Introduction
Cancer is the predominant cause of mortality in the
United States and various other countries worldwide (1). Lung cancer is a major health issue
worldwide and is primarily caused by tobacco smoking (2–5).
Clinically, lung cancer may be categorized into two subtypes: Small
cell lung cancer (SCLC) and non-SCLC (NSCLC) (6). For the majority of lung cancer cases,
the average survival time from diagnosis is only 8 months (7). In China, lung cancer has been the most
common cancer diagnosis and leading cause of cancer-associated
mortality for a number of years (8),
with previous studies describing an increasing trend (9,10). At
present, treatment for lung cancer includes surgery, chemotherapy
and radiotherapy. Whilst surgery is considered to be the optimal
choice, only 20–25% of lung tumors are suitable for potentially
curative resection (11). Two
individual participant data meta-analyses reported that
postoperative chemotherapy, with or without radiotherapy, improved
survival (11). Preoperative
chemotherapy has the potential to reduce tumor size, increase
operability and eradicate micrometastases. However, chemotherapy
may also be ineffective, resulting in delayed surgery with tumors
possibly becoming unresectable (11,12).
Therefore, exploiting novel chemicals is important to potentially
improve the treatment of lung cancer.
N,
N′-di-(m-methylphenyi)-3,6-dimethyl-1,4-dihydro-1,2,4,
5-tetrazine-1,4-dicarboamide (ZGDHu-1) is a novel tetrazine
derivative synthesized by Wei-xiao Hu (Pharmaceutical College of
Zhejiang University of Technology, China) who obtained a patent for
this chemical in China (13,14). Previous studies have demonstrated that
ZGDHu-1 inhibits proliferation, induces apoptosis (15,16) and
markedly suppresses the cell cycle at the G2/mitotic (M)
phase (17) in leukemia cells.
Furthermore, it has been reported that ZGDHu-1 possesses anti-tumor
activity, and may induce apoptosis and inhibit proliferation in
lung cancer cells (18). However, the
mechanisms by which ZGDHu-1 functions to inhibit the cell cycle in
human lung cancer cells remain to be elucidated.
The cell cycle is a complex and precise process, and
includes M, G1, S and G2 phases. Regulation
of the cell cycle predominantly depends on the regulatory network,
which includes cyclin-dependent kinases (CDKs), cyclins and
cyclin-dependent kinase inhibitors (CKIs) (19,20).
G2/M is important for the entrance of cells into M
phase, and has also been associated with resistance of tumor cells
to chemotherapy (21). During the
G2/M arrest, the expression of the Cdc2/cyclin B1 (also
known as CDK1) complex is altered, resulting in incomplete mitosis
and mitotic catastrophe, which induces cell death (17).
The current study aimed to investigate the mechanism
by which ZGDHu-1 induces apoptosis and G2/M phase arrest
in A549 and RERF-LC-MA lung cancer cells.
Materials and methods
Cell culture
The A549 and RERF-LC-MA human lung cancer cell lines
were provided by Dr. Hong Wang (Department of Respiratory Medicine,
Zhejiang Provincial People's Hospital, Hangzhou, China). The cells
were cultured in RPMI 1640 medium containing 10% fetal bovine serum
(FBS), HEPES, 100 U/ml penicillin and 100 µg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a
humidified atmosphere with 5% CO2 at 37°C.
Preparation of ZGDHu-1
ZGDHu-1 was provided by the Pharmaceutical
Engineering Research Institute, College of Pharmaceutical Science,
Zhejiang University of Technology (Hangzhou, China). ZGDHu-1 was
dissolved in dimethyl sulfoxide as a stock solution (1 mg/ml) and
stored at −20°C. For the experiment, the final working
concentration (10 µg/ml) was resuspended in the RPMI 1640 media
supplemented with 10% FBS.
Flow cytometry cell cycle and DNA
ploidy analysis
DNA Prep™ reagent system (Beckman Coulter, Inc.,
Indianapolis, IN, USA) was used to analyze cell cycle alterations
and DNA ploidy in A549 and RERF-LC-MA cells, respectively. Firstly,
A549 or RERF-LC-MA cells (2×108 cells/l) were seeded
into 6-well plates overnight and exposed to various concentrations
of ZGDHu-1 (2, 10, 50, 100, 200 and 500 µg/l), RPMI 1640 medium
(negative control) or fluorouracil (5-FU; 5 ng/l; positive control;
Tianjin Jinyao Amino Acid Co., Ltd., Tianjin, China) for 24 h or 48
h. Cells were subsequently harvested with trypsin, collected by
centrifugation (192 × g for 5 min) and washed twice with cold PBS.
The pellet was incubated with 50 µl DNA PREP LPR (containing RNase;
Beckman Coulter, Inc.) for 1 min and then treated with DNA PREP
stain [containing propidium iodide (PI), Beckman Coulter, Inc.] in
a dark place for 5 min at room temperature. Following incubation,
the samples were analyzed by flow cytometry (Cytomics FC 500;
Beckman Coulter, Inc.) and MultiCycle AV software (Phoenix Flow
Systems, San Diego, CA, USA).
Western blot analysis
To study the potential molecular mechanism of
ZGDHu-1 treatment on A549 and RERF-LC-MA cells, the expression
levels of relative proteins was measured by western blot. The A549
and RERF-LC-MA cells were seeded in dishes at a density of
2×108 cells/l, and were cultured overnight.
Subsequently, the A549 and RERF-LC-MA cells were treated with
different concentrations of ZGDHu-1 (0, 100, 200 and 500 µg/l) for
48 h. The cells were then collected and lysed using
radioimmunoprecipitation assay lysis buffer (Beijing Dingguo
Changsheng Biotechnology Co., Ltd., Beijing, China). Protein was
extracted and quantified using the BCA Protein Quantitation kit
(Beijing Dingguo Changsheng Biotechnology Co., Ltd.) following the
manufacturer's protocol. For each sample, a total of 50 µg protein
was separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (10–12%) and transferred onto a polyvinylidene
fluoride membrane (Beijing Dingguo Changsheng Biotechnology Co.,
Ltd.). The membranes were blocked with 10% non-fat dry milk in
Tris-buffered saline with Tween-20 (TBST) for 2 h and then
incubated with primary antibodies overnight at 4°C individually.
After washing with TBST three times, the membranes were hybridized
with a horseradish peroxidase (HRP)-conjugated secondary antibody
at room temperature for 2 h. Detection was performed using Western
Blotting Luminol Reagent (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA). All protein levels were normalized to β-actin (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). The following antibodies
were used: Monoclonal mouse anti-Cdc2 (#9116), monoclonal rabbit
anti-human cell division cycle 25c (Cdc25c; #4688), monoclonal
mouse anti-cyclin B1 (#4135), polyclonal anti-IκBα (#9242),
polyclonal anti-nuclear factor (NF)-κB (#3034) (Cell Signaling
Technology, Inc., Danvers, MA, USA), monoclonal mouse anti-p53
(#3036; Biovision, Inc., Milpitas, CA, USA), HRP-conjugated goat
anti-mouse immunoglobulin (Ig)G (h+l) and HRP-conjugated goat
anti-goat immunoglobulin (Ig)G (h+l) [MultiSciences (Lianke)
Biotech Co., Ltd., Hangzhou, China]. The antibodies were diluted by
1:3,000 in TBST.
Statistical analysis
All statistical calculations were performed using
SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA). Results are
expressed as the mean ± standard deviation. The differences between
treated and control groups were analyzed using t-test. Differences
were considered to be statistically significant at values of
P<0.05.
Results
ZGDHu-1 induces A549 and RERF-LC-MA
cell apoptosis through the detection of sub-G1
hypodiploid cells
An increased population of sub-G1
hypodiploid cells serves as a typical marker of apoptosis (22,23). In
the present study, following incubation with various concentrations
of ZGDHu-1 (2, 10, 50, 100, 200 and 500 µg/l), RPMI 1640 medium
(negative control) and 5-FU (5 ng/l; positive control) for 48 h,
the populations of sub-G1 hypodiploid A549 and
RERF-LC-MA cells were analyzed by flow cytometry. The results
demonstrated that the sub-G1 hypodiploid cell population
increased significantly with increasing concentrations of ZGDHu-1
in the A549 and RERF-LC-MA cells (P<0.01; Table I). In addition, it was observed that
the sub-G1 hypodiploid population in the A549 cells was
greater than that in the RERF-LC-MA cells. These results suggest
that apoptosis is induced by ZGDHu-1, and it may be different in
A549 and RERF-LC-MA cells.
 | Table I.Population of sub-G1
hypodiploid A549 and RERF-LC-MA cells with increasing
concentrations of ZGDHu-1. |
Table I.
Population of sub-G1
hypodiploid A549 and RERF-LC-MA cells with increasing
concentrations of ZGDHu-1.
| Concentration,
µg/l | A549 | RERF-LC-MA |
|---|
| 2 | 10.4±2.2a |
5.2±1.5a |
| 10 | 14.2±2.4a |
9.2±2.1a |
| 50 | 25.5±2.6a | 11.4±2.6a |
| 100 | 29.2±3.5a |
16.2±3.3a |
| 200 |
30.9±4.6a |
27.9±4.1a |
| 500 |
41.3±4.8a |
33.2±3.3a |
| 5-FU |
25.6±4.3a |
62.6±5.2a |
| Control |
5.1±0.6 |
3.6±1.5 |
ZGDHu-1 induces cell cycle arrest at
the G2/M phase and modulates cell cycle-related protein
levels in the A549 and RERF-LC-MA cells
To determine whether cell cycle changes are involved
in ZGDHu-1-induced cell apoptosis, cell cycle phase distribution
was detected by flow cytometry. Following treatment of the A549 and
RERF-LC-MA cells with various concentrations of ZGDHu-1 (0, 100 and
500 µg/l) for 48 h, the results indicated that the number of A549
and RERF-LC-MA cells decreased during G0/G1
phase and increased during G2/M phase with increasing
concentrations of ZGDHu-1 (Fig. 1).
Furthermore, to investigate the molecular mechanism of
ZGDHu-1-induced G2/M arrest in A549 and RERF-LC-MA
cells, the expression levels of cell cycle-related proteins,
including cyclin B1, Cdc2, Cdc25c and p53, were analyzed by western
blotting. The results demonstrated that the protein levels of
cyclin B1, Cdc2 and Cdc25c were downregulated in the A549 and
RERF-LC-MA cells following treatment with increasing concentrations
of ZGDHu-1, whilst the expression of p53 was upregulated (Fig. 2).
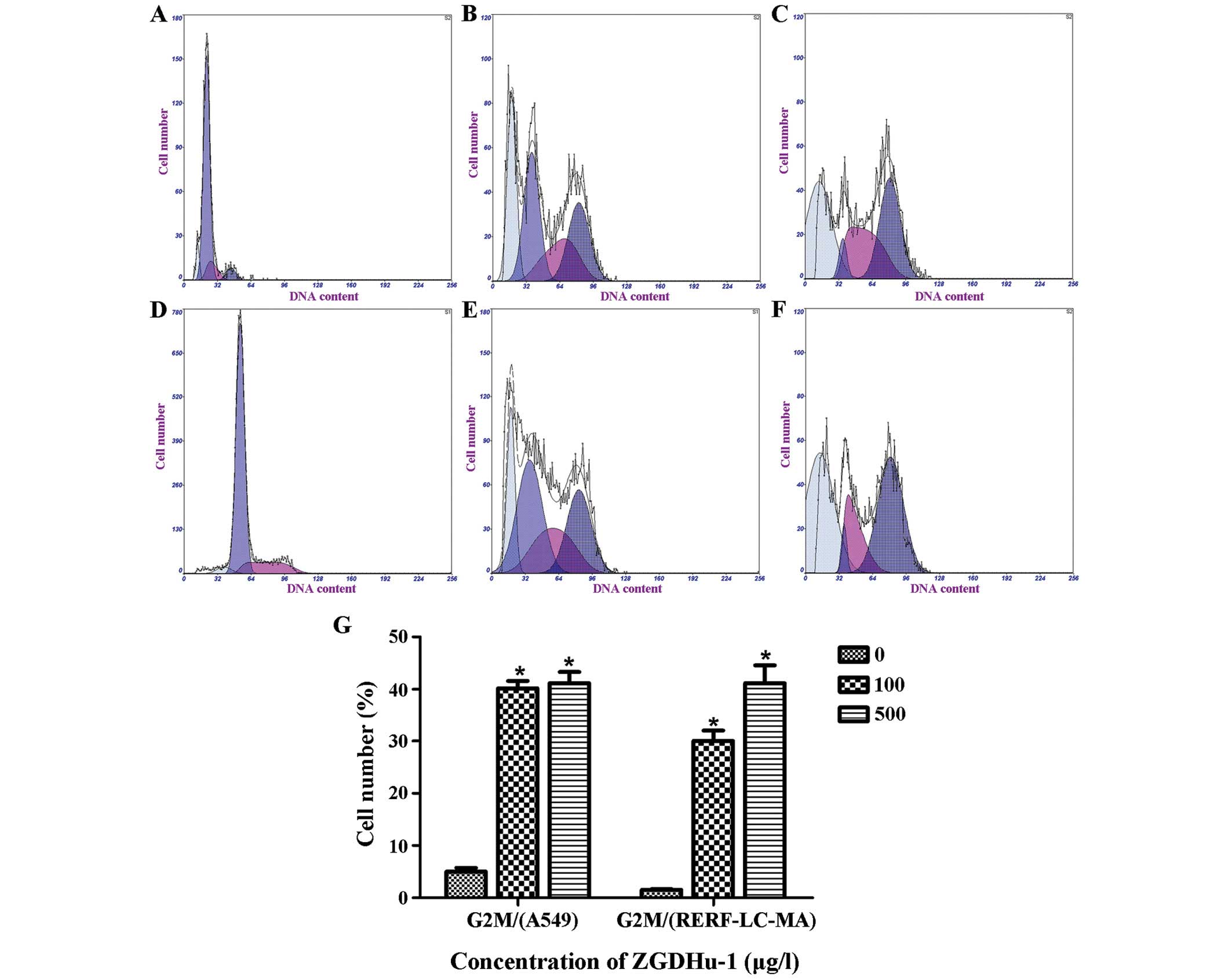 | Figure 1.ZGDHu-1 induced G2/M cell
cycle arrest in A549 and RERF-LC-MA cells. Cell cycle distribution
was analyzed by flow cytometry subsequent to staining with PI. The
number of cells in G2/M phase significantly increased
with increasing concentrations of ZGDHu-1, and the cell number was
5.02, 44.2 and 41.2% at G2 by (A) 0, (B) 100 and (C) 500µg/l
ZGDHu-1 in A549, respectively. The number of cells in
G2/M phase significantly increased with increasing
concentrations of ZGDHu-1, and the cell number was 0, 30 and 37.8%
at G2 by (D) 0, (E) 100 and (F) 500µg/l ZGDHu-1 in RERF-LC-MA,
respectively. (G) Percentage of cells in G2/M phase in
the A549 and RERF-LC-MA cells. Data are expressed as the mean ±
standard deviation, and experiments were repeated three times.
*P<0.05 vs. control. ZGDHu-1, N,
N′-di-(m-methylphenyi)-3,6-dimethyl-1,4-dihydro-1,2,4,
5-tetrazine-1,4-dicarboamide; M, mitotic; PI, propidium iodide. |
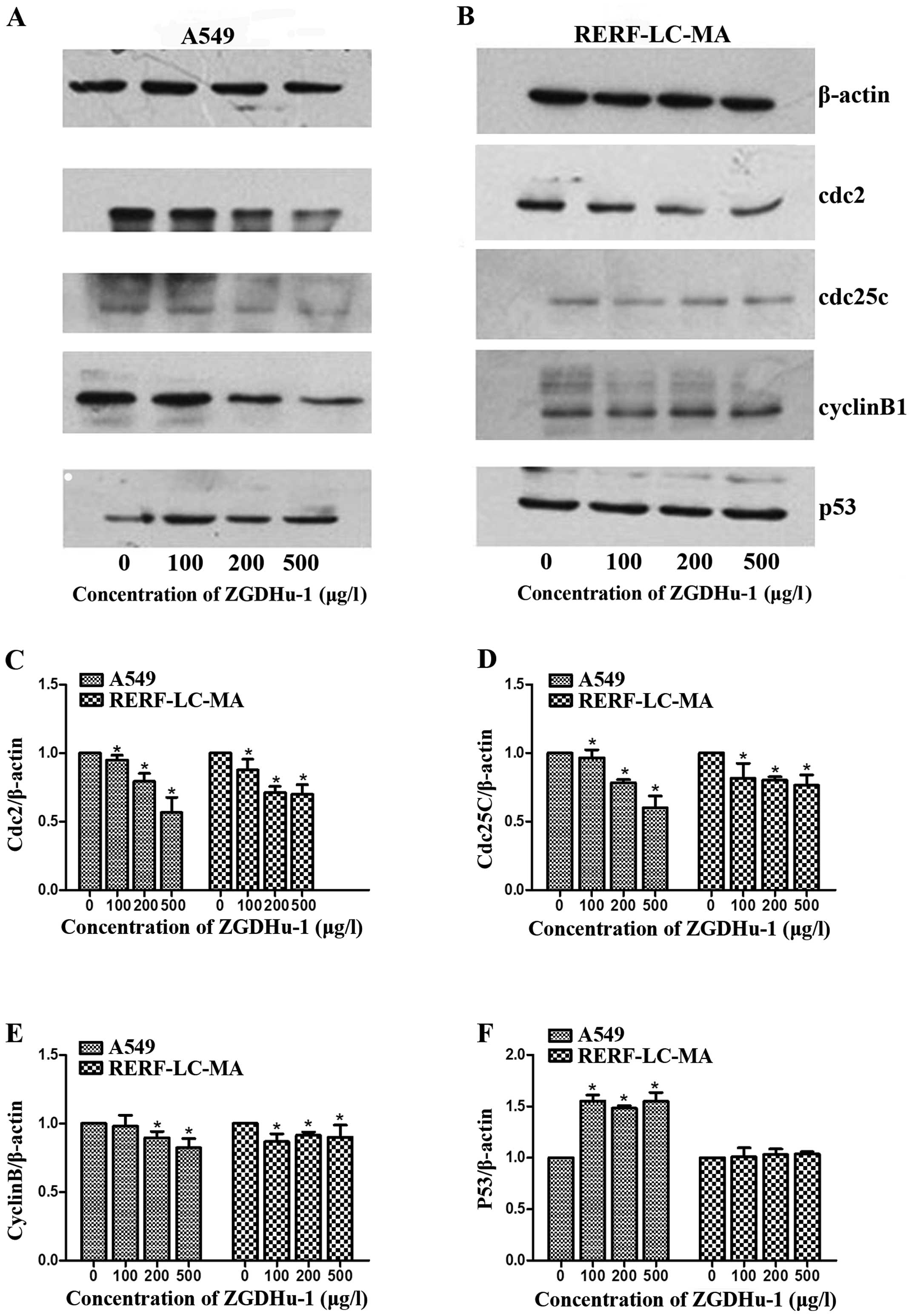 | Figure 2.ZGDHu-1 modulates cell cycle-related
protein levels in A549 and RERF-LC-MA cells. Western blot analysis
of G2/M cell cycle control proteins (cyclin B1, Cdc2,
Cdc25c and p53) levels in (A) A549 and (B) RERF-LC-MA cells.
Quantification of (C) cdc2, (D) cdc25c,(E) cyclinB1 and (F) P53,
respectively. *P<0.05, compared with control The cells were
treated with various concentrations of ZGDHU-1 (0, 100, 200 and 500
µg/l) for 48 h. β-actin was used as a loading control. ZGDHu-1, N,
N′-di-(m-methylphenyi)-3,6-dimethyl-1,4-dihydro-1,2,4,
5-tetrazine-1,4-dicarboamide; M, mitotic; Cdc25c, cell division
cycle 25c. |
ZGDHu-1 downregulates the expression
of NF-κB and upregulates the expression of IκB in A549 and
RERF-LC-MA cells
NF-κB is a nuclear transcription factor, which
regulates a number of genes and serves an important role in
cellular proliferation, apoptosis, invasion and differentiation.
IκB is an inhibitory factor that suppresses the activity of NF-κB
(24). In the present study,
following incubation with different concentrations of ZGDHu-1 (0,
100, 200 and 500 µg/l), the expression levels of NF-κB and IκB were
detected by western blotting. The results demonstrated that the
expression of IκB elevated with the increasing concentrations of
ZGDHu-1 in the A549 and RERF-LC-MA cells, whilst the expression of
NF-κB decreased (Fig. 3). This
suggests that the expression of IκB and NF-κB were altered through
the induction of apoptosis in A549 and RERF-LC-MA cells.
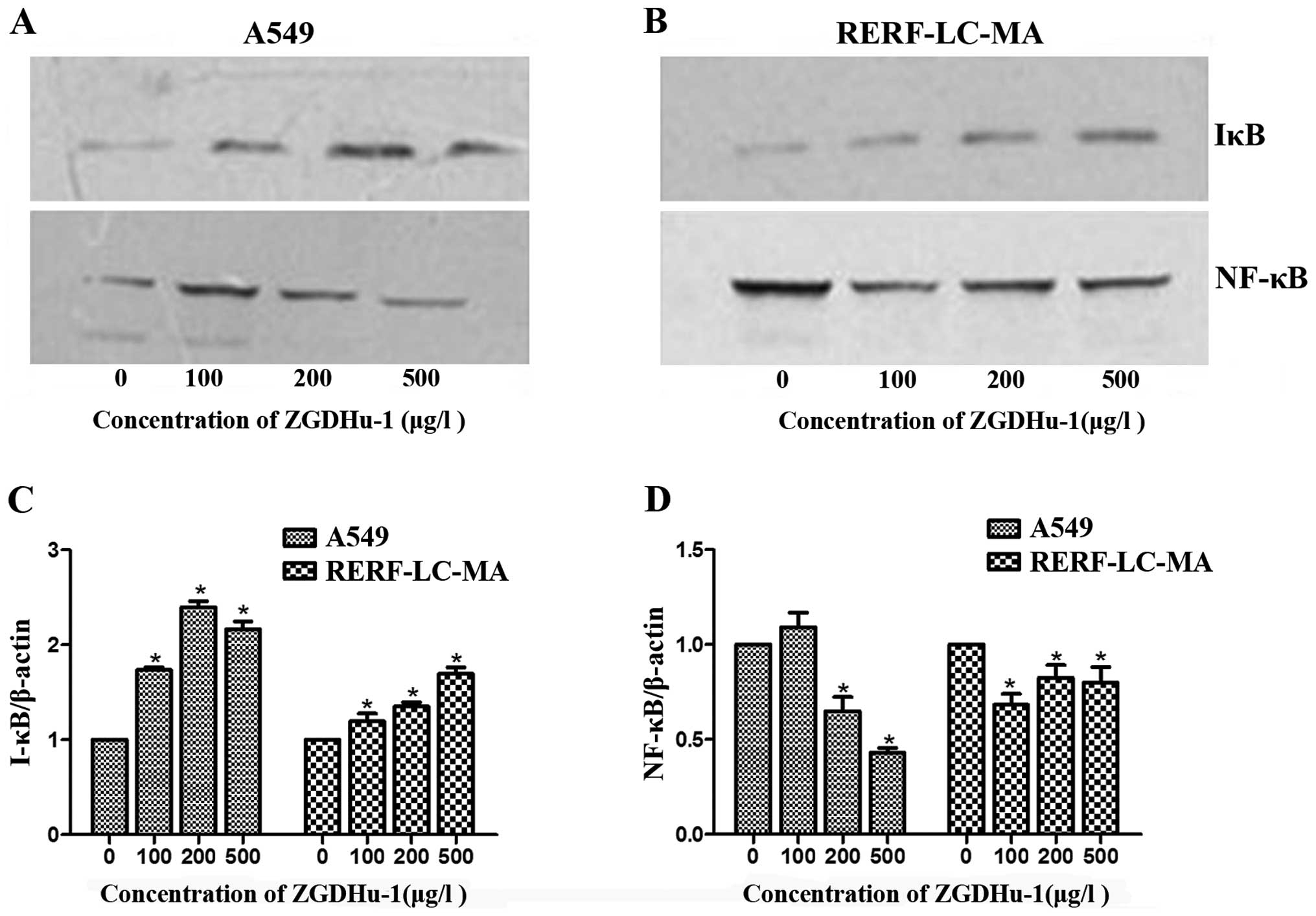 | Figure 3.The effect of ZGDHu-1 on the NF-κB
pathway in A549 and RERF-LC-MA cells. (A) ZGDHu-1 downregulated the
expression of NF-κB and upregulated the expression of IκB in the
A549 cells following incubation with various concentrations of
ZGDHU-1 (0, 100, 200 and 500 µg/l) for 48 h. β-actin was used as a
loading control. (B) ZGDHu-1 downregulated the expression of NF-κB
and upregulated the expression of IκB in the RERF-LC-MA cells
following incubation with various concentrations of ZGDHU-1 (0,
100, 200 and 500 µg/l) for 48 h. Quantification of (C) IκB and (D)
NF-κB, respectively. *P<0.05, compared with control. β-actin was
used as a loading control. ZGDHu-1, N,
N′-di-(m-methylphenyi)-3,6-dimethyl-1,4-dihydro-1,2,4,
5-tetrazine-1,4-dicarboamide; NF-κB, nuclear factor-κB. |
Discussion
Previous studies have reported that ZGDHu-1 is able
to inhibit proliferation and induce apoptosis in leukemia cells
(15,16), in addition to markedly inhibiting the
cell cycle at the G2/M phase (17). Furthermore, it has been demonstrated
that ZGDHu-1 inhibits proliferation and induces apoptosis in lung
cancer cells (18). However, the
mechanisms by which ZGDHu-1 functions to inhibit the cell cycle in
human lung cancer cells has not yet been elucidated. In the present
study, ZGDHu-1 induced apoptosis through the increased population
of sub-G1 hypodiploid cells. In apoptotic cells, DNA is
partially degraded, which leaves nucleosomal and oligonucleosomal
DNA fragments. PI is a fluorogenic compound. It binds to nucleic
acids, meaning that fluorescence emission is proportional to the
DNA content of a cell. When apoptotic cells are stained with PI and
analyzed with a flow cytometer, they exhibit a broad hypodiploid
(sub-G1) peak, which may be easily discriminated from
the narrow peak of cells with normal (diploid) DNA content.
Overall, the results of the present study suggested that ZGDHu-1
may induce A549 and RERF-LC-MA cells to undergo apoptosis.
Furthermore, we found an interesting phenomenon that the population
of sub-G1 hypodiploid were significantly differences in
A549 and RERF-LC-MA. Clinically, lung cancer is classified into two
subtypes: SCLC (for example, RERF-LC-MA cells) and NSCLC (for
example, A549 cells). The SCLC cases are associated with greater
chemosensitivity than NSCLC (12,25), thus
A549 and RERF-LC-MA cells may exert varying levels of resistance
against ZGDHu-1.
The cell cycle may be divided into four stages:
G1, S, G2 and M phases. G2/M is
important for the entrance of cells into the M phase and is also
associated with tumor cell resistance (21). In the present study, it was
demonstrated that ZGDHu-1 arrested the cell cycle of the A549 and
RERF-LC-MA cells at G2/M phase in a
concentration-dependent manner. The regulation of the cell cycle
primarily depends on a number of proteins and kinases, which
include CDKs, cyclins and CKIs (19,20). The
activity of the CDK1 complex is key for the transition from
G2 to M in eukaryotic cells (26). Cdc25c is also a CDK, which phosphatase
is responsible for dephosphorylating resulting in the activation of
the CDK1 complex at the G2/M checkpoint (27). In the present study, expression of
cyclin B1, Cdc2 and Cdc25c was downregulated following cell
treatment with ZGDHu-1, which suggests that Cdc25c was decreased to
inactivate the CDK1 complex, resulting in obstruction of mitotic
entry in the A549 and RERF-LC-MA cells. p53, a notable tumor
suppressor, is capable of inducing either apoptosis or cell cycle
arrest at the cell cycle checkpoints (27,28).
Furthermore, p21, a CDK inhibitor, is able to inhibit the
CDK-cyclin complexes that are transcriptionally activated by p53
(27). The present study demonstrated
that the expression of p53 was upregulated by ZGDHu-1 in a
concentration-dependent manner; therefore, p53 was activated and
the CDK1 complex was inhibited. These results indicate that
apoptosis and G2/M arrest were induced by ZGDHu-1 in the
A549 and RERF-LC-MA cells in a concentration-dependent manner.
NF-κB is a heterodimer consisting of two subunits,
and is bound to and retained in the cytoplasm by the inhibitor, IκB
(29). NF-κB serves a critical role
in the promotion of cell growth and proliferation, and the
inhibition of apoptosis (30,31). Notably, previous studies have reported
that high levels of NF-κB were activated in lung cancer, and
inhibition of NF-κB by IκB may suppress lung cancer cell survival
and proliferation (32,33). In addition, studies have reported that
ZGDHu-1 may upregulate the expression of IκB and downregulate the
expression of NF-κB (17). This
suggests that IκB levels may have been upregulated by ZGDHu-1 to
suppress the function of NF-κB, subsequently inducing apoptosis and
G2/M arrest in the A549 and RERF-LC-MA cells in the
present study.
In conclusion, the current study demonstrated that
ZGDHu-1 is able to induce apoptosis and G2/M arrest in
A549 and RERF-LC-MA cells. Notably, cell cycle- and
apoptosis-related proteins are key factors that contribute to the
inhibitory effects of ZGDHu-1. The present results indicate that
ZGDHu-1 may function as a potential, novel drug to treat lung
cancer in the future.
Acknowledgements
The present study was supported by a funded project
from the Department of Higher Education of Zhejiang Province (grant
no. FW2013008).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frauenfelder T, Puhan MA, Lazor R, von
Garnier C, Bremerich J, Niemann T, Christe A, Montet X, Gautschi O,
Weder W, et al: Early detection of lung cancer: A statement from an
expert panel of the Swiss university hospitals on lung cancer
screening. Respiration. 87:254–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naimi AI, Cole SR, Hudgens MG and
Richardson DB: Estimating the effect of cumulative occupational
asbestos exposure on time to lung cancer mortality: Using
structural nested failure-time models to account for healthy-worker
survivor bias. Epidemiology. 25:246–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Basmy A: Profile of lung cancer in
Kuwait. Asian Pac J Cancer Prev. 14:6181–6184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Dai M, Chen Y, Zhang S, Chen W, Dai
Z and Zou X: Estimates of lung cancer mortality at the province
level in China. Zhongguo Fei Ai Za Zhi. 14:120–126. 2011.(In
Chinese). PubMed/NCBI
|
|
6
|
Ellis J: The impact of lung cancer on
patients and carers. Chron Respir Dis. 9:39–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Subirana M, Lopez C and Pascual A:
Non-invasive interventions for improving well-being and quality of
life in patients with lung cancer. Clin J Oncol Nurs. 14:81–82.
2012.
|
|
8
|
Zheng R, Zeng H, Zhang S, Fan Y, Qiao Y,
Zhou Q and Chen W: Lung cancer incidence and mortality in China,
2010. Thorac Cancer. 5:330–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han R, Zheng R, Zhang S, Wu M and Chen W:
Trend analyses on the differences of lung cancer incidence between
gender, area and average age in China during 1989–2008. Zhongguo
Fei Ai Za Zhi. 16:445–451. 2013.(In Chinese). PubMed/NCBI
|
|
10
|
Chen W, Zhang S and Zou X: Evaluation on
the incidence, mortality and tendency of lung cancer in China.
Thorac Cancer. 1:35–40. 2010. View Article : Google Scholar
|
|
11
|
NSCLC Meta-analysis Collaborative Group:
Preoperative chemotherapy for non-small-cell lung cancer: A
systematic review and meta-analysis of individual participant data.
Lancet. 383:1561–1571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bambang IF, Lu D, Li H, Chiu LL, Lau QC,
Koay E and Zhang D: Cytokeratin 19 regulates endoplasmic reticulum
stress and inhibits ERp29 expression via p38 MAPK/XBP-1 signaling
in breast cancer cells. Exp Cell Res. 315:1964–1974. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rao GW and Hu WX: Synthesis, X-ray
crystallographic analysis, and antitumor activity of
1-acyl-3,6-disubstituted phenyl-1,4-dihydro-1,2,4,5-tetrazines.
Bioorg Med Chem Lett. 15:3174–3176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao GW and Hu WX: Synthesis, structure
analysis, and antitumor activity of
3,6-disubstituted-1,4-dihydro-1,2,4,5-tetrazine derivatives. Bioorg
Med Chem Lett. 16:3702–3705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou YL, Lü YP, Hu WX, Qiu LN, Wang WS,
Liu JD and Wu JG: ZGDHu-1-inducing apoptosis of SHI-1 leukemia
cells and its molecular mechanism. Zhongguo Shi Yan Xue Ye Xue Za
Zhi. 15:483–489. 2007.(In Chinese). PubMed/NCBI
|
|
16
|
Qiu LN, Zhou YL, Wang ZN, Huang Q and Hu
WX: ZGDHu-1 promotes apoptosis of chronic lymphocytic leukemia
cells. Int J Oncol. 41:533–540. 2012.PubMed/NCBI
|
|
17
|
Xia J, Chen SF, Lv YP, Lu LN, Hu WX and
Zhou YL: ZGDHu-1 induces G2/M phase arrest and apoptosis
in Kasumi-1 cells. Mol Med Rep. 11:3398–3404. 2015.PubMed/NCBI
|
|
18
|
Zhou YL, Hu WX, Lü YP, Qiu LN, Wang WS,
Yang ZY, Liu JD and Rao GW: Effect of ZGDHu-1 on proliferation and
apoptosis of A549 cells in vitro and antitumor activity in vivo.
Yao Xue Xue Bao. 42:26–34. 2007.(In Chinese). PubMed/NCBI
|
|
19
|
Murray AW: Recycling the cell cycle:
Cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: Role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
21
|
Chen T, Stephens PA, Middleton FK and
Curtin NJ: Targeting the S and G2 checkpoint to treat cancer. Drug
Discov Today. 17:194–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Riccardi C and Nicoletti I: Analysis of
apoptosis by propidium iodide staining and flow cytometry. Nat
Protoc. 1:1458–1461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin DY, Kim GY, Hwang HJ, Kim WJ and Choi
YH: Diallyl trisulfide-induced apoptosis of bladder cancer cells is
caspase-dependent and regulated by PI3K/Akt and JNK pathways.
Environ Toxicol Pharmacol. 37:74–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tchoghandjian A, Jennewein C, Eckhardt I,
Momma S, Figarella-Branger D and Fulda S: Smac mimetic promotes
glioblastoma cancer stem-like cell differentiation by activating
NF-κB. Cell Death Differ. 21:735–747. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krohn A, Ahrens T, Yalcin A, Plönes T,
Wehrle J, Taromi S, Wollner S, Follo M, Brabletz T, Mani SA, et al:
Tumor cell heterogeneity in Small Cell Lung Cancer (SCLC):
Phenotypical and functional differences associated with
Epithelial-Mesenchymal Transition (EMT) and DNA methylation
changes. PLoS One. 9:e1002492014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stark GR and Taylor WR: Control of the
G2/M transition. Mol Biotechnol. 32:227–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen CY, Oliner JD, Zhan Q, Fornace AJ Jr,
Vogelstein B and Kastan MB: Interactions between p53 and MDM2 in a
mammalian cell cycle checkpoint pathway. Proc Natl Acad Sci USA.
91:2684–2688. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abraham E: NF-kappaB activation. Crit Care
Med. 28(Suppl): N100–N104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen F, Lu Y, Zhang Z, Vallyathan V, Ding
M, Castranova V and Shi X: Opposite effect of NF-kappa B and c-Jun
N-terminal kinase on p53-independent GADD45 induction by arsenite.
J Biol Chem. 276:11414–11419. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karin M and Lin A: NF-kappaB at the
crossroads of life and death. Nat Immunol. 3:221–227. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin X, Wang Z, Qiu L, Zhang D, Guo Z, Gao
Z, Deng C, Wang F, Wang S and Guo C: Potential biomarkers involving
IKK/RelA signal in early stage non-small cell lung cancer. Cancer
Sci. 99:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Chen W and Lin Y: Sensitization of
TNF-induced cytotoxicity in lung cancer cells by concurrent
suppression of the NF-kappaB and Akt pathways. Biochem Biophys Res
Commun. 355:807–812. 2007. View Article : Google Scholar : PubMed/NCBI
|

















