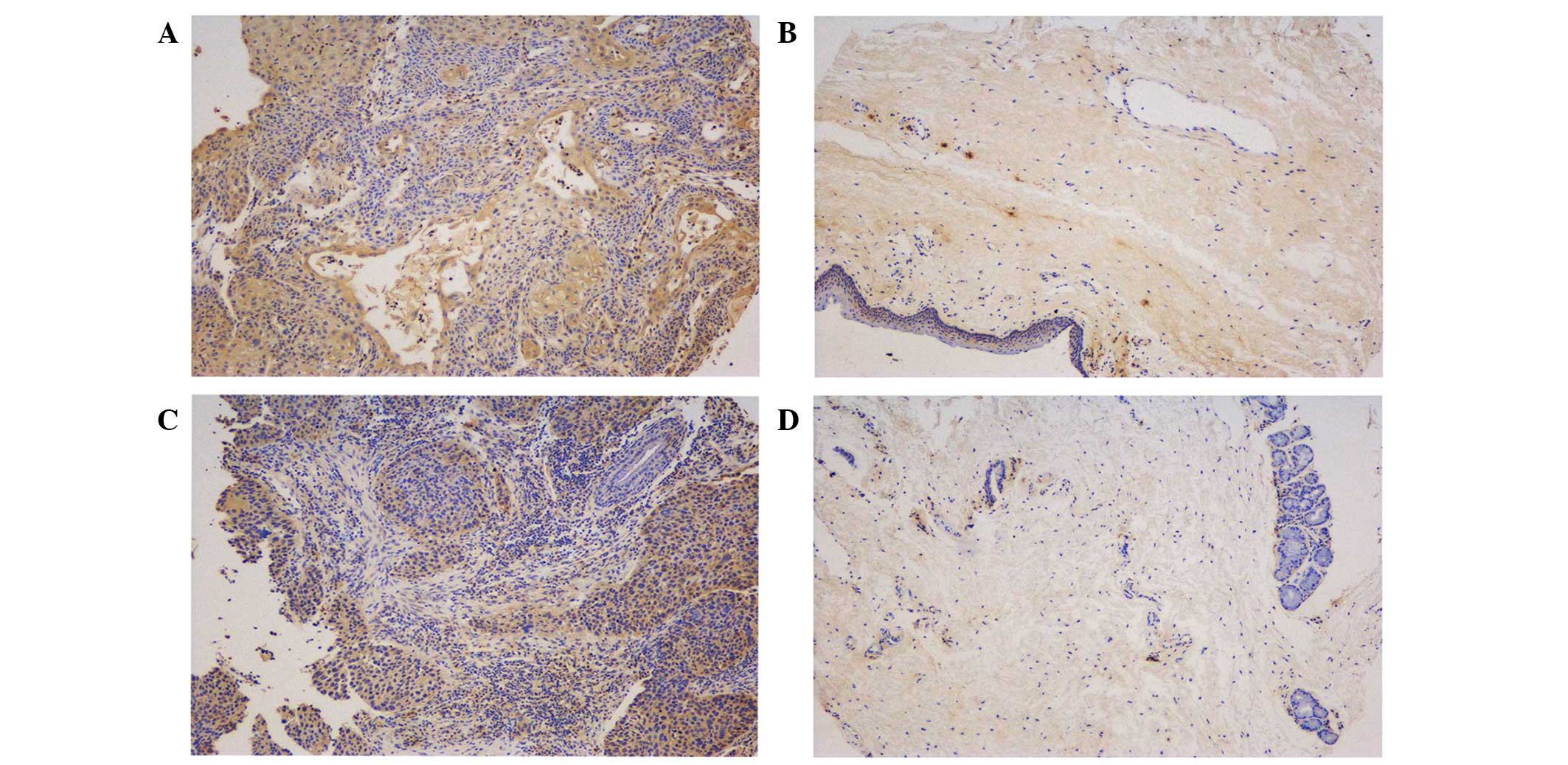
Representative images are shown in Fig.
3. The tumor and paracancerous tissues expressed EZH2; however,
the expression levels were significantly different between the two
groups (P=0.0004). EZH2 was diffusely distributed throughout the
primary tumor tissues, but was mainly expressed in the basal layer
in the paracancerous tissue samples, which indicated that EZH2 may
be involved in cell proliferation. By comparing the immunoreactive
scores for the two types of tissues, EZH2 was found to be highly
expressed in the tumor tissues, which supported the RT-qPCR
results. To investigate the association between the level of EZH2
expression and the clinical characteristics of the laryngeal
squamous cells cancers, the level of EZH2 expression in tumors of
various clinical stages and locations was compared. EZH2 was
expressed more highly in glottic cancers compared with nonglottic
cancers (P=0.007), but there was no significant difference in the
levels of EZH2 expression between T1-T2 stage and T3-T4 stage
tumors (P=0.982; Table I).
 | Table I.Clinical indices and EZH2
expression. |
Table I.
Clinical indices and EZH2
expression.
|
| EZH2
expression |
|---|
|
|
|
|---|
| Clinical index | Negative | Positive | P-value |
|---|
| TNM stage |
|
|
|
|
I–II | 38 | 21 |
0.982 |
|
III–IV | 11 | 6 |
|
| Tumor location |
|
|
|
|
Glottic | 37 | 13 |
0.007 |
|
Nonglottic | 11 | 15 |
|
| Tissue type |
|
|
|
| Tumor
tissue | 48 | 28 | <0.001 |
| Normal
tissue | 63 | 8 |
|
EZH2 overexpression stimulates the
proliferation of AMC-HN-8 cells through promoting entry into the
synthesis phase of the cell cycle
Through transfection of an EZH2 overexpression
lentiviral vector, a cell line that stably overexpressed EZH2 was
established. In this cell line, the transfection effects were
confirmed by western blot analysis at protein level (Fig. 1B), the level of EZH2 expression was
30-fold greater compared with the control group (P=0.011; Fig. 1C) and the efficacy of this expression
was not reduced by passaging the cells. To investigate the role of
EZH2 in the proliferation of AMC-HN-8 cells, a proliferation assay
was performed using a CCK-8. The results showed that the
transfected cells exhibited an increased proliferative ability
compared with the control group (Fig.
1D). In particular, the proliferative capacity of the
transfected cells was significantly greater compared with the
AMC-HN-8 cells between the fifth (P=0.012) to the seventh (P=0.004)
of culture (Table II), suggesting
that EZH2 overexpression enhanced the proliferative capacity of the
AMC-HN-8 cells. To examine the effect of EZH2 overexpression on
AMC-HN-8 cells, cell cycle and apoptosis analyses were conducted
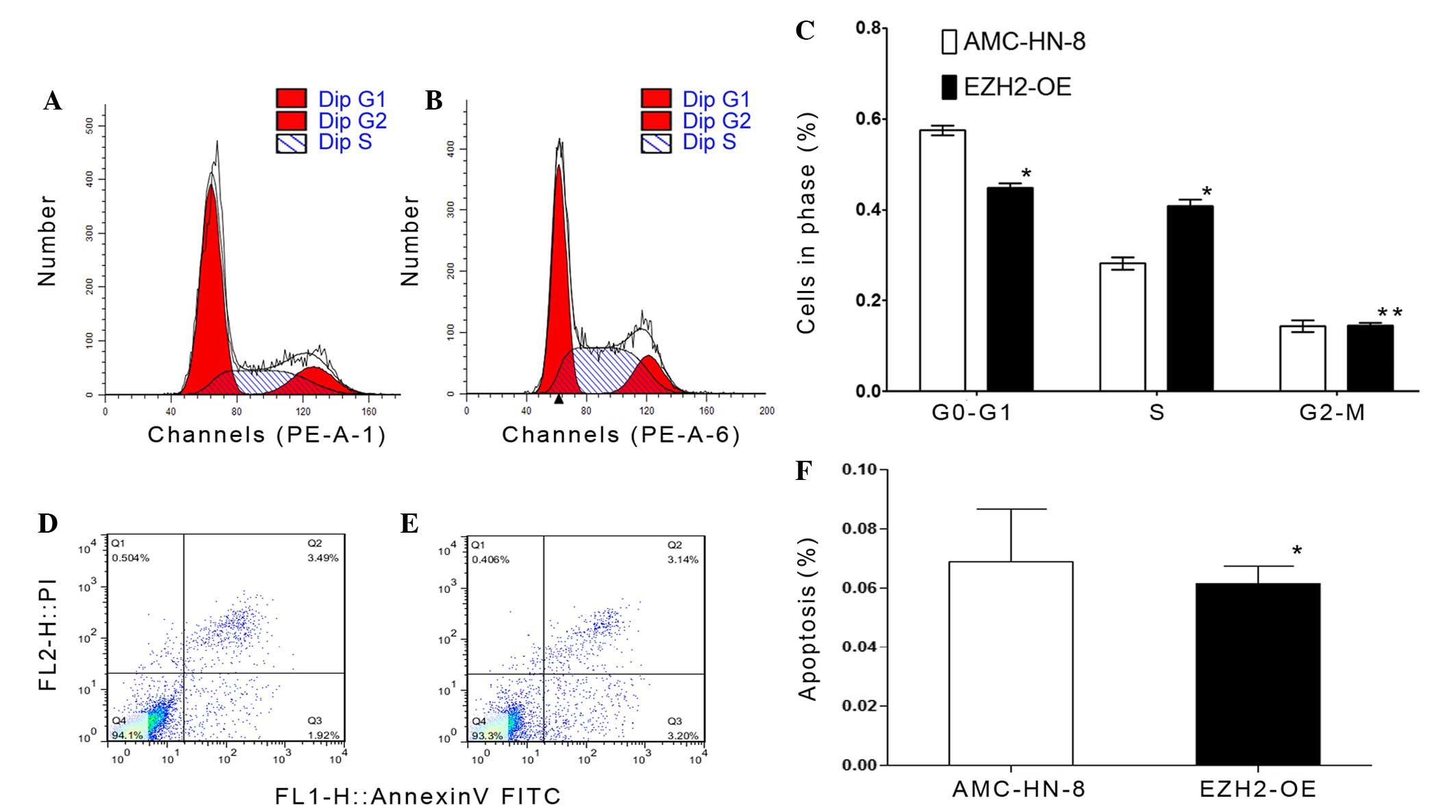
using flow cytometry. The cell cycle assay revealed that fewer
EZH2-overexpressing cells had accumulated in the G0-G1 phase
(P=0.001), but that more had accumulated in the synthesis phase
(P=0.001) compared with the control cells (Fig. 4A-C). Subsequently, an apoptosis assay
was performed by staining cells with anti-annexin-V and PI
(Fig. 4D and E). The results showed
that the average apoptosis rate of EZH2-overexpressing cells was
6.870±1.803%, whereas the rate for the control group was
6.150±0.583% (Fig. 4F), and these
rates were not significantly different (P=0.545).
 | Table II.Absorbance of the AMC-HN-8 cells and
the EZH2-OE cells. |
Table II.
Absorbance of the AMC-HN-8 cells and
the EZH2-OE cells.
|
| Day |
|---|
|
|
|
|---|
| Type | 1a | 3b | 5c | 7d |
|---|
| EZH2-OE | 0.3242±0.0636 | 0.5148±0.1100 | 0.9573±0.0870 | 1.7426±0.1932 |
| AMC-HN-8 | 0.2365±0.0451 | 0.3711±0.0235 | 0.6345±0.0396 | 0.9988±0.0925 |
EZH2 overexpression induces
chemotherapy resistance
To evaluate the chemotherapy sensitivity of control
and EZH2-overexpressing AMC-HN-8 cells, cisplatinum was applied to
the cells and the rate of the tumor cell growth inhibition was
determined. As shown in Fig. 5, the
rate of growth inhibition of the two types of cells increased with
the increasing dose of cisplatin. The rate of inhibition of the
growth of the transfected tumor cells was significantly decreased
compared with the control AMC-HN-8 cells, and the statistical
analysis showed that the rates were significantly different in the
3 µg/ml (P=0.027) and 6 µg/ml (P=0.006) groups (Table III). Therefore, EZH2 overexpression
increased the level of cisplatin resistance in AMC-HN-8 cells.
 | Table III.Growth inhibition rates of AMC-HN-8
cells and EZH2-OE cells exposed to cisplatinum, a chemotherapeutic
drug. |
Table III.
Growth inhibition rates of AMC-HN-8
cells and EZH2-OE cells exposed to cisplatinum, a chemotherapeutic
drug.
|
| Dose |
|---|
|
|
|
|---|
| Type | 3
µg/mla | 6
µg/mlb | 12
µg/mlc |
|---|
| EZH2-OE | 0.4996±0.0540 | 0.6022±0.0331 | 0.7063±0.0201 |
| AMC-HN-8 | 0.2856±0.0940 | 0.3662±0.0680 | 0.5305±0.0842 |
EZH2 overexpression promotes
tumorigenesis in vivo
To investigate whether EZH2 overexpression affected
tumorigenesis in vivo, NOD mice were used to perform a
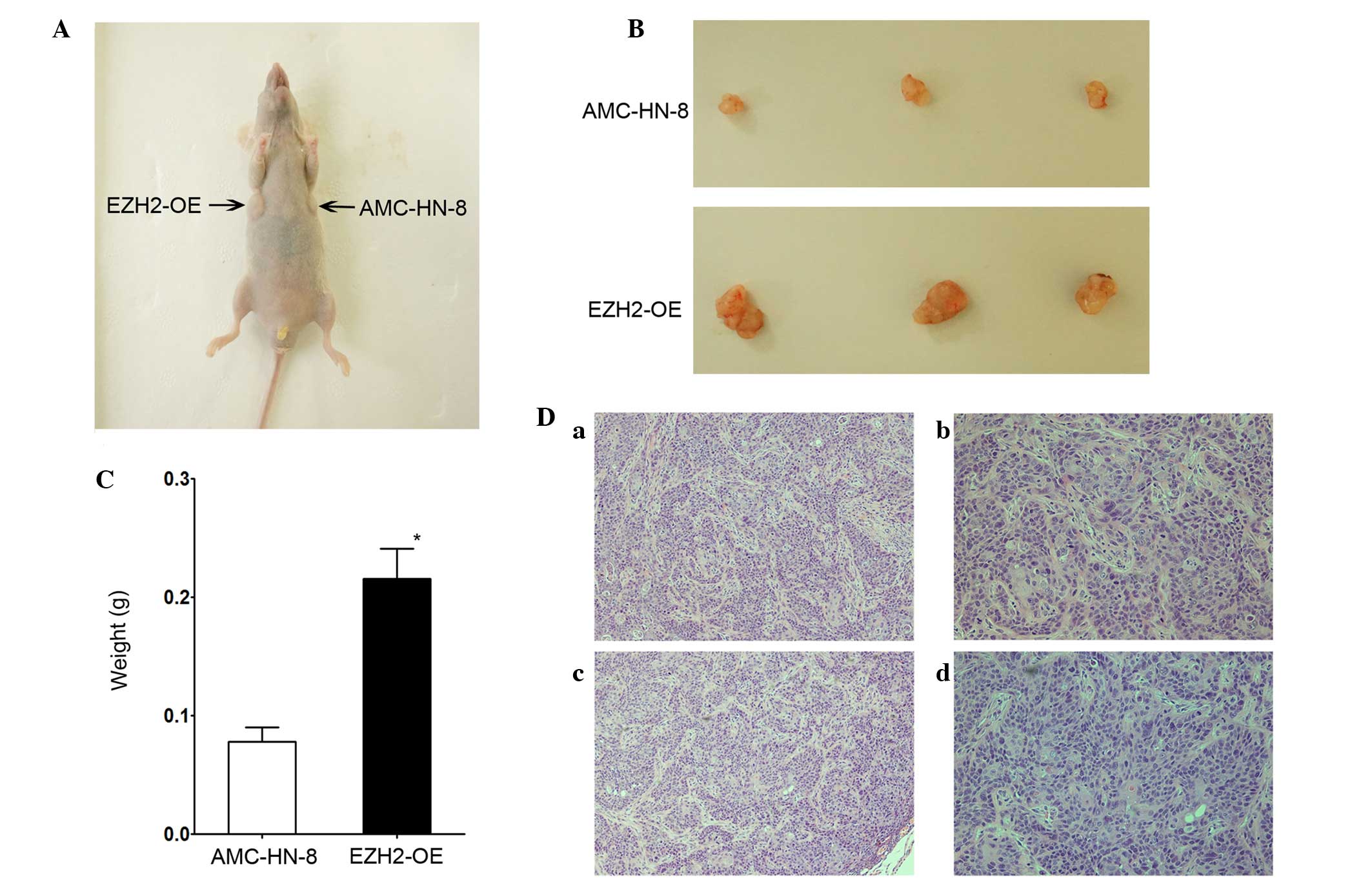
tumorigenesis assay. Fig. 6A shows an
image of a representative mouse, 20 days subsequent to being
injected with control and transfected AMC-HN-8 cells. Tumors were
present in each armpit of the mice. At 20 days post-injection, the
mice were sacrificed, and the tumors were surgically removed.
Fig. 6B shows images of the two types
of tumors that grew in the mice. Fig.
6C shows that the tumors derived from the EZH2-overexpressing
cells were significantly larger compared with those derived from
the AMC-HN-8 cells. The mean tumor weight in the transfected group
was greater compared with the AMC-HN-8 group, with values of
0.2157±0.0256 and 0.0780±0.0303 g for tumors derived from
EZH2-overexpressing cells and the control AMC-HN-8 cells (P=0.001),
respectively. Fig. 6D shows images of
hematoxylin-eosin stained tumor sections. A professional
pathologist determined that the histological characteristics of the
sections were those of tumor tissues. In summary, EZH2
overexpression promoted the tumorigenesis of laryngeal squamous
cell carcinoma cells in vivo.
Discussion
Patients with advanced stage laryngeal cancers
demonstrate a low rate of successful treatment when treated using
traditional therapies, including surgery, chemotherapy and
radiotherapy (11). In order to
optimize the effects of the traditional therapies, novel
treatments, including gene-targeted therapies, are currently being
explored. EZH2, a core catalytic subunit of PRC2, has been
previously reported to mediate the proliferation and
differentiation of hematopoietic (12), skeletal-muscle (13) and neural stem cells (14). In addition, EZH2 has been reported to
be involved in sustaining the proliferative capacity and preventing
the apoptosis of prostate cancer stem cell-like lines (6). EZH2 is highly expressed in numerous
malignant tumors, including inflammatory breast cancer (15), lung cancer (16), renal cell carcinoma (17), cutaneous melanoma and prostate and
endometrial cancer (18). EZH2 is
also highly expressed in head and neck squamous cell cancers
(19,20). Kidani et al (19) reported that high-level EZH2 expression
is associated with a poor prognosis of oral squamous cell cancers.
Another study reported that EZH2 is highly expressed in certain
nasopharyngeal carcinomas and is associated with a poor clinical
outcome (20). These studies
indicated that EZH2 may be a useful biomarker for making future
clinical diagnoses and for predicting the clinical outcome of these
diseases. The mechanisms of EZH2 have been examined in numerous
studies, and to date, EZH2 has been reported to affect the
proliferation, apoptosis, cell cycle and invasion of cancer cells
(18–23). Therefore, EZH2 is categorized as an
oncogene in certain types of tumors, which may influence
pharmaceutical companies to develop a drug that specifically
targets it. However, the expression level and function of EZH2 in
laryngeal cancers is unknown. The present study revealed that the
expression levels of EZH2 in primary laryngeal tumor tissues were
increased compared with paracancerous epithelial tissues, and
indicated that the upregulation of EZH2 expression promoted
AMC-HN-8 cell proliferation in vitro by inducing the cells
to pass the G0-G1 checkpoint. Notably, the present study
demonstrated that EZH2 overexpression diminished sensitivity to
cisplatin and facilitated the in vivo tumorigenicity of the
cells.
In the present study, the expression of EZH2 was
evaluated in specimens collected from patients that suffered from
laryngeal cancers and had received surgery at the Eye, Ear, Nose
and Throat Hospital of Fudan University. RT-qPCR and tissue
microarray assays revealed that EZH2 was expressed at a
significantly greater level in tumor tissues compared with the
paracancerous epithelial tissues. However, unlike the findings
regarding other polycomb-group proteins, such as BMI1
proto-oncogene, polycomb ring finger (BMI1) (24), the present study found that the
increased expression level of EZH2 was associated with the location
but not the stage of the tumors. In addition, glottic cancers had
greater EZH2 expression levels compared with nonglottic cancers.
However, the difference in the levels of EZH2 expression in tumors
of various stages was not significant, the reasons for which are
unknown and require further investigation. In the present study,
clear differences were observed between the EZH2 expression levels
of individual tumors in the same group, which indicated that the
value of EZH2 as a clinical biomarker in laryngeal squamous cell
cancers may be limited. Increased numbers of specimens must be
examined in the future to expand the sample size and verify the
results of the present study. The tissue microarrays may provide
additional information, including potential correlations between
the level of EZH2 expression and the 5-year survival rate, but this
cannot be determined for a few years. In summary, the present study
demonstrated that EZH2 was highly expressed in laryngeal cancers,
which is consistent with previous findings regarding other types of
cancer. However, the use of EZH2 expression as a diagnostic or
prognostic factor requires additional assessment.
Several studies have reported that EZH2 is essential
to the renewal and differentiation of numerous types of stem cells
(25,26). The enhanced expression of EZH2 allows
these cells to more easily undergo malignant transformation. In
contrast, the knockdown of EZH2 expression suppresses the
proliferation of cancer cells in numerous malignancy models,
including prostate cancer (6), breast
cancer (27) and lymphoma (28). However, certain studies have reported
that the knockdown of EZH2 and BMI1 expression does not prevent
osteosarcoma cell proliferation (29). In the case of head and neck squamous
cell cancers, certain studies have demonstrated that EZH2 affects
cancer cell proliferation. For example, Zhao et al reported
that the suppression of EZH2 expression reduces the proliferative
ability of oral squamous cell carcinoma (10). In another study, Alajez et al
(20) reported that the targeted
depletion of EZH2 decreases the viability of nasopharyngeal
carcinoma cells. These studies suggest that EZH2 may affect the
stem cell-like properties of cancer cells. Therefore, the present
tested whether this hypothesis was valid for laryngeal squamous
cell carcinomas. EZH2 expression was upregulated through lentiviral
transfection, and RT-qPCR and western blotting were used to verify
the upregulation of EZH2. The findings demonstrated that the
upregulation of EZH2 expression significantly enhanced the
proliferation of AMC-HN-8 cells in vitro and in vivo.
These results were consistent with those of previous reports
regarding head and neck squamous cell cancers (19,20).
Cell cycle progression and apoptosis affect the rate
of cell proliferation. Previous studies have shown that EZH2 is
important for the cell cycle and apoptosis by affecting other
signaling pathways. Wu et al (30) reported that the depletion of EZH2
results in defective G1 and G2/M cell cycle checkpoints and that
EZH2 depletion promotes apoptosis. Zhang et al (31)reported that the downregulation of EZH2
expression increases the rate of the docetaxel-induced apoptosis of
prostate cancer cells. Thus, the present study used cell cycle and
apoptosis assays to evaluate the effect of EZH2 overexpression. The
results revealed that EZH2 overexpression shortened the G0-G1
phase, increased the percentage of cells entering S phase and
induced more efficient cell proliferation. Consistent with the
results of previous studies, the present results demonstrated that
EZH2 affected the cell cycle. However, although EZH2 overexpression
did not affect the rate of apoptosis, whether the suppression of
EZH2 expression promotes apoptosis remains unknown.
Cancer stem cells are responsible for the
chemotherapy resistance of tumors. EZH2 is a cancer stem
cell-specific gene, so the effect of EZH2 on the chemotherapy
resistance of tumors is worth studying (32). Hu et al (33) reported that EZH2 overexpression
contributes to the acquired cisplatin resistance of ovarian cancer
cells in vitro and vivo. Meng et al (34) reported that EZH2 overexpression is
associated with a decreased 5-year survival rate of rectal cancer
patients treated with neoadjuvant chemotherapy. Therefore, the
cisplatin sensitivity of control and EZH2-overexpressing AMC-HN-8
cells was assessed in the present study. Consistent with the
results of previous studies, the present findings showed that EZH2
had a similar effect on the drug resistance of laryngeal squamous
cancer cells, indicating that EZH2 can enhance the drug resistance
of cancer cells. This finding suggests that EZH2 may provide a
novel approach for promoting the effect of chemotherapy on
laryngeal cancer patients.
The results of the present study indicated that EZH2
may be an important target in laryngeal cancer stem cells. However,
additional studies regarding the role of EZH2 in other properties
of cancer stem cells are required. For example, studies have
indicated that EZH2 promotes the invasion and angiogenesis of
certain types of cancer cells (21,35). In
addition, in the preliminary experiments of the present study, the
downregulation of EZH2 expression was observed to enhance the
invasive ability of AMC-HN-8 cells. Thus, EZH2 could increase the
invasive ability of laryngeal cancer cells, and high-level
expression may result in a poor outcome for laryngeal cancer
patients. The authors intend to investigate the association between
EZH2 expression and the invasion of laryngeal cancer cells in the
future. Since non-coding RNA has become a topic of intense
research, various studies have focused on the association between
EZH2 and non-coding RNA. Benetatos et al reported that
non-coding RNAs affect the expression of protein-coding genes and
establish feedback loops through interacting with EZH2 (36). Non-coding RNAs have also been
indicated to participate in networks involving upstream and
downstream factors that require EZH2 (36). Notably, a recent study indicated that
the expression of several non-coding RNAs, including long
non-coding RNAs CDKN2B antisense RNA 1, HOX transcript antisense
RNA and metastasis associated lung adenocarcinoma transcript 1, was
dramatically decreased with increasing concentrations of cisplatin
and longer treatment durations in patients with laryngeal cancer,
who received cisplatin therapy (37).
Thus, EZH2 and several non-coding RNAs affect the level of
chemotherapy resistance of tumors. Additional studies should
therefore be performed in order to determine the involvement of
these proteins and to investigate the relevance of the signaling
pathways associated with this process.
In conclusion, the present study evaluated the
association between EZH2 expression and laryngeal squamous cell
cancers. EZH2 was demonstrated to be highly expressed in laryngeal
squamous cell cancer tissues and to promote the proliferation of
AMC-HN-8 cells in vitro and in vivo. Furthermore, the
overexpression of EZH2 was found to accelerate the cell cycle of
AMC-HN-8 cells and to enhance their resistance to cisplatin.
Therefore, the present study demonstrated that EZH2 is a factor
that regulates the proliferation of laryngeal squamous cancer cells
and is a potential chemotherapeutic target for the treatment of
such cancers.
Acknowledgements
The authors would like to thank Dr Zhang Duo for his
assistance with the qPCR experiment. The present study was
supported by the Shanghai Science and Technology Foundation,
Shanghai, China (grant no., 08JC1404000).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simon JA and Lange CA: Roles of the EZH2
histone methyltransferase in cancer epigenetics. Mutat Res.
647:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vire E, Brenner C, Deplus R, Blanchon L,
Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden
JM, et al: The Polycomb group protein EZH2 directly controls DNA
methylation. Nature. 439:871–874. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee TI, Jenner RG, Boyer LA, Guenther MG,
Levine SS, Kumar RM, et al: Control of developmental regulators by
polycomb in human embryonic stem cells. Cell. 125:301–313. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ezhkova E, Pasolli HA, Parker JS, Stokes
N, Su IH, Hannon G, Tarakhovsky A and Fuchs E: Ezh2 orchestrates
gene expression for the stepwise differentiation of tissue-specific
stem cells. Cell. 136:1122–1135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li KQ, Liu C, Zhou BF, Bi L, Huang H, Lin
T and Xu K: Role of EZH2 in the growth of prostate cancer stem
cells isolated from LNCaP cells. Int J Mol Sci. 14:11981–11993.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rizzo S, Hersey JM, Mellor P, Dai W,
Santos-Silva A, Liber D, Luk L, Titley I, Carden CP, Box G, Hudson
DL, Kaye SB and Brown R: Ovarian cancer stem cell-like side
populations are enriched following chemotherapy and overexpress
EZH2. Mol Cancer Ther. 10:325–335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sobin LH, Gospodarowicz MK and Wittekind
C: UICC TNM classification of malignant tumours (7th).
Wiley-Blackwell. Oxford: 63–72. 2009.
|
|
9
|
Shi Y, Gong HL, Zhou L, Tian J and Wang Y:
CD24: A novel cancer biomarker in laryngeal squamous cell
carcinoma. ORL J Otorhinolaryngol Relat Spec. 74:78–85. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao LB, Yu Y, Wu J, Bai J, Zhao Y, Li C,
Sun W and Wang X: Role of EZH2 in oral squamous cell carcinoma
carcinogenesis. Gene. 537:197–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blake JL, Runhua S, Vikas M, Glenn M,
Federico A and Cherie-Ann ON: Improvements in survival and
disparities for advanced-staged laryngeal cancer. JAMA Otolaryngol
Head Neck Surg. 141:169–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kamminga LM, Bystrykh LV, de Boer A,
Houwer S, Douma J, Weersing E, Dontje B and de Haan G: The polycomb
group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood.
107:2170–2179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Juan AH, Derfoul A, Feng X, Ryall JG,
Dell'Orso S, Pasut A, Zare H, Simone JM, Rudnicki MA and Sartorelli
V: Polycomb EZH2 controls self-renewal and safeguards the
transcriptional identity of skeletal muscle stem cells. Gene Dev.
25:789–794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sher F, Rössler R, Brouwer N,
Balasubramaniyan V, Boddeke E and Copray S: Differentiation of
neural stem cells into oligodendrocytes: Involvement of the
polycomb group protein Ezh2. Stem Cells. 26:2875–2883. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong Y, Huo L, Liu P, Sneige N, Sun X,
Ueno NT, Lucci A, Buchholz TA, Valero V and Cristofanilli M:
Polycomb group protein EZH2 is frequently expressed in inflammatory
breast cancer and is predictive of worse clinical outcome. Cancer.
117:5476–5484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wan L, Li X, Shen H and Bai X:
Quantitative analysis of EZH2 expression and its correlations with
lung cancer patients' clinical pathological characteristics. Clin
Transl Oncol. 15:132–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wagener N, Macher-Goeppinger S, Pritsch M,
Hüsing J, Hoppe-Seyler K, Schirmacher P, Pfitzenmaier J, Haferkamp
A, Hoppe-Seyler F and Hohenfellner M: Enhancer of zeste homolog 2
(EZH2) expression is an independent prognostic factor in renal cell
carcinoma. Bmc Cancer. 10:5242010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bachmann IM, Halvorsen OJ, Collett K,
Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP, et al:
EZH2 expression is associated with high proliferation rate and
aggressive tumor subgroups in cutaneous melanoma and cancers of the
endometrium, prostate and breast. J Clin Oncol. 24:268–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kidani K, Osaki M, Tamura T, Yamaga K,
Shomori K, Ryoke K and Ito H: High expression of EZH2 is associated
with tumor proliferation and prognosis in human oral squamous cell
carcinomas. Oral Oncol. 45:39–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alajez NM, Shi W, Hui AB, Bruce J,
Lenarduzzi M, Ito E, Yue S, O'Sullivan B and Liu F: Enhancer of
Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal
carcinoma and is regulated by miR-26a, miR-101 and miR-98. Cell
Death Dis. 1:e852010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rao ZY, Cai MY, Yang GF, He LR, Mai SJ,
Hua WF, Liao YJ, Deng HX, Chen YC, Guan XY, et al: EZH2 supports
ovarian carcinoma cell invasion and/or metastasis via regulation of
TGF-beta1 and is a predictor of outcome in ovarian carcinoma
patients. Carcinogenesis. 31:1576–1583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tong ZT, Cai MY, Wang XG, Kong LL, Mai SJ,
Liu YH, Zhang HB, Liao YJ, Zheng F, Zhu W, et al: EZH2 supports
nasopharyngeal carcinoma cell aggressiveness by forming a
co-repressor complex with HDAC1/HDAC2 and Snail to inhibit
E-cadherin. Oncogene. 31:583–594. 2012.PubMed/NCBI
|
|
23
|
Crea F, Fornaro L, Bocci G, Sun L, Farrar
WL, Falcone A and Danesi R: EZH2 inhibition: Targeting the
crossroad of tumor invasion and angiogenesis. Cancer Metast Rev.
31:753–761. 2012. View Article : Google Scholar
|
|
24
|
Chen H, Zhou L, Wan G, Dou T and Tian J:
BMI1 promotes the progression of laryngeal squamous cell carcinoma.
Oral Oncol. 47:472–481. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suvà ML, Riggi N, Janiszewska M,
Radovanovic I, Provero P, Stehle JC, Baumer K, Le Bitoux MA, Marino
D, Cironi L, et al: EZH2 Is Essential for glioblastoma cancer stem
cell maintenance. Cancer Res. 69:9211–9218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Majewski IJ, Blewitt ME, de Graaf CA,
McManus EJ, Bahlo M, Hilton AA, Hyland CD, Smyth GK, Corbin JE,
Metcalf D, et al: Polycomb repressive complex 2 (PRC2) restricts
hematopoietic stem cell activity. PLoS Biol. 6:e932008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Vlerken LE, Kiefer CM, Morehouse C, Li
Y, Groves C, Wilson SD, Yao Y, Hollingsworth RE and Hurt EM: EZH2
is required for breast and pancreatic cancer stem cell maintenance
and can be used as a functional cancer stem cell reporter. Stem
Cells Transl Med. 2:43–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McCabe MT, Ott HM, Ganji G, Korenchuk S,
Thompson C, Van Aller GS, Liu Y, Graves AP, Della Pietra A III,
Diaz E, et al: EZH2 inhibition as a therapeutic strategy for
lymphoma with EZH2-activating mutations. Nature. 492:108–112. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sasaki H, Setoguchi T, Matsunoshita Y, Gao
H, Hirotsu M and Komiya S: The knock-down of overexpressed EZH2 and
BMI-1 does not prevent osteosarcoma growth. Oncol Rep. 23:677–684.
2010.PubMed/NCBI
|
|
30
|
Wu Z, Lee ST, Qiao Y, Li Z, Lee PL, Lee
YJ, Jiang X, Tan J, Aau M, Lim CZ and Yu Q: Polycomb protein EZH2
regulates cancer cell fate decision in response to DNA damage. Cell
Death Differ. 18:1771–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Q, Padi SK, Tindall DJ and Guo B:
Polycomb protein EZH2 suppresses apoptosis by silencing the
proapoptotic miR-31. Cell Death Dis. 5:e14862014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Serguei V and Xin W: Cancer stem cells and
drug resistance: The potential of nanomedicine. Nanomedicine.
7:597–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu S, Yu L, Li Z, Shen Y, Wang J, Cai J,
Xiao L and Wang Z: Overexpression of EZH2 contributes to acquired
cisplatin resistance in ovarian cancer cells in vitro and in vivo.
Cancer Biol Ther. 10:788–795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng X, Huang Z, Wang R, Jiao Y, Li H, Xu
X, Feng R, Zhu K, Jiang S, Yan H and Yu J: The prognostic role of
EZH2 expression in rectal cancer patients treated with neoadjuvant
chemoradiotherapy. Radiat Oncol. 9:1882014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu CH, Han HD, Mangala LS, Ali-Fehmi R,
Newton CS, Ozbun L, Armaiz-Pena GN, Hu W, Stone RL, Munkarah A, et
al: Regulation of tumor angiogenesis by EZH2. Cancer Cell.
18:185–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benetatos L, Voulgaris E, Vartholomatos G
and Hatzimichael E: Non-coding RNAs and EZH2 interactions in
cancer: Long and short tales from the transcriptome. Int J Cancer.
133:267–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen H, Xin Y, Zhou L, Huang JM, Tao L,
Cheng L and Tian J: Cisplatin and paclitaxel target significant
long noncoding RNAs in laryngeal squamous cell carcinoma. Med
Oncol. 31:2462014. View Article : Google Scholar : PubMed/NCBI
|