Introduction: Comparing HER-2 targeted
therapies in human and canine cancer
Human epidermal growth factor receptor-2 (HER-2) is
overexpressed in 25–30% of human breast cancers due to gene
amplification, thus defining ‘HER-2-positive disease’ (1–3).
HER-2-overexpression in human breast cancer has been associated
with increased metastatic potential (4), poor disease-free and overall survival,
and poor responsiveness to chemotherapy in vitro and in
vivo (5,6). Besides surgery, radiation and the usual
chemotherapy protocols, therapies used most successfully in
HER-2-overexpressing cancers are those addressing HER-2 as a target
on malignantly transformed cells (7,8). These so
called ‘targeted therapies’ are well established in human
HER-2-positive breast cancer and several agents have been approved
for this indication over the last years (9,10).
Examples of such targeted therapies that significantly improved
disease outcome are trastuzumab (Herceptin®; Hoffmann-La Roche AG,
Basel, Switzerland) and pertuzumab (Perjeta®; Hoffmann-La Roche AG)
(11), two monoclonal antibodies used
for passive immunotherapy in combination with different
chemotherapy protocols (12), but
tyrosine kinase inhibitors like lapatinib (Tyverb®; GlaxoSmithKline
plc., London, UK) have also been proven to be effective (13).
Hardly any of these targeted therapies have been
adapted for the use in canine patients or have yet been studied in
veterinary clinical trials. To date, only the tyrosine kinase
inhibitors masitinib (Masivet®; AB Science S.A., Paris, France) and
toceranib (Palladia®; Pfizer Inc., New York, NY, USA), each
targeting c-Kit, have been successfully tested in clinical trials
and have been approved for canine mast cell tumor patients
(14,15). In the case of toceranib, it was the
efficacy of the human counterpart substance sunitinib (Sutent®;
Pfizer Inc.) that led to the independent development of a similar
drug in dogs (15,16).
A recent study revealed that in addition to a
similar ErbB-2 overexpression rate in canine mammary gland tumors
compared with the human disease counterpart (17–20), there
was also an amino acid identity of 92% and a homology of 95%
between canine dog epidermal growth factor receptor-2 (DER-2) and
human HER-2 (21). Moreover, it was
demonstrated that the targeting of DER-2 with trastuzumab led to
the growth inhibition of canine tumor cells (21), indicating a similar biology in canine
mammary carcinomas as that of the HER-2 system in human patients
(19). The DER-2 status of mammary
tumors in dogs is not yet considered in veterinary medicine,
although its expression could be important in terms of
carcinogenesis and disease severity, as well as in the development
of novel targeted drugs (20,22).
HER-2 mimotope vaccines - a novel treatment
approach
Trastuzumab, as aforementioned, is a humanized
monoclonal antibody used for passive immunotherapy in human
HER-2-positive breast cancer (12).
In its humanized form, it is not applicable in comparative medicine
studies, e.g., in canine models, as it represents a xenogeneic and
highly immunogenic protein that is capable of inducing
hypersensitivity (23–25). This risk can be minimized if the
constant region domains are adapted to the given species (24,26). Only
two such chimeric canine antibodies have been reported to date. One
is based on cetuximab (Erbitux®; Merck KGaA, Darmstadt, Germany), a
mouse-human chimeric anti-epidermal growth factor receptor (EGFR)
antibody (26), and the second is a
rituximab-like (MabThera®; Hoffman-La Roche AG, Basel, Switzerland)
antibody targeting the B-cell antigen cluster of differentiation
(CD)20 (27). However, these
approaches are cost intensive, which may limit their market value
in comparative medical studies and veterinary oncology. The cost
factor, as well as the induction of autologous antibodies would
favor active immunotherapies such as a vaccine over passive
immunotherapy, not only for translational or comparative studies,
but also for human patients (28,29).
A tumor vaccine acts as an active immunotherapy,
training the immune system to induce polyclonal antibodies against
a tumor-specific antigen. Several tumor vaccines are currently
under clinical development in human breast cancer, aiming for the
induction of antibodies against different tumor-associated
proteins, e.g., mucin-1, telomerase reverse transcriptase or
carcinoembryonic antigen (30).
Correspondingly, several anti-HER-2 vaccines are also being studied
in clinical trials, including NeuVax™, a CD8(+) T-cell-eliciting
vaccine (31) and the AdHER2/neu
dendritic cell vaccine, which is currently being tested in a phase
I study (32).
Mimotopes, i.e. peptides mimicking protein,
carbohydrate or lipid epitopes represent another novel and feasible
option for cancer vaccines (33,34). In
previous studies, we developed a series of mimotope vaccines
against important tumor targets, including EGFR (35) and HER-2 (36). HER-2 specific mimotopes were generated
via biopanning of phage display libraries with trastuzumab, and the
deduced peptides were coupled to immunogenic carriers, such as
keyhole limpet hemocyanin, for vaccination (37), or they were expressed as fusion
proteins with carriers, such as adeno-associated viruses (AAV) or
AAV-like particles (AAVLPs), with predictably high safety (38). The antigenicity, immunogenicity and
tumoricidic effects of the HER-2 mimotope vaccines were
demonstrated in vitro and in vivo in previous studies
(37). The vaccine-induced HER-2
reactive trastuzumab-like antibodies also showed significant tumor
inhibitory effects on HER-2-overexpressing human SK-BR-3 cell lines
due to growth signal inhibition and growth receptor downregulation
by internalization. More recently, an AAV-HER-2 vaccine resulted in
significantly slower tumor growth in a BALB/c mouse model engrafted
with D2F2E2 tumor cells, which expressed the HER-2 transgene
(39). Notably, none of the mice
showed any signs of vaccine-related side effects, including local
or systemic reactions, or the well-described trastuzumab-associated
cardiotoxicity (40).
Canine mammary carcinomas resemble human
disease
Neoplasms of the mammary gland complex are the most
common tumors in dogs, particularly occurring in non-spayed female
individuals with a median age of first occurrence of around nine
years (41,42). The incidence in female dogs of any
breed is estimated at 50%, of which 40 to 50% are diagnosed as
malignant (43). All of these
malignant tumors have the potential to metastasize, either
lymphogenously to the regional lymph nodes and the lung, or
haematogenously directly to the lung and other distant organs
(44). Metastasizing tumors and a
tumor size of >3 cm result in a poor prognosis in terms of
survival (44). Other prognostic
factors are histological grade, differentiation of the tumor, and
the presence of estrogen and/or progesterone receptors (45,46).
Corresponding with human breast cancer, steroid-hormone receptor
expression is frequent in canine mammary gland tumors and these
receptors are important players in tumor development (44,47–49). In
addition, other factors, including p53 overexpression and
mutations, HER-2 overexpression or the immunological
microenvironment of the tumor, are markedly comparable and show
similar clinical correlations in dogs as in the human disease
(17–21,44,50–53).
Based on the discussed pathophysiological similarities between
canine and human mammary gland carcinoma, dog cancer patients could
serve as potential model patients for the study of disease biology,
and particularly for the development of novel immunotherapies.
Usual treatment options at present are primarily
surgery, radiation or chemotherapy (42,45).
However, these are far from optimal and are often associated with
serious side effects (54–56). The therapy of canine mammary
carcinomas is therefore limited and the recurrence of the disease
is frequent (44,57,58).
In summary, no optimal therapy for this indication
exists to date, and treatment options that are commonly used in
human clinical oncology, such as passive immunotherapy, have not
yet been implemented in standard veterinary care.
Due to the high molecular homology, we anticipate
that a vaccine targeted against HER-2, such as the aforementioned
AAV HER-2 mimotope vaccine developed for human patients, could
induce functional anti-HER-2 antibodies in dogs that are
cross-reactive with the canine DER-2 and result in tumoricidic
effects. Hence, such an active immunotherapy could be a novel and
alternative approach, by inducing a polyclonal immune response with
high antibody specificity and by additionally inducing
immunological memory (33). In dog
model patients, combinatory treatment strategies could be favorably
tested. It is likely that a vaccine study in canine cancer patients
with spontaneous HER-2-positive mammary gland carcinomas
will deliver more robust results for human patients than animal
experiments with induced or grafted tumors.
Translational relevance
It is clear that dogs with naturally occurring
cancer would not only gain individual benefit by participating in a
clinical trial, but could also act as animal model patients for
human disease (16,46). Currently, the development of novel
anticancer drugs is quite an inefficient procedure, where only
10.4% of novel agents that enter clinical phase I trials receive
market approval by the Food and Drug Administration (59). The situation is even worse for novel
cancer drugs, where this rate drops to only 6.7%, mostly due to the
low success rate of anticancer biologicals (59). Another obstacle is that the
development of a compound can take up to 15 years (60). The usual procedure of drug-development
starts with designing a novel compound on the computer (computer
aided drug design) (61) and testing
it in vitro. Even though there is a legal requirement to
replace animal experiments with alternative, ‘animal-free’ methods
where possible (62), each drug has
to be tested for toxicity in at least two animal species (61). Such animal models are also used to
gain important information on pharmacokinetics and efficacy prior
to being used in clinical trials in humans (63). If a drug has passed all preclinical
tests, the clinical development starts with phase 0/I studies in
healthy volunteers (or patients in oncological phase I trials),
followed by further clinical phase II–IV trials in human patients
(64,65).
However, animal patients with similar spontaneous
diseases to humans are usually left out during this process of drug
development, even though they could serve as ‘real life’ models for
human diseases and are a missing link between the laboratory
setting of animal experimentation and the ‘real life’ conditions
(66). Companion animals, and
particularly dogs, not only present with a similar pathophysiology
of diseases, but also share their human owners' environmental
surrounding and lifestyle, and thus are prone to develop similar
diseases to humans, including cancer (67–69).
Drug efficacy and safety should be addressed in
naturally occurring cancer, as these questions would be difficult
to answer in rodent models or in human clinical trials alone.
Research in animal patients could thus serve not only to complete
data generation in human medicine, but also to establish novel
drugs for usage in veterinary medicine itself (70). As described by Paoloni and Khanna
(16), the parallel development of
the compound SU11654 in dogs and sunitinib (Sutent®; Pfizer Inc.)
in human cancer patients could act as an example of how the two
disciplines could co-operate in respect to the translational
development of agents in human and veterinary oncology.
An additional advantage of using the dog cancer
patient as an animal model for human disease is the relatively
shorter lifespan and thus a shorter time of disease development and
response to treatment. This remains true even though the timespan
to the conclusion of veterinary clinical trials is longer than
experiments in rodent models (16,70). This
time aspect would be further supported by the fact that clinical
trials in pet patients are not constrained by the usual phase I–III
trial designs in human medicine, and novel drugs can be offered to
animal patients prior to any other conventional treatment being
provided (16). Results that would
take years to obtain in human clinical trials could be revealed in
a relatively short timespan with dog patients, and then translated
to humans. However, testing novel treatment strategies, and
specifically targeted therapies, in veterinary oncology within
clinical trials is not as common as in human oncology. Funding
opportunities are rare and the difference to animal experimentation
is legally rarely acknowledged (71).
Overall, the clinical development of a drug in human
medicine is cost- and time-intensive. With regard to HER-2-positive
breast cancer, the high homology of DER-2-positive mammary
carcinoma and its responsiveness to specific targeting (21) suggest that canine cancer patients
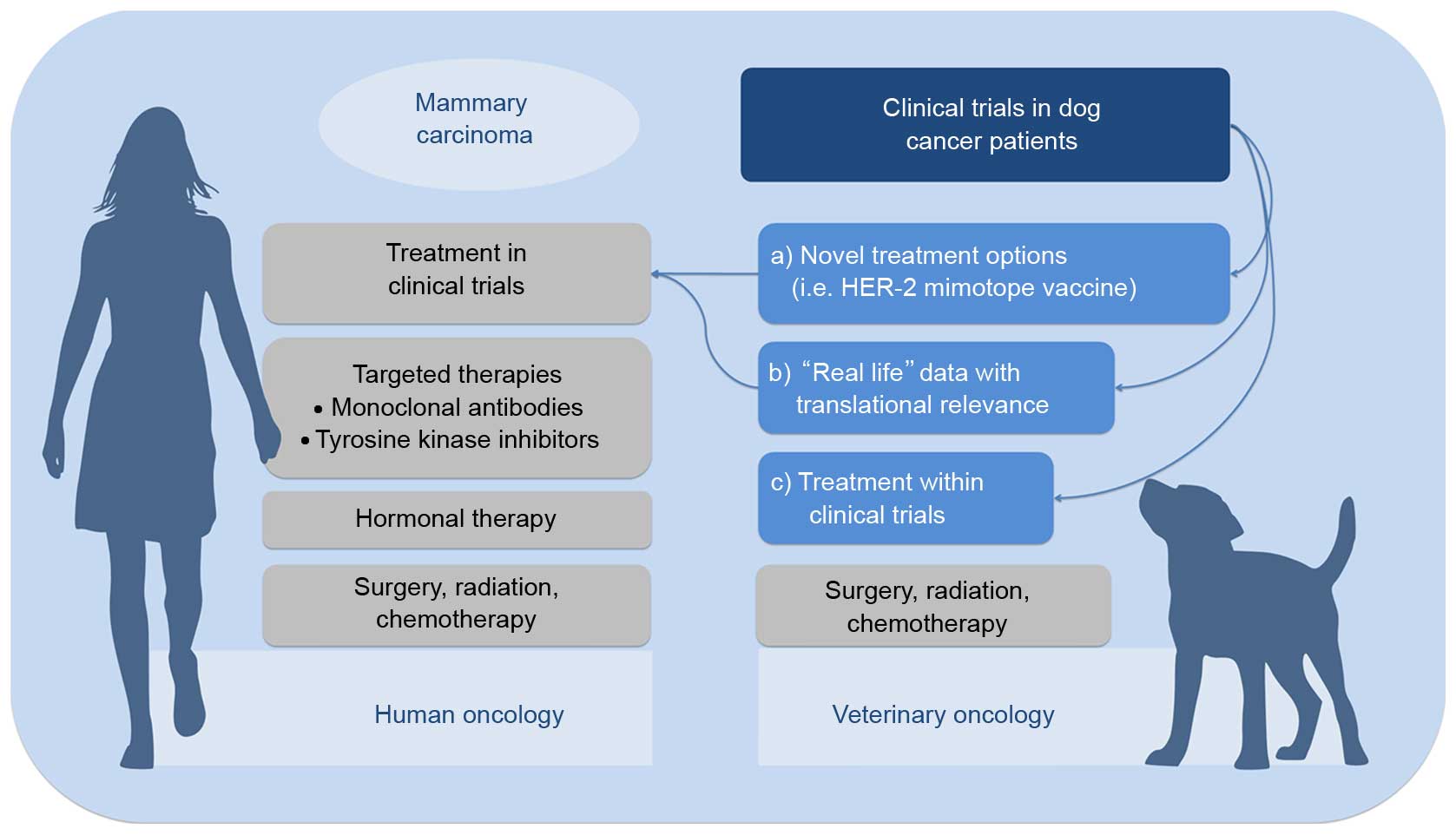
could act as a ‘real-life model’ for human cancer patients.
Furthermore, translating well-established therapeutic strategies
from human to veterinary oncology would improve the currently
available therapeutic options (Fig.
1).
Conclusion: Where we stand
The differences in the therapies available to humans
compared to those available to animals are very evident,
particularly in oncology. Present cancer treatments for pet animals
are frequently one step behind human medicine. Thus, surgery,
chemotherapy and, if available, radiation therapy represent the
most commonly applied treatment options for companion animals. In
the meantime, targeted therapies have become indispensable
treatment options in human oncology, either as registered
therapeutics or as drugs under clinical development. A particularly
important change has occurred with regard to implications for the
HER-2-positive breast cancer diagnosis in women. HER-2-positivity
used to be regarded as an indicator of a bad prognosis (3), however, this evaluation has changed
completely in the last 17 years due to the availability of
personalized anti-HER-2 therapies that have significantly improved
treatment outcomes (1,8,12).
Comparative medicine is systematically revealing
more and more similarities in the pathophysiology of a number of
diseases, particularly in the comparison between humans and pet
animals, such as the example of HER-2-positive mammary tumors in
dogs (17–21). This knowledge can be used to gain
benefits in each field by simultaneous and thus faster development
of novel drugs (72,73).
Since cancer incidence rates are also increasing in
veterinary medicine (74), the
development of targeted therapies is greatly required. However,
simply translating human monoclonal antibodies into veterinary
medicine is not an option; they have to be specifically adapted for
each species in order to prevent immunogenicity and adverse
reactions. Only two antibodies have thus far been ‘caninized’ for
use in dog cancer patients (26,27).
Therefore, the idea of developing an AAVLP HER-2 mimotope vaccine
that induces trastuzumab-like immunoglobulins would be a
cost-effective and species-independent alternative, and also of
great interest to human oncology. The clinical development of such
a novel treatment option for animal patients would gain robust
clinical ‘real life’ data with a higher predictive value for
translation into human medicine as well.
In conclusion, clinical trials in comparative
oncology settings may be of increasing importance in the future,
but not only for animals. The trials could provide translational
evidence for applications in humans, and allow the fast and
efficient verification of novel combinatorial treatments.
Anticancer vaccines, such as the AAVLP-HER-2 mimotope approach
could be particularly effective; they can be applied independent of
species as long as the homology of the target antigen is high, such
as in the case of HER-2 and DER-2.
Acknowledgements
The present study was supported by the Austrian
Science Fund projects P 23398-B11, by W1205-B09 (doctoral program
Cell Communication in Health and Disease), and by Biomedical
International R+D GmbH (Vienna, Austria). The authors would like to
thank Ms. Amelia Wein (Interuniversity Messerli Research Institute,
Vienna, Austria) for proofreading the original manuscript.
Glossary
Abbreviations
Abbreviations:
|
AAV
|
adeno-associated virus
|
|
AAVLP
|
AAV-like particle
|
|
DER-2
|
dog epidermal growth factor
receptor-2
|
|
EGFR
|
epidermal growth factor receptor
|
|
HER-2
|
human epidermal growth factor
receptor-2
|
References
|
1
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan M, Yao J and Yu D: Overexpression of
the c-erbB-2 gene enhanced intrinsic metastasis potential in human
breast cancer cells without increasing their transformation
abilities. Cancer Res. 57:1199–1205. 1997.PubMed/NCBI
|
|
5
|
Yu D and Hung MC: Overexpression of ErbB2
in cancer and ErbB2-targeting strategies. Oncogene. 19:6115–6121.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knuefermann C, Lu Y, Liu B, Jin W, Liang
K, Wu L, Schmidt M, Mills GB, Mendelsohn J and Fan Z:
HER2//PI-3K//Akt activation leads to a multidrug resistance in
human breast adenocarcinoma cells. Oncogene. 22:3205–3212. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hudis CA: Trastuzumab-mechanism of action
and use in clinical practice. N Engl J Med. 357:39–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perez EA, Romond EH, Suman VJ, Jeong JH,
Davidson NE, Geyer CE Jr, Martino S, Mamounas EP, Kaufman PA and
Wolmark N: Four-year follow-up of trastuzumab plus adjuvant
chemotherapy for operable human epidermal growth factor receptor
2-positive breast cancer: Joint analysis of data from NCCTG N9831
and NSABP B-31. J Clin Oncol. 29:3366–3373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh JC, Jhaveri K and Esteva FJ:
HER2-positive advanced breast cancer: Optimizing patient outcomes
and opportunities for drug development. Br J Cancer. 111:1888–1898.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rimawi MF, Schiff R and Osborne CK:
Targeting HER2 for the treatment of breast cancer. Annu Rev Med.
66:111–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Swain SM, Baselga J, Kim SB, Ro J,
Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A,
Heeson S, et al: Pertuzumab, trastuzumab, and docetaxel in
HER2-positive metastatic breast cancer. N Engl J Med. 372:724–734.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukohara T: Role of HER2-targeted agents
in adjuvant treatment for breast cancer. Chemother Res Pract.
2011:7303602011.PubMed/NCBI
|
|
13
|
deAzambuja E, Holmes AP, Piccart-Gebhart
M, Holmes E, Di Cosimo S, Swaby RF, Untch M, Jackisch C, Lang I,
Smith I, et al: Lapatinib with trastuzumab for HER2-positive early
breast cancer (NeoALTTO): Survival outcomes of a randomised,
open-label, multicentre, phase 3 trial and their association with
pathological complete response. Lancet Oncol. 15:1137–1146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hahn KA, Ogilvie G, Rusk T, Devauchelle P,
Leblanc A, Legendre A, Powers B, Leventhal PS, Kinet JP, Palmerini
F, et al: Masitinib is safe and effective for the treatment of
canine mast cell tumors. J Vet Intern Med. 22:1301–1309. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
London CA, Malpas PB, Wood-Follis SL,
Boucher JF, Rusk AW, Rosenberg MP, Henry CJ, Mitchener KL, Klein
MK, Hintermeister JG, et al: Multi-center, placebo-controlled,
double-blind, randomized study of oral toceranib phosphate
(SU11654), a receptor tyrosine kinase inhibitor, for the treatment
of dogs with recurrent (either local or distant) mast cell tumor
following surgical excision. Clin Cancer Res. 15:3856–3865. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paoloni M and Khanna C: Translation of new
cancer treatments from pet dogs to humans. Nat Rev Cancer.
8:147–156. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gamba CO, Dias EJ, Ribeiro LG, Campos LC,
Estrela-Lima A, Ferreira E and Cassali GD: Histopathological and
immunohistochemical assessment of invasive micropapillary mammary
carcinoma in dogs: A retrospective study. Vet J. 196:241–246. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ressel L, Puleio R, Loria GR, Vannozzi I,
Millanta F, Caracappa S and Poli A: HER-2 expression in canine
morphologically normal, hyperplastic and neoplastic mammary tissues
and its correlation with the clinical outcome. Res Vet Sci.
94:299–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Muhammadnejad A, Keyhani E, Mortazavi P,
Behjati F and Haghdoost IS: Overexpression of her-2/neu in
malignant mammary tumors; translation of clinicopathological
features from dog to human. Asian Pac J Cancer Prev. 13:6415–6421.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JH, Im KS, Kim NH, Yhee JY, Nho WG and
Sur JH: Expression of HER-2 and nuclear localization of HER-3
protein in canine mammary tumors: Histopathological and
immunohistochemical study. Vet J. 189:318–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singer J, Weichselbaumer M, Stockner T,
Mechtcheriakova D, Sobanov Y, Bajna E, Wrba F, Horvat R, Thalhammer
JG, Willmann M and Jensen-Jarolim E: Comparative oncology: ErbB-1
and ErbB-2 homologues in canine cancer are susceptible to cetuximab
and trastuzumab targeting. Mol Immunol. 50:200–209. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peña L, Gama A, Goldschmidt MH, Abadie J,
Benazzi C, Castagnaro M, Díez L, Gärtner F, Hellmén E, Kiupel M, et
al: Canine mammary tumors: A review and consensus of standard
guidelines on epithelial and myoepithelial phenotype markers, HER2,
and hormone receptor assessment using immunohistochemistry. Vet
Pathol. 51:127–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pichler WJ: Adverse side-effects to
biological agents. Allergy. 61:912–920. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Corominas M, Gastaminza G and Lobera T:
Hypersensitivity reactions to biological drugs. J Investig Allergol
Clin Immunol. 24:212–225. 2014.PubMed/NCBI
|
|
25
|
Baldo BA: Adverse events to monoclonal
antibodies used for cancer therapy: Focus on hypersensitivity
responses. Oncoimmunology. 2:e263332013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singer J, Fazekas J, Wang W,
Weichselbaumer M, Matz M, Mader A, Steinfellner W, Meitz S,
Mechtcheriakova D, Sobanov Y, et al: Generation of a canine
anti-EGFR (ErbB-1) antibody for passive immunotherapy in dog cancer
patients. Mol Cancer Ther. 13:1777–1790. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rue SM, Eckelman BP, Efe JA, Bloink K,
Deveraux QL, Lowery D and Nasoff M: Identification of a candidate
therapeutic antibody for treatment of canine B-cell lymphoma. Vet
Immunol Immunopathol. 164:148–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jensen-Jarolim E and Singer J: Cancer
vaccines inducing antibody production: More pros than cons. Expert
Rev Vaccines. 10:1281–1289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Milani A, Sangiolo D, Montemurro F,
Aglietta M and Valabrega G: Active immunotherapy in HER2
overexpressing breast cancer: Current status and future
perspectives. Ann Oncol. 24:1740–1748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anderson KS: Tumor vaccines for breast
cancer. Cancer Invest. 27:361–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sears AK, Perez SA, Clifton GT, Benavides
LC, Gates JD, Clive KS, Holmes JP, Shumway NM, Van Echo DC,
Carmichael MG, et al: AE37: A novel T-cell-eliciting vaccine for
breast cancer. Expert Opin Biol Ther. 11:1543–1550. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
NCT01730118, . Ad/HER2/Neu dendritic cell
cancer vaccine testing. https://clinicaltrials.gov/Accessed. August
31–2015
|
|
33
|
Knittelfelder R, Riemer AB and
Jensen-Jarolim E: Mimotope vaccination-from allergy to cancer.
Expert Opin Biol Ther. 9:493–506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ashok BT, David L, Chen YG, Garikapaty VP,
Chander B, Kanduc D, Mittelman A and Tiwari RK: Peptide mimotopes
of oncoproteins as therapeutic agents in breast cancer. Int J Mol
Med. 11:465–471. 2003.PubMed/NCBI
|
|
35
|
Riemer AB, Kurz H, Klinger M, Scheiner O,
Zielinski CC and Jensen-Jarolim E: Vaccination with cetuximab
mimotopes and biological properties of induced anti-epidermal
growth factor receptor antibodies. J Natl Cancer Inst.
97:1663–1670. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Riemer AB, Kraml G, Scheiner O, Zielinski
CC and Jensen-Jarolim E: Matching of trastuzumab (Herceptin)
epitope mimics onto the surface of Her-2/neu-a new method of
epitope definition. Mol Immunol. 42:1121–1124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Riemer AB, Klinger M, Wagner S, Bernhaus
A, Mazzucchelli L, Pehamberger H, Scheiner O, Zielinski CC and
Jensen-Jarolim E: Generation of Peptide mimics of the epitope
recognized by trastuzumab on the oncogenic protein Her-2/neu. J
Immunol. 173:394–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manzano-Szalai K, Thell K, Willensdorfer
A, Weghofer M, Pfanzagl B, Singer J, Ritter M, Stremnitzer C,
Flaschberger I, Michaelis U and Jensen-Jarolim E: Adeno-associated
virus-like particles as new carriers for B-cell vaccines: Testing
immunogenicity and safety in BALB/c mice. Viral Immunol.
27:438–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singer J, Manzano-Szalai K, Fazekas J,
Thell K, Bentley-Lukschal A, Stremnitzer C, Roth-Walter F, Weghofer
M, Ritter M, Tossi KP, et al: Proof of concept study with a HER-2
mimotope anti-cancer vaccine deduced from a novel AAV-mimotope
library platform. Oncoimmunology. e11714462016. View Article : Google Scholar
|
|
40
|
Valicsek E, Kószó R, Dobi Á, Uhercsák G,
Varga Z, Vass A, Jebelovszky É and Kahán Z: Cardiac surveillance
findings during adjuvant and palliative trastuzumab therapy in
patients with breast cancer. Anticancer Res. 35:4967–4973.
2015.PubMed/NCBI
|
|
41
|
Dobson JM, Samuel S, Milstein H, Rogers K
and Wood JL: Canine neoplasia in the UK: Estimates of incidence
rates from a population of insured dogs. J Small Anim Pract.
43:240–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Arnold-Gloor S, Hubler M and Reichler I:
Diseases of the mammary glandPlacement of teh dog clinic. Suter PF,
Arnold-Gloor S and Niemand HG: Parey; Stuttgart: pp. 881–883. 2006,
(In German).
|
|
43
|
Baba AI, Câtoi C and Baba AI: Mammary
gland tumours-comparative oncology. The publishing house of the
Romanian academy; Bucharest: pp. 1online resource. 2007, https://www.ncbi.nlm.nih.gov/books/NBK9557/Accessed
August 31, 2015.
|
|
44
|
Sorenmo K: Canine mammary gland tumors.
Vet Clin North Am Small Anim Pract. 33:573–596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Novosad CA: Principles of treatment for
mammary gland tumors. Clin Tech Small Anim Pract. 18:107–109. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Queiroga FL, Raposo T, Carvalho MI, Prada
J and Pires I: Canine mammary tumours as a model to study human
breast cancer: Most recent findings. In vivo. 25:455–465.
2011.PubMed/NCBI
|
|
47
|
de Las Mulas JM, Millán Y and Dios R: A
prospective analysis of immunohistochemically determined estrogen
receptor alpha and progesterone receptor expression and host and
tumor factors as predictors of disease-free period in mammary
tumors of the dog. Vet Pathol. 42:200–212. 2005. View Article : Google Scholar
|
|
48
|
Queiroga FL, Pérez-Alenza MD, Silvan G,
Peña L, Lopes C and Illera JC: Role of steroid hormones and
prolactin in canine mammary cancer. J Steroid Biochem Mol Biol.
94:181–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Torres CG, Pino AM and Sierralta WD: A
cyclized peptide derived from alpha fetoprotein inhibits the
proliferation of ER-positive canine mammary cancer cells. Oncol
Rep. 21:1397–1404. 2009.PubMed/NCBI
|
|
50
|
Wakui S, Muto T, Yokoo K, Yokoo R,
Takahashi H, Masaoka T, Hano H and Furusato M: Prognostic status of
p53 gene mutation in canine mammary carcinoma. Anticancer Res.
21:611–616. 2001.PubMed/NCBI
|
|
51
|
Lee CH and Kweon OK: Mutations of p53
tumor suppressor gene in spontaneous canine mammary tumors. J Vet
Sci. 3:321–325. 2002.PubMed/NCBI
|
|
52
|
Carvalho MI, Pires I, Prada J and Queiroga
FL: A role for T-lymphocytes in human breast cancer and in canine
mammary tumors. Biomed Res Int. 2014:1308942014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Haga S, Nakayama M, Tatsumi K, Maeda M,
Imai S, Umesako S, Yamamoto H, Hilgers J and Sarkar NH:
Overexpression of the p53 gene product in canine mammary tumors.
Oncol Rep. 8:1215–1219. 2001.PubMed/NCBI
|
|
54
|
Tao JJ, Visvanathan K and Wolff AC: Long
term side effects of adjuvant chemotherapy in patients with early
breast cancer. Breast. 24(Suppl 2): S149–S153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bracha S, Walshaw R, Danton T, Holland S,
Ruaux C and Obradovich J: Evaluation of toxicities from combined
metronomic and maximal-tolerated dose chemotherapy in dogs with
osteosarcoma. J Small Anim Pract. 55:369–374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Selmic LE, Burton JH, Thamm DH, Withrow SJ
and Lana SE: Comparison of carboplatin and doxorubicin-based
chemotherapy protocols in 470 dogs after amputation for treatment
of appendicular osteosarcoma. J Vet Intern Med. 28:554–563. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Stratmann N, Failing K, Richter A and
Wehrend A: Mammary tumor recurrence in bitches after regional
mastectomy. Vet Surg. 37:82–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Betz D, Schoenrock D, Mischke R,
Baumgärtner W and Nolte I: Postoperative treatment outcome in
canine mammary tumors. Multivariate analysis of the prognostic
value of pre- and postoperatively available information. Tierarztl
Prax Ausg K Kleintiere Heimtiere. 40:235–242. 2012.(In English,
German). PubMed/NCBI
|
|
59
|
Hay M, Thomas DW, Craighead JL, Economides
C and Rosenthal J: Clinical development success rates for
investigational drugs. Nat Biotechnol. 32:40–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Marchetti S and Schellens JH: The impact
of FDA and EMEA guidelines on drug development in relation to Phase
0 trials. Br J Cancer. 97:577–581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Stapff M: Drug trials: An introduction to
clinical trials for doctors, students, medical assistants and
interested laymen. 5th. Zuckschwerdt; Munich: 2008
|
|
62
|
Directive 2010/63/EU, . Directive
2010/63/EU of the European Parliament and of the Council of 22
September 2010 on the protection of animals used for scientific
purposes. 2010, simpleeur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDFAccessed
May 17, 2016.
|
|
63
|
European Medicines Agency. ICH-GCP: ICH
Topic E6 (R1) Guideline for Good Clinical Practice. 2002,
simpleeur-lex.europa.eu/legalcontent/EN/TXT/?uri=celex%3A32010L0063Accessed
August 31, 2015.
|
|
64
|
National Institutes of Health. U.S.
National Library of Medicine: Clinical Trial Phases. 2015,
simpleclinicaltrials.govAccessed August 31,
2015.
|
|
65
|
Ciociola AA, Cohen LB and Kulkarni P:
FDA-Related Matters Committee of the American College of
Gastroenterology: How drugs are developed and approved by the FDA:
Current process and future directions. Am J Gastroenterol.
109:620–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Khanna C, London C, Vail D, Mazcko C and
Hirschfeld S: Guiding the optimal translation of new cancer
treatments from canine to human cancer patients. Clin Cancer Res.
15:5671–5677. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Monath TP, Kahn LH and Kaplan B:
Introduction: One health perspective. ILAR J. 51:193–198. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Séveré S, Marchand P, Guiffard I, Morio F,
Venisseau A, Veyrand B, Le Bizec B, Antignac JP and Abadie J:
Pollutants in pet dogs: A model for environmental links to breast
cancer. Springerplus. 4:272015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kelsey JL, Moore AS and Glickman LT:
Epidemiologic studies of risk factors for cancer in pet dogs.
Epidemiol Rev. 20:204–217. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hansen K and Khanna C: Spontaneous and
genetically engineered animal models; use in preclinical cancer
drug development. Eur J Cancer. 40:858–880. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fürdös I, Fazekas J, Singer J and
Jensen-Jarolim E: Translating clinical trials from human to
veterinary oncology and back. J Transl Med. 13:2652015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jensen-Jarolim E: Definition of
comparative medicine: History and new identityComparative Medicine.
Anatomy and Physiology. Springer; Wien: pp. 1–18. 2014
|
|
73
|
Singer J and Jensen-Jarolim E: IgE-based
immunotherapy of cancer-a comparative oncology approach. J Carcinog
Mutagen. 5:10001762014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Merlo DF, Rossi L, Pellegrino C, Ceppi M,
Cardellino U, Capurro C, Ratto A, Sambucco PL, Sestito V, Tanara G
and Bocchini V: Cancer incidence in pet dogs: Findings of the
Animal Tumor Registry of Genoa, Italy. J Vet Intern Med.
22:976–984. 2008. View Article : Google Scholar : PubMed/NCBI
|