Introduction
Alzheimer's disease (AD) is the most prevalent
chronic neurodegenerative disorder, demonstrating widespread
degeneration in numerous types of neuron. Individuals with AD
exhibit memory loss and severe cognitive decline caused by the
formation of amyloid plaques in the brain parenchyma, particularly
in the hippocampus and cerebral cortex. The increased accumulation
of misfolded amyloid-β (Aβ) peptides in the senile plaque core is
the main cause of the neurodegenerative action of AD (1). Thus, preventing the deposition of Aβ in
the brain could be a feasible strategy in the therapy of AD.
Increasing evidence has demonstrated that the
presence of extensive oxidative stress plays an essential role in
the initiation and progression of AD. On the one hand, the elevated
oxidative stress may be induced by Aβ aggregation resulting in
mitochondrial dysfunction and lipid peroxidation (2,3). Aβ
treatment has been demonstrated to enhance the hydrogen peroxide
and lipid peroxides levels in cell models (4). In addition, oxidative modifications of
proteins and lipids were increased in a AbPP/PS1 transgenic mouse
model associated with Aβ accumulation (5,6). The
soluble Aβ oligomers induced the reactive oxygen species (ROS),
causing synaptic impairment and neuronal loss in hippocampal
neuronal cells (7). These studies
demonstrated that Aβ contributes to increased oxidative stress in
AD models. Conversely, a significant number of studies have
suggested that oxidative stress is involved in the production of
Aβ. It was reported that enhanced oxidative stress caused by
defects in the antioxidant defense system notably increased the
deposition of Aβ in APP overexpression transgenic mice (8). In addition, the antioxidant supplement
ameliorated the brain Aβ plaque burden and cognitive dysfunction
(9). Several studies revealed that
oxidative stress induced the expression of β-secretases BACE1 and
PS1 and the activity of γ-secretase, while it decreased the
activity of α-secretase for Aβ production from APP (10,11).
Previously, the Butterfield laboratory reported that protein
oxidation and lipid peroxidation were associated with AD brain
regions with abundant Aβ but not in Aβ-poor cerebellum, indicating
that Aβ (1–42)-associated oxidative stress is responsible for the
pathogenesis and progression of AD (12). Since oxidative stress-mediated
toxicity is a key factor contributing to neurodegenerative events,
developing efficient antioxidant protection is an attractive
strategy for AD therapy.
Current developments in mesenchymal stem cell (MSC)
technology have stimulated new therapies for neurodegenerative
disorders including AD. Collected evidence indicates that stem
cell-based approaches have potential for use in the treatment of
neurodegenerative diseases AD (13).
MSCs have been demonstrated to play an effective role in
neuroprotection through decreasing apoptosis and oxidative stress
(14). In stroke-prone spontaneously
hypertensive rats, the superoxide, apoptotic cells and by-products
of lipid peroxidation were decreased following MSC treatment
(15). Human umbilical cord
blood-derived MSCs exhibited low antioxidant enzyme activity by
decreasing the gene expression levels of ROS scavenging enzymes,
including catalase, superoxide dismutase (SOD) and glutathione
peroxidase (GPx) (16). MSCs could be
employed as an antioxidant to prevent the progression of AD by its
antioxidant activity.
Amniotic membrane has the potential for the
generation of MSCs due to the convenience of its acquirement from
fetal tissue without any ethical conflict and its high efficiency
in the production of MSCs. In contrast with other mesenchymal
cells, including human bone marrow-derived MSCs and umbilical cord
blood-derived MSCs, human amniotic membrane-derived MSCs (hAMMSCs)
do not possess the major histocompatibility complex class I
molecule and may exhibit immunological tolerance.
However, whether hAMMSCs are capable of ameliorating
oxidative stress, which is inextricably linked with several major
pathological processes in AD, remains unknown. In the present
study, we investigated the effects of intravenous infusions of
hAMMSCs in a transgenic mouse model of AD for the first time.
Materials and methods
Isolation and in vitro culture of
hAMMSCs
Human term placentas were collected with informed
consent from healthy females following Caesarean section and washed
immediately several times with phosphate-buffered saline (PBS)
containing 200 U/ml penicillin and 100 µg/ml streptomycin. The
study procedure was approved by the ethics committee of Zhengzhou
University, China. The isolation of hAMMSCs was performed according
to a previously described method (17). Amniotic membrane was bluntly dissected
from the chorion and cut into small pieces. The minced amnion was
subjected to 60-min digestion with 0.25% trypsin (Sigma-Aldrich,
Steinheim, Germany) and collagenase I solution (0.75 mg/ml) in
Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich) at 37°C.
Following the centrifugation of supernatant at 252 × g for 10 min,
the pellet was suspended in DMEM containing 10% heat-inactivated
fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin and
2 mM L-glutamine (Sigma-Aldrich) and was cultured at 37°C in 5%
CO2. The harvest cells were analyzed by flow cytometry
to confirm stem cell characteristics previously described (18).
Animals and injection of hAMMSCs
The full-length mutant APP cDNA 695 V 717 I gene of
humans was transferred into C57BL/6J mice (C57BL/6J-APP mice) to
create a transgenic mouse model of AD (19), which was employed to examine the
effect of hAMMSC transplantation. The C57BL/6J-APP mice were
obtained from the Institute of Experimental Animals, Chinese
Academy of Medical Sciences (Beijing, China). All experimental
animals were housed under a 12/12-h dark/light cycle in specific
pathogen-free conditions and handled following the provisions and
general recommendations of Chinese Experimental Animal
Administration legislation.
For intravenous injection, 500 µl amounts of cell
suspension (~1×106 cells) were injected into the tail
vein (hAMMSC-injected group). The mice of the control group
received an injection of 500 µl PBS into the tail vein. Adult
C57BL/6J-APP mice (11 months old; n=10 per group) were used for the
behavioral experiments and for pathological analysis at 3 weeks
after transplantation. Wild-type littermate mice (11 months old;
n=9) were used as the negative control group.
Tissue preparation and staining
Following anesthetization with chloral hydrate, mice
were immediately cardiac-perfused with 0.9% saline solution
followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Following
perfusion, the brains of the mice were excised and postfixed
overnight at 4°C and mounted on slides. The slides were washed in
PBS three times and 80% (v/v) methanol, then pretreated with 0.3%
hydrogen peroxide/methanol for 25 min in order to quench the
endogenous peroxidase activity. The sections were blocked with 10%
normal goat serum in PBS for 30 min to prevent nonspecific protein
binding, then washed and incubated with anti-Aβ monoclonal antibody
(6E10, 1:100; Covance Inc., Princeton, NJ, USA) in 3% bovine serum
albumin (BSA)/PBS overnight at 4°C in a humid chamber. After
washing with PBS three times, the sections were incubated with
appropriate biotin-conjugated secondary antibody for 1 h, and then
horseradish peroxidase-labeled streptavidin was added. The tissues
were washed and stained using the diaminobenzidine substrate method
and counterstained with hematoxylin.
Behavior test
The modified Morris water maze test was used to
assess the spatial memory performance of mice (20). For spatial acquisition tests, mice
were placed into a pool and were given 60 sec to find a hidden
platform. Mice that failed to find the platform within 60 sec were
guided to the platform. Four trials were performed for each mouse
every day. In the basic acquisition training, the starting
positions were north, east, southeast and northwest, and the
platform was placed in the southwest quadrant. After that, the
platform was removed and mice were located at a new starting
position (northeast) in the maze and allowed to swim for 60
sec.
Cell proliferation assay
Following the third passage, hAMMSCs were seeded at
1000 cells/cm2 in a 6-well plate (Corning, Inc.,
Corning, NY, USA). After 4, 7, 10, 14, 17 and 21 days' culture,
cells were collected by 0.25% Trypsin-EDTA solution at 37°C for 2
min and counted using a hemocytometer. Trypan blue staining
(Sigma-Aldrich) was used to exclude the dead cells. All experiments
were carried out in triplicate.
Measurement of Aβ40 and Aβ42 levels by
enzyme-linked immunosorbent assay (ELISA)
Following the behavior test, brain samples were
isolated from the C57BL/6J-APP transgenic mice and stored at −80°C
until use. The Aβ40 and Aβ42 levels were assayed by ELISA according
to a previously described method (21). Briefly, one hemisphere was homogenized
in eight volumes of ice-cold guanidine buffer (5.0 M guanidine-HCl,
50 mM Tris-HCl, pH 8.0) and incubated at room temperature for 4 h.
Ice-cold reaction buffer BSAT-DPBS (Dulbecco phosphate-buffered
saline, with 5% BSA, 0.03% Tween-20, 0.2 g/l KCl, 0.2 g/l
KH2PO4, 8.0 g/l NaCl, 1.150 g/l
Na2HPO4, pH 7.4) containing 1X protease
inhibitor cocktail (Invitrogen Life Technologies, Carlsbad, CA,
USA) was employed to dilute the homogenate to 1:20. The mixture was
centrifugated at 25,200 × g at 4°C for 20 min and the supernatant
was used to quantify the Aβ40 and Aβ42 levels using Aβ40 and Aβ42
ELISA kits (Invitrogen Life Technologies) according to the
manufacturer's instructions.
Measurement of antioxidant
capacity
Brain homogenates were added to nine volumes of
ice-cold 0.9% saline and centrifugated at 100,710 × g for 10 min at
4°C. The supernatant in the brain homogenate was used to determine
the levels of glutathione (GSH) and glutathione disulfide (GSSG)
using commercial kits (Beyotime Institute of Biotechnology,
Shanghai, China) as per the manufacturer's instructions (22). The optical density was read at 405 nm
on a microplate reader. The activity of SOD and malonaldehyde (MDA)
in the brain homogenate was detected using assay kits (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) (23).
Statistical analysis
All data are shown as the means ± standard error,
and were obtained from at least three separate experiments (with
the exception of behavioral data). Student's t-test was performed
to determine the difference between two groups, and a one-way
analysis of variance (ANOVA) test was used to compare one-variable
data for more than two groups. The two-way repeated-measures ANOVA
was utilized to value the statistical significance for two-variable
experiments. P≤0.05 was considered to indicate a statistically
significant difference.
Results
Isolation and characterization of
hAMMSCs
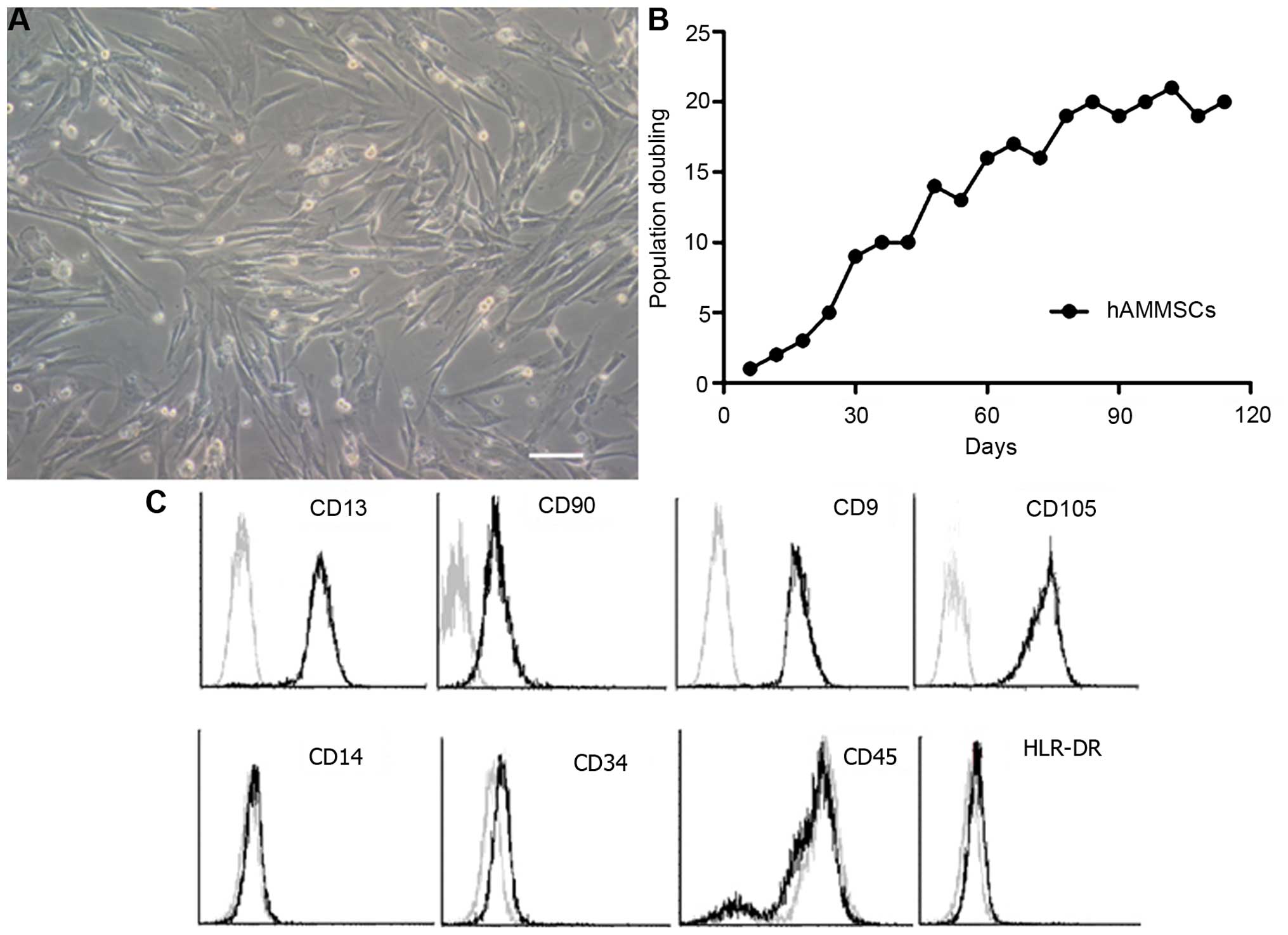
The morphology of harvested hAMMSCs at passage 4 of
culture is shown in Fig. 1A. hAMMSCs
exhibited strong viability by MTT assay (Fig. 1B) with ~20 population doublings,
suggesting that amniotic membrane is an abundant source for the
generation of mesenchymal cells. In order to estimate the purity of
hAMMSC cultures, flow cytometry analysis was used to analyze the
immunophenotypic surface profile of the hAMMSCs. Surface marker
analysis revealed that hAMMSCs were positive for mesenchymal
lineage markers CD13, CD90, CD9 (a nontrophoblast marker) and
CD105, and negative for hematopoietic lineage markers CD14, CD34,
CD45 and HLR-DR (Fig. 1C).
Transplantation of hAMMSCs attenuates
spatial learning and memory function in AD transgenic mice
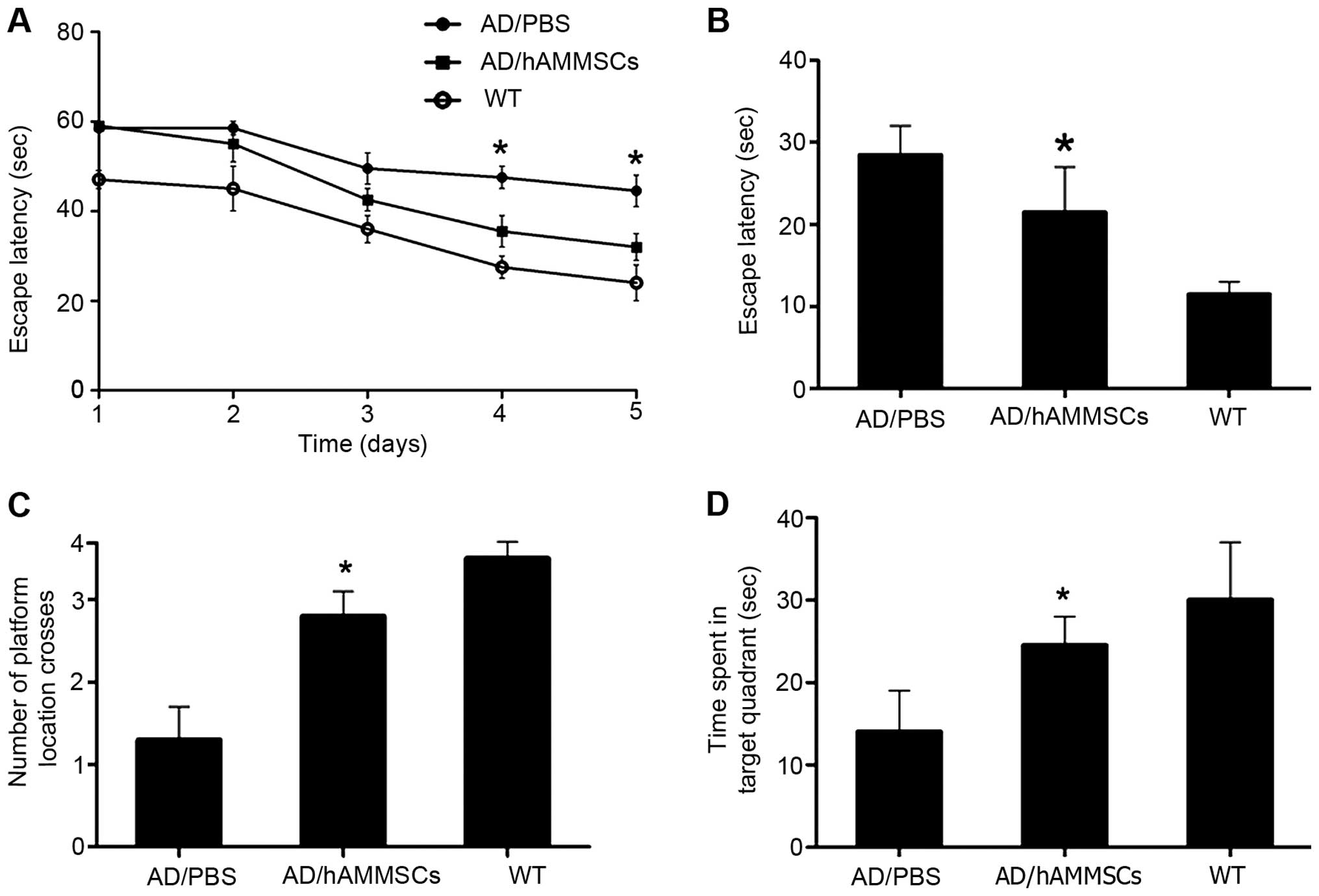
To investigate the effect of hAMMSC transplantation
on the learning and memory of AD transgenic mice, a Morris water
maze test was conducted 3 weeks after hAMMSC transplantation.
C57BL/6J-APP transgenic mice overexpress the APP695 gene and
secrete endogenous Aβ, contributing to the deposition of Aβ mainly
in the neurons of the cerebral cortex and hippocampus. During the
acquisition training phase of the water maze test, the PBS-injected
control group exhibited notable learning and memory dysfunction
compared with the normal control mice, whereas the hAMMSCs-injected
group located the hidden platform significantly faster than the
PBS-injected control group, and demonstrated no significantly
difference from the normal control group, indicating significantly
improved learning and memory function (Fig. 2A). Then, we performed a probe test one
day after the last training trial to examine the spatial memory.
The platform was removed and the time taken to reach the same zone
was recorded within 60 sec. The PBS-injected control group spent
less time in the target quadrant than the hAMMSC group and the
normal mice (P<0.01; Fig. 2B-D),
demonstrating that the deficit in spatial memory was rescued by
hAMMSC transplantation.
Attenuated Aβ deposition by hAMMSC
transplantation
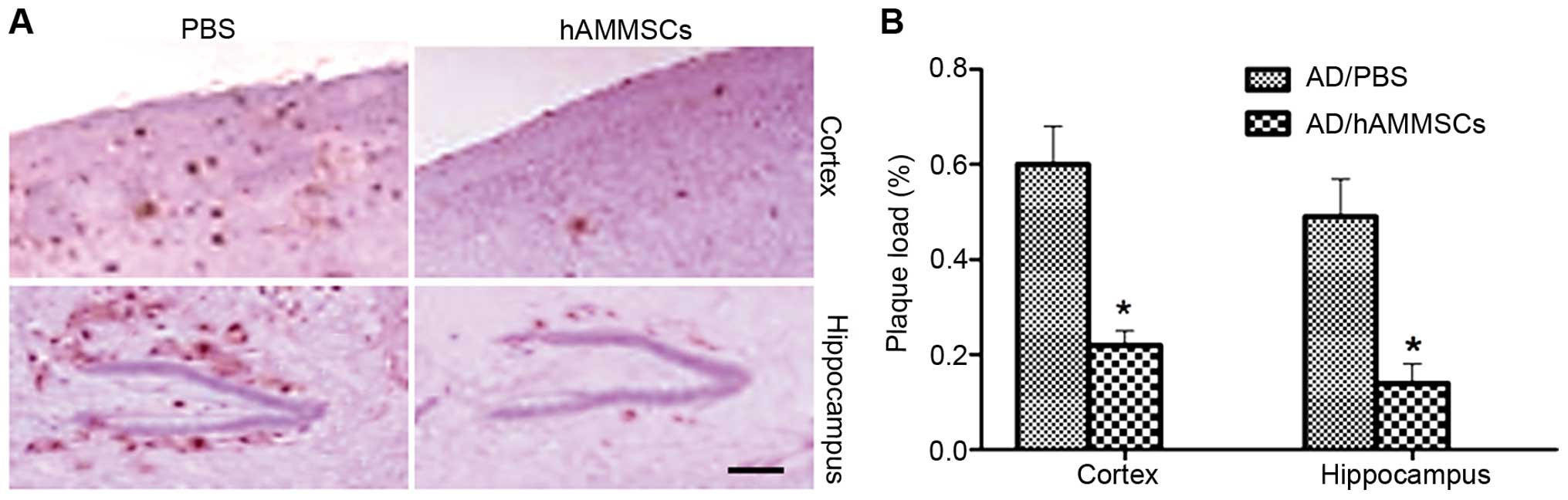
Since Aβ deposition is the main pathogenic cause of
AD inducing cognitive deficits, brain tissue excised from mice
following the water maze test was incubated with anti-Aβ 6E10
antibody to observe the changes in Aβ deposition. hAMMSC
transplantation notably decreased the Aβ plaques in the cortex and
hippocampus of mice compared with the PBS-injected control group
(Fig. 3A). Plaque load was qualified
by morphometric analysis and is shown as the percentage of the
total area demonstrating immunoreactivity of Aβ. The PBS-treated AD
mice demonstrated severe Aβ deposition with plaque levels of 60%
and 49% in the cortex and hippocampus, whereas the hAMMSC group
demonstrated a reduced plaque load of 22% and 14% in the cortex and
hippocampus (Fig. 3B).
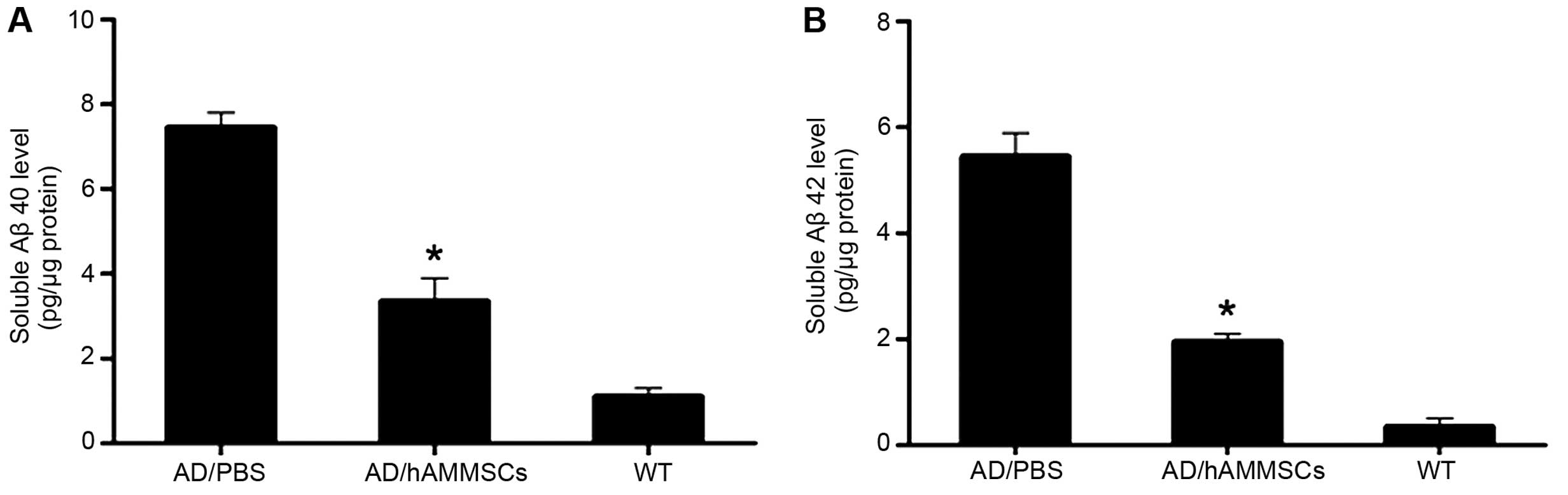
Full-length or N-truncated Aβ40 and Aβ42 are two
significant factors contributing to Aβ aggregation, and the ratio
of Aβ40/42 has a notable effect on the neurotoxicity of Ab fibrils,
being associated with the onset of familial AD (24). ELISA assay revealed that there were
significant differences in the soluble Aβ level between the
PBS-injected control group and the hAMMSC group.
hAMMSC-transplanted mice exhibited a notably decreased Aβ level
compared with PBS-infused mice (Fig. 4A
and B). Collectively, these results indicate that hAMMSC
transplantation attenuates the deposition of Aβ in a transgenic
mice model.
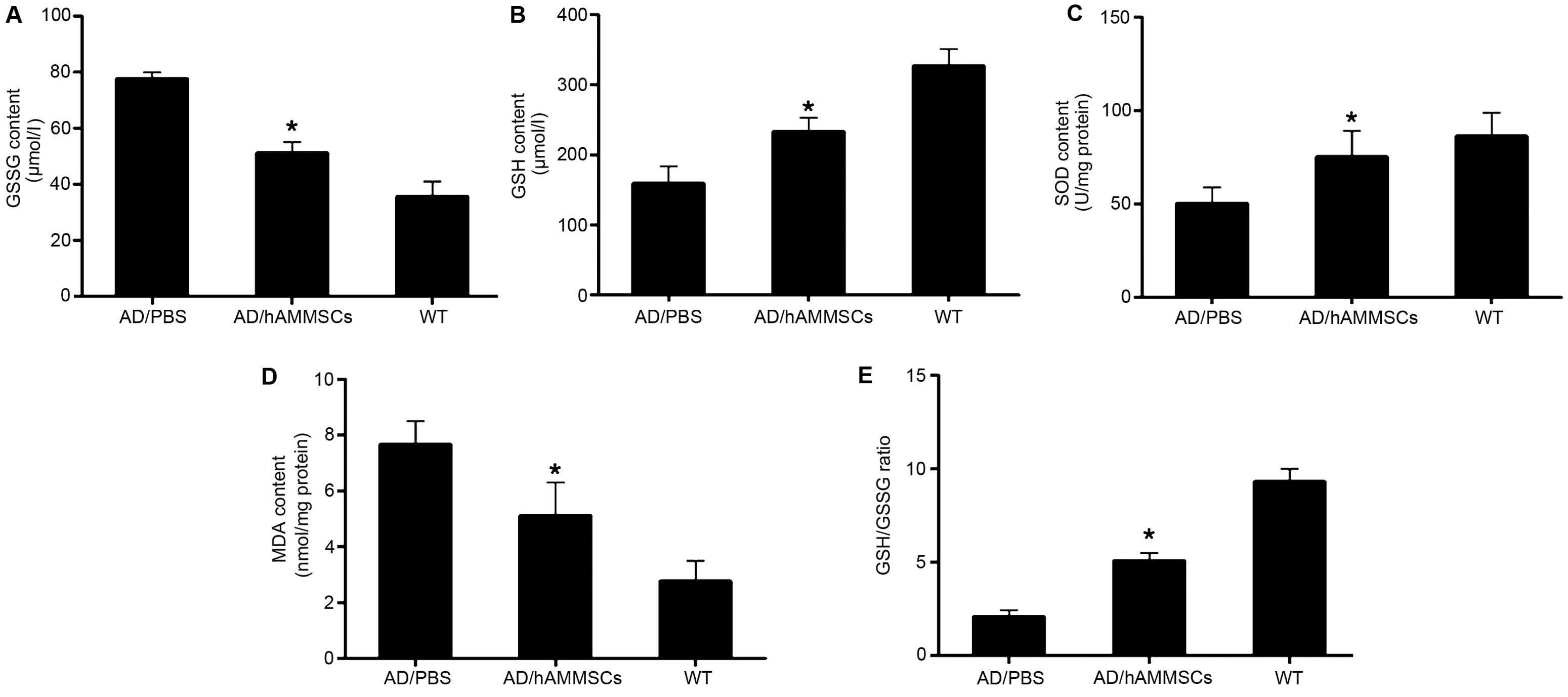
hAMMSCs reduce oxidative stress in AD
transgenic mice
Oxidative stress was proposed as an essential factor
contributing to Aβ neurotoxicity, which occurs at the prophase of
AD prior to the onset of clinical and pathological symptoms
(25). Aβ deposition in the brain
parenchyma causes lipid peroxidation and protein oxidation,
damaging the mitochondria and resulting in the loss of oxidative
function of significant proteins in numerous pathways, including
glucose metabolic proteins and death of neurons (26). Since hAMMSC transplantation attenuated
the deposition of Aβ, we further investigated whether hAMMSCs could
decrease oxidative stress in AD mice. GSH acts as a crucial
cellular antioxidant against oxidative stress by reducing hydrogen
peroxides and hydroperoxides in addition to protecting protein
thiol groups against oxidation (27).
GSH is converted into GSSG by GPx to detoxify peroxides, and the
reaction could be converted back by glutathione reductase. It is
considered that the ratio of GSH/GSSG reflects the intracellular
antioxidant level. Following the transplantation of hAMMSCs in AD
transgenic mice, the GSH level was increased significantly but the
GSSG level was only slightly lowered in brain homogenates (Fig. 5A and B). The GSH/GSSG ratio in the
hAMMSC group was significantly elevated compared with that of the
PBS group (Fig. 5C).
As a vital antioxidant enzyme, SOD catalyzes
superoxide radical anions to H2O2, inducing
the oxidization of polyunsaturated fatty acids and lipid
peroxidation (28). MDA is an end
product of lipid peroxidation, which is toxic to neurons. hAMMSC
treatment notably enhanced the SOD activity and diminished the MDA
level in the brain of AD transgenic mice in contrast with the PBS
control group (Fig. 5D and E).
Discussion
Studies on the therapeutic effect of MSCs on AD
indicate that the transplantation of MSCs could improve cognitive
decline in AD mice. In this study, our data revealed that amniotic
membrane could be used as a powerful cellular source for the
generation of stem cells. The differentiation potential and the
number of human bone marrow-derived MSCs decreased during culture.
Derived from the epiblast following fertilization, amniotic
membrane has a number of advantages over bone marrow and embryos.
Amniotic membrane cells express low levels of myosin heavy chain
class I antigens (29). It is
reported that amniotic membrane cells could be induced to neural
cells in special conditions and discharge neurotransmitters,
including acetylcholine, norepinephrine and dopamine (30). Multipotent MSCs have previously been
isolated from various placental tissues. Moreover, hAMMSCs exhibit
inducible angiogenic potential and may be useful in therapeutic
strategy for vascular diseases (31).
Our results indicated that the cultured hAMMSCs had powerful
vitality at passage 4, which ensured the subsequent experimental
requirements.
Before performing the water maze test, there was no
statistical difference in the basic locomotor and anxiety-like
behaviors observed in the three groups of this study (the normal
control group, the PBS-injected group and the hAMMSC-injected
group). The escape latency observed from the PBS-injected group was
poor compared with that of the normal control group. However, the
hAMMSC group demonstrated improved escape latency and was not
significantly different to the normal group. The hAMMSC group
demonstrated an improved performance in memory function compared
with the PBS group. The amount of Aβ plaque was decreased
significantly as the spatial learning and memory function
ameliorated following hAMMSC transplantation, which was not
observed in the PBS group. Our findings provide evidence that the
improved cognitive function may be caused by the decreased amount
of Aβ plaques.
It is considered that Aβ (1–42) oligomer is a toxic
species and is correlated with learning and memory in the brain of
mice (32). Whether the total plaque
burden correlates well with the phenotypic variability of AD has
not yet been ascertained. However, the molecular and structural
composition of Aβ, including the ratio of Aβ40/42, is responsible
for neurotoxicity (24). Studies have
reported that the Aβ40/42 ratio has a great impact on neurotoxicity
and is associated with the onset of familial AD (33). Our findings demonstrated that
cognitive function improved significantly as the Aβ level
decreased. Intracerebroventricular transplantation of hAMMSCs
increased acetylcholine concentration and the number of hippocampal
cholinergic neurites in AbPP/PS1 transgenic AD mice (34). Following hAMMSC injection, the number
of ED1-positive phagocytic microglial cells associated with Aβ
plaques was enhanced, the expression levels of proinflammatory
cytokines were decreased, and those of anti-inflammatory cytokines
were increased (35).
In the progression of AD, Aβ induces cytotoxicity
correlated with oxidative stress, ROS production and mitochondrial
dysfunction (36). Natural
antioxidants, including EGb 761 and curcumin, decrease the
Aβ-induced ROS generation and neuronal apoptosis to protect neuron
function (37). As major antioxidant
enzymes, SOD and GSH-Px remove harmful peroxide metabolites and
block lipid peroxidation chain reaction to prevent cell damage by
free radicals (28). The SOD activity
and the level of GSH and GSSG are indicators of oxidative stress,
and the level of MDA represents lipid peroxidation. The present
study revealed that the GSH level and the GSH/GSSG ratio were
higher in the hAMMSC group than in the PBS group. The SOD activity
and MDA level were improved significantly as the level of Aβ
decreased, but there was no such trend in the PBS group. This
finding provides further evidence that the improvement of
antioxidative function may be triggered by a reduction in the
amount of amyloid plaques following hAMMSC transplantation.
In summary, our findings have demonstrated that
hAMMSC transplantation into an AD transgenic mice model attenuates
the oxidative stress supported by the increased level of
antioxidative enzymes and the decreased level of lipid peroxidation
product, which is correlated with low levels of Aβ. Consequently,
intravenous injection of hAMMSCs elevated spatial learning ability
and memory function in the AD mouse model by stimulating
antioxidative function, indicating that hAMMSCs are useful in the
prevention and treatment of AD.
Acknowledgements
This study was supported by The Second Youth
Innovation Fund of the Affiliated Hospital of Zhengzhou University
(grant no. 2011YN01008) and The College and University Major
Scientific Research Program of Henan Educational Committee (grant
no's. 15A310011 and 16A310008).
References
|
1
|
Caughey B and Lansbury PT: Protofibrils,
pores, fibrils, and neurodegeneration: separating the responsible
protein aggregates from the innocent bystanders. Annu Rev Neurosci.
26:267–298. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de la Monte SM and Wands JR: Molecular
indices of oxidative stress and mitochondrial dysfunction occur
early and often progress with severity of Alzheimer's disease. J
Alzheimers Dis. 9:167–181. 2006.PubMed/NCBI
|
|
3
|
Perry VH and Holmes C: Microglial priming
in neurodegenerative disease. Nat Rev Neurol. 10:217–224. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Behl C, Davis JB, Lesley R and Schubert D:
Hydrogen peroxide mediates amyloid beta protein toxicity. Cell.
77:817–827. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manczak M, Anekonda TS, Henson E, Park BS,
Quinn J and Reddy PH: Mitochondria are a direct site of A beta
accumulation in Alzheimer's disease neurons: implications for free
radical generation and oxidative damage in disease progression. Hum
Mol Genet. 15:1437–1449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Apelt J, Bigl M, Wunderlich P and Schliebs
R: Aging-related increase in oxidative stress correlates with
developmental pattern of beta-secretase activity and beta-amyloid
plaque formation in transgenic Tg2576 mice with Alzheimer-like
pathology. Int J Dev Neurosci. 22:475–484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Felice FG, Velasco PT, Lambert MP,
Viola K, Fernandez SJ, Ferreira ST and Klein WL: Abeta oligomers
induce neuronal oxidative stress through an N-methyl-D-aspartate
receptor-dependent mechanism that is blocked by the Alzheimer drug
memantine. J Biol Chem. 282:11590–11601. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li F, Calingasan NY, Yu F, Mauck WM,
Toidze M, Almeida CG, Takahashi RH, Carlson GA, Flint Beal M, Lin
MT and Gouras GK: Increased plaque burden in brains of APP mutant
MnSOD heterozygous knockout mice. J Neurochem. 89:1308–1312. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishida Y, Yokota T, Takahashi T, Uchihara
T, Jishage K and Mizusawa H: Deletion of vitamin E enhances
phenotype of Alzheimer disease model mouse. Biochem Biophys Res
Commun. 350:530–536. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Na R, Gu M, Richardson A and Ran
Q: Lipid peroxidation up-regulates BACE1 expression in vivo: a
possible early event of amyloidogenesis in Alzheimer's disease. J
Neurochem. 107:197–207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quiroz-Baez R, Rojas E and Arias C:
Oxidative stress promotes JNK-dependent amyloidogenic processing of
normally expressed human APP by differential modification of
alpha-, beta- and gamma-secretase expression. Neurochem Int.
55:662–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Butterfield DA: The 2013 SFRBM discovery
award: selected discoveries from the Butterfield laboratory of
oxidative stress and its sequela in brain in cognitive disorders
exemplified by Alzheimer disease and chemotherapy induced cognitive
impairment. Free Radic Biol Med. 74:157–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindvall O and Kokaia Z: Stem cells in
human neurodegenerative disorders - time for clinical translation?
J Clin Invest. 120:29–40. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lanza C, Morando S, Voci A, Canesi L,
Principato MC, Serpero LD, Mancardi G, Uccelli A and Vergani L:
Neuroprotective mesenchymal stem cells are endowed with a potent
antioxidant effect in vivo. J Neurochem. 110:1674–1684. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Calió ML, Marinho DS, Ko GM, Ribeiro RR,
Carbonel AF, Oyama LM, Ormanji M, Guirao TP, Calió PL, Reis LA, et
al: Transplantation of bone marrow mesenchymal stem cells decreases
oxidative stress, apoptosis, and hippocampal damage in brain of a
spontaneous stroke model. Free Radic Biol Med. 70:141–154. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ko E, Lee KY and Hwang DS: Human umbilical
cord blood-derived mesenchymal stem cells undergo cellular
senescence in response to oxidative stress. Stem Cells Dev.
21:1877–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsuji H, Miyoshi S, Ikegami Y, Hida N,
Asada H, Togashi I, Suzuki J, Satake M, Nakamizo H, Tanaka M, et
al: Xenografted human amniotic membrane-derived mesenchymal stem
cells are immunologically tolerated and transdifferentiated into
cardiomyocytes. Circ Res. 106:1613–1623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Wu X, Qin C, Qi J, Ma S, Zhang H,
Kong Q, Chen D, Ba D and He W: A novel recombinant adeno-associated
virus vaccine reduces behavioral impairment and beta-amyloid
plaques in a mouse model of Alzheimer's disease. Neurobiol Dis.
14:365–379. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vorhees CV and Williams MT: Morris water
maze: procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Bai M, Xi Y, Hao J, Liu L, Mao N,
Su C, Miao J and Li Z: Early memory deficits precede plaque
deposition in APPswe/PS1dE9 mice: involvement of oxidative stress
and cholinergic dysfunction. Free Radic Biol Med. 52:1443–1452.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong HS, Li L, Song ZH, Tang J, Xu B, Zhai
XW, Sun LL, Zhang P, Li ZB, Pan QJ, et al: Premeiotic fetal murine
germ cells cultured in vitro form typical oocyte-like cells but do
not progress through meiosis. Theriogenology. 72:219–231. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu D, Cao Y, He R, Han N, Liu Z, Miao L
and Yin J: Schizandrin, an antioxidant lignan from Schisandra
chinensis, ameliorates Aβ1-42-induced memory impairment in
mice. Oxid Med Cell Longev. 2012:7217212012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuperstein I, Broersen K, Benilova I,
Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten
I, Van Der Werf K, Subramaniam V, et al: Neurotoxicity of
Alzheimer's disease Aβ peptides is induced by small changes in the
Aβ42 to Aβ40 ratio. EMBO J. 29:3408–3420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Onyango IG and Khan SM: Oxidative stress,
mitochondrial dysfunction, and stress signaling in Alzheimer's
disease. Curr Alzheimer Res. 3:339–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Behl C: Oxidative stress in Alzheimer's
disease: implications for prevention and therapy. Subcell Biochem.
38:65–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Khan RA, Khan MR and Sahreen S: Protective
effects of rutin against potassium bromate induced nephrotoxicity
in rats. BMC Complement Altern Med. 12:2042012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chauhan V and Chauhan A: Oxidative stress
in Alzheimer's disease. Pathophysiology. 13:195–208. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakuragawa N, Tohyama J and Yamamoto H:
Immunostaining of human amniotic epithelial cells: possible use as
a transgene carrier in gene therapy for inborn errors of
metabolism. Cell Transplant. 4:343–346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kakishita K, Elwan MA, Nakao N, Itakura T
and Sakuragawa N: Human amniotic epithelial cells produce dopamine
and survive after implantation into the striatum of a rat model of
Parkinson's disease: a potential source of donor for
transplantation therapy. Exp Neurol. 165:27–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alviano F, Fossati V, Marchionni C,
Arpinati M, Bonsi L, Franchina M, Lanzoni G, Cantoni S, Cavallini
C, Bianchi F, et al: Term amniotic membrane is a high throughput
source for multipotent mesenchymal stem cells with the ability to
differentiate into endothelial cells in vitro. BMC Dev Biol.
7:112007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klein WL: Synaptotoxic amyloid-β
oligomers: a molecular basis for the cause, diagnosis, and
treatment of Alzheimer's disease? J Alzheimers Dis. (33 Suppl 1):
S49–S65. 2013.PubMed/NCBI
|
|
33
|
Kumar-Singh S, Theuns J, Van Broeck B,
Pirici D, Vennekens K, Corsmit E, Cruts M, Dermaut B, Wang R and
Van Broeckhoven C: Mean age-of-onset of familial alzheimer disease
caused by presenilin mutations correlates with both increased
Abeta42 and decreased Abeta40. Hum Mutat. 27:686–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xue S, Chen C, Dong W, Hui G, Liu T and
Guo L: Therapeutic effects of human amniotic epithelial cell
transplantation on double-transgenic mice co-expressing APPswe and
PS1ΔE9-deleted genes. Sci China Life Sci. 55:132–140. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim KS, Kim HS, Park JM, Kim HW, Park MK,
Lee HS, Lim DS, Lee TH, Chopp M and Moon J: Long-term
immunomodulatory effect of amniotic stem cells in an Alzheimer's
disease model. Neurobiol Aging. 34:2408–2420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Camilleri A, Zarb C, Caruana M, Ostermeier
U, Ghio S, Högen T, Schmidt F, Giese A and Vassallo N:
Mitochondrial membrane permeabilisation by amyloid aggregates and
protection by polyphenols. Biochim Biophys Acta. 1828:2532–2543.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao Y and Zhao B: Natural antioxidants in
prevention and management of Alzheimer's disease. Front Biosci
(Elite Ed). 4:794–808. 2012. View
Article : Google Scholar : PubMed/NCBI
|