Introduction
Lung cancer is a malignant tumor with a high
incidence and mortality rate; it is currently the number one cause
of cancer-associated mortality worldwide, with ~1.4 million deaths
annually (1). Lung cancer is
diagnosed during its advanced stages in the majority of patients,
which is the primary reason underlying the high mortality rate
associated with this disease (2).
Non-small cell lung cancer (NSCLC), including adenocarcinoma,
squamous cell carcinoma, large cell carcinoma and
bronchioloalveolar carcinoma, accounts for ~85% of all lung cancer
cases (3). Despite numerous available
adjuvant therapies, the overall five-year survival rate remains
poor for lung cancer patients (4),
and the most frequent causes of cancer-associated mortality are the
occurrence of local invasion or distant metastasis, rather than the
primary tumors (5). Therefore,
investigating novel markers and the molecular mechanisms that are
involved in NSCLC is of great importance for improving the
diagnosis and treatment of NSCLC.
Retinoids regulate the growth, differentiation, and
apoptosis of healthy cells during embryonic development and of
premalignant and malignant cells during carcinogenesis (6). It has been proposed that nuclear
retinoid receptors may mediate these effects via the regulation of
gene transcription (7). The
regulation of gene transcription is controlled by the activation of
two types of nuclear receptors: Retinoic acid receptors (RARα, RARβ
and RARγ) and retinoid X receptors (RXRα, RXRβ and RXRγ) (8). Tazarotene, a synthetic retinoid that
binds RARβ and RARγ, is utilized in the treatment of psoriasis
(9). Tazarotene upregulates three
genes: Tazarotene-induced gene (TIG)1, TIG2 and TIG3, which may
lead to an anti-proliferative effect (10). Initially, TIG2 was identified in a
subtraction hybridization assay, which observed upregulated genes
of human skin raft cultures by treatment with the anti-psoriatic
retinoid drug tazarotene (11). TIG2
is expressed in the skin, pancreas, liver, spleen, prostate, ovary,
small intestine and colon (12). A
small amount of evidence has demonstrated that TIG2 may be a tumor
suppressor gene (13); however, an
association between TIG2 expression, clinicopathological
characteristics and the prognosis in NSCLC remains to be
elucidated.
The present study sought to demonstrate TIG2
expression in patients with NSCLC using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
western blotting and immunohistochemical analysis. Subsequently,
the present study investigated the association between TIG2
expression and clinicopathological parameters to establish whether
TIG2 may serve as a novel prognostic biomarker in NSCLC
patients.
Materials and methods
Patients and specimens
Relevant clinicopathological data from 98 lung
cancer patients who underwent surgical resection at the Affiliated
Hospital of Nantong University (Nantong, China) between January
2006 and December 2008 were collected and retrospectively analyzed.
These human NSCLC tissue samples had been formalin-fixed,
paraffin-embedded and histopathologically diagnosed. Additionally,
for RT-qPCR and western blot analysis, the present study collected
32 paired fresh NSCLC tumor tissue samples and corresponding
adjacent noncancerous tissue samples from patients who underwent
surgery between January 2013 and December 2014. None of the
patients received preoperative chemotherapy or radiation therapy. A
follow-up evaluation was performed every 2 months for the initial 2
years following the operation, every 3 months in the third year and
every 6 months after this. Informed consent was obtained from all
patients, and the study was approved by the Institutional Hospital
Ethics Committee.
RT-qPCR
Total RNA was extracted from tissues using TRIzol
reagent according to the manufacturer's protocol (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA was reverse
transcribed using an Omniscript Reverse Transcription kit (Qiagen,
Inc., Valencia, CA, USA) according to the manufacturer's protocol.
The PCR amplification was performed at 96°C for 2 min, followed by
40 cycles of 96°C for 15 sec and 60°C for 1 min, on a Mastercycler
ep realplex (Eppendorf, Hamburg, Germany) with 1.0 µl of cDNA and
2X Fast EvaGreen™ qPCR Master Mix (Biotium, Inc.,
Fremont, CA, USA). The expression level of each candidate gene was
internally normalized against that of glyceraldehyde-3-phosphate
dehydrogenase (GAPDH; internal control). The relative quantitative
value was expressed by the 2−ΔΔCq method (14). Each experiment was performed in
triplicate. The primer sequences used were as follows: TIG2
forward, AATGGGAGGAAACGGAAATGC and reverse,
GCGAACTGTCCAGGGAAGTAGAA; and GAPDH forward,
5′-AACTTCCGTTGCTGCCAT-3′ and reverse, 5′-TTTCTTCCACAGGGCTTTG-3′.
Negative controls (no cDNA) and RT controls (no RT) were
performed.
Western blot analysis
The frozen tissue samples from patients with NSCLC,
including the tumor tissues and adjacent noncancerous tissues, were
homogenized in radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China), and the
lysates were cleared by centrifugation (13,523 × g) at 4°C for 15
min. Protein samples (20 µg) were separated by 10% SDS-PAGE and
subsequently transferred to nitrocellulose membranes. The membranes
were blocked with 5% non-fat dry milk in Tris-Buffered Saline and
Tween 20 (TBST) buffer for 1 h at room temperature and incubated
with a monoclonal mouse anti-human TIG2 antibody (dilution, 1:500;
cat. no. sc-373797; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) at 4°C overnight. Following washing three times for 5 min each
in TBST, the membranes were incubated with a horseradish peroxidase
(HRP)-conjugated goat anti-mouse secondary antibody (dilution,
1:5,000; cat. no. L3032-2; Signalway Antibody, Nanjing, China) for
2 h at room temperature. Following washing 3 times for 5 min with
TBST, protein bands were visualized by scanning with an
Odyssey® CLx Infrared Imaging System (LI-COR
Biosciences, Lincoln, NE, USA). The acquired band intensity was
measured by densitometry using Quantity One 4.62 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The protein levels were
normalized to that of GAPDH.
Immunohistochemistry
Paraffin-embedded sections of surgically resected
specimens were routinely dewaxed through a series of xylene and
were rehydrated with graded ethanol. Sections were pretreated by
high-pressure mediated antigen retrieval buffer (Abcam, Cambridge,
UK) with 0.01 M sodium citrate-hydrochloric acid (pH=6.0) at 100
Kpa for 5 min. Slides were blocked in 3% H2O2
for 10 min. The sections were incubated with monoclonal mouse
anti-human TIG2 antibody (dilution, 1:100) overnight at 4°C. After
washing three times for 5 min each with TBST, the sections were
incubated with HRP-conjugated goat anti-mouse secondary antibody
(dilution, 1:5,000) at 4°C overnight, and then visualized with
3,3′-diaminobenzidine solution. All of the slides were
counterstained with hematoxylin. Immunostaining of TIG2 was scored
following a semiquantitative scale by evaluating representative
tumor areas, intensity and percentage of cells. Staining was
classified as positive or negative, and was scored as follows:
Intensity (0, negative; 1, weak; 2, moderate; and 3, strong); and
percentage of positive tumor cells (0, <5%; 1, <25%; 2,
26–50%; 3, 51–75%; and 4, >75%). The scores for intensity and
percentage of positive tumor cells of each sample were multiplied
(range 0–12), and the total expression of TIG2 was determined as:
Low (negative, <1; weak, 1–4); or high (moderate, 5–8; strong,
9–12). All stained sections were evaluated and scored individually
by two pathologists with no prior knowledge of the
clinicopathological consequences of the patients.
Statistical analysis
Statistical analyses were performed using SPSS
version 18.0 (SPSS, Inc., Chicago, IL, USA). Correlation between
TIG2 expression and clinicopathological characteristics was
evaluated using the χ2 test. The 5-year survival rates
following tumor resection were calculated using the Kaplan-Meier
method, and differences in overall survival curves were analyzed
using the log-rank test. Multivariate analysis was performed using
the Cox proportional hazards regression model on several prognostic
factors. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression levels of TIG2 in
NSCLC
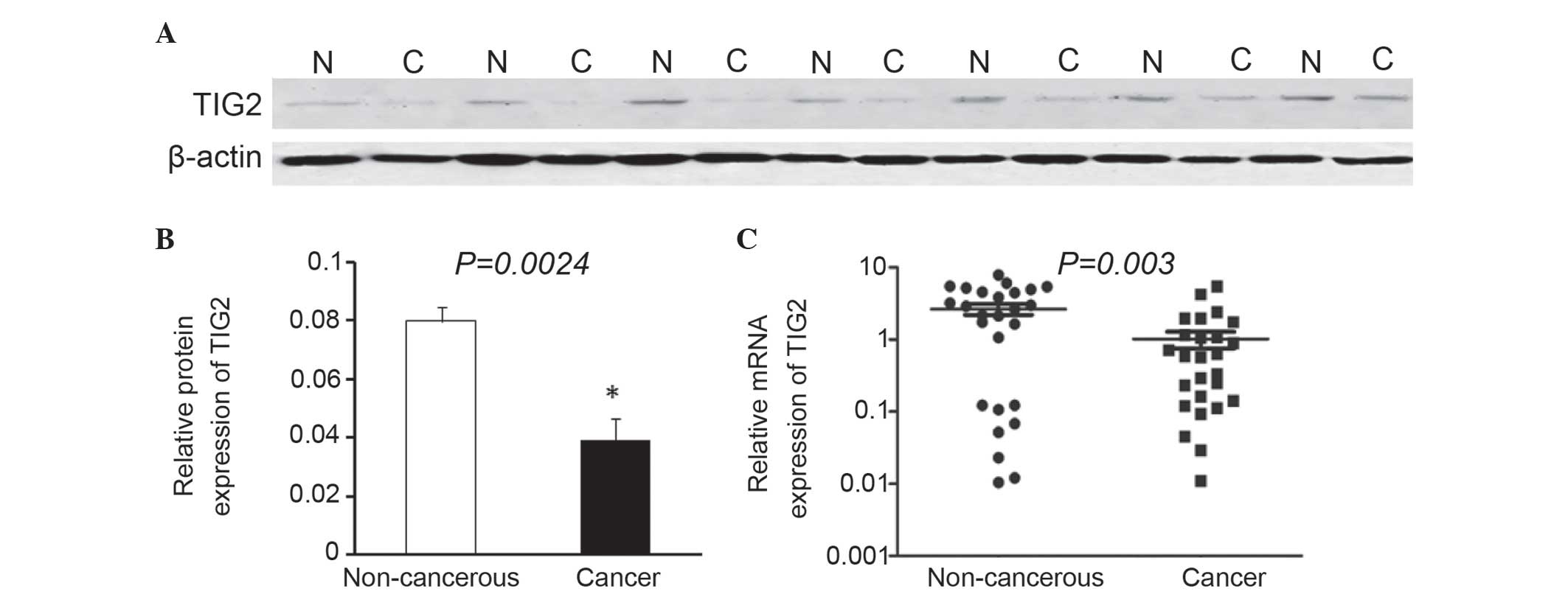
Western blot analysis revealed that the expression
level of TIG2 was markedly decreased in 16/20 NSCLC tissues
compared with the corresponding adjacent noncancerous tissues. A
total of 7 representative western blot analysis results are
presented in Fig. 1A. The average
TIG2 protein level in 20 NSCLC tissues was significantly reduced
compared with that of TIG2 in adjacent normal tissues (P=0.0024;
Fig. 1B). In addition, RT-qPCR was
performed to detect the expression levels of TIG2. The results of
the present study demonstrated that TIG2 mRNA expression was
downregulated in 18/26 NSCLC samples compared with corresponding
adjacent noncancerous tissues. The mean expression value of mRNA in
NSCLC tissues was significantly reduced compared with the value in
paired adjacent noncancerous tissues (P=0.003; Fig. 1C).
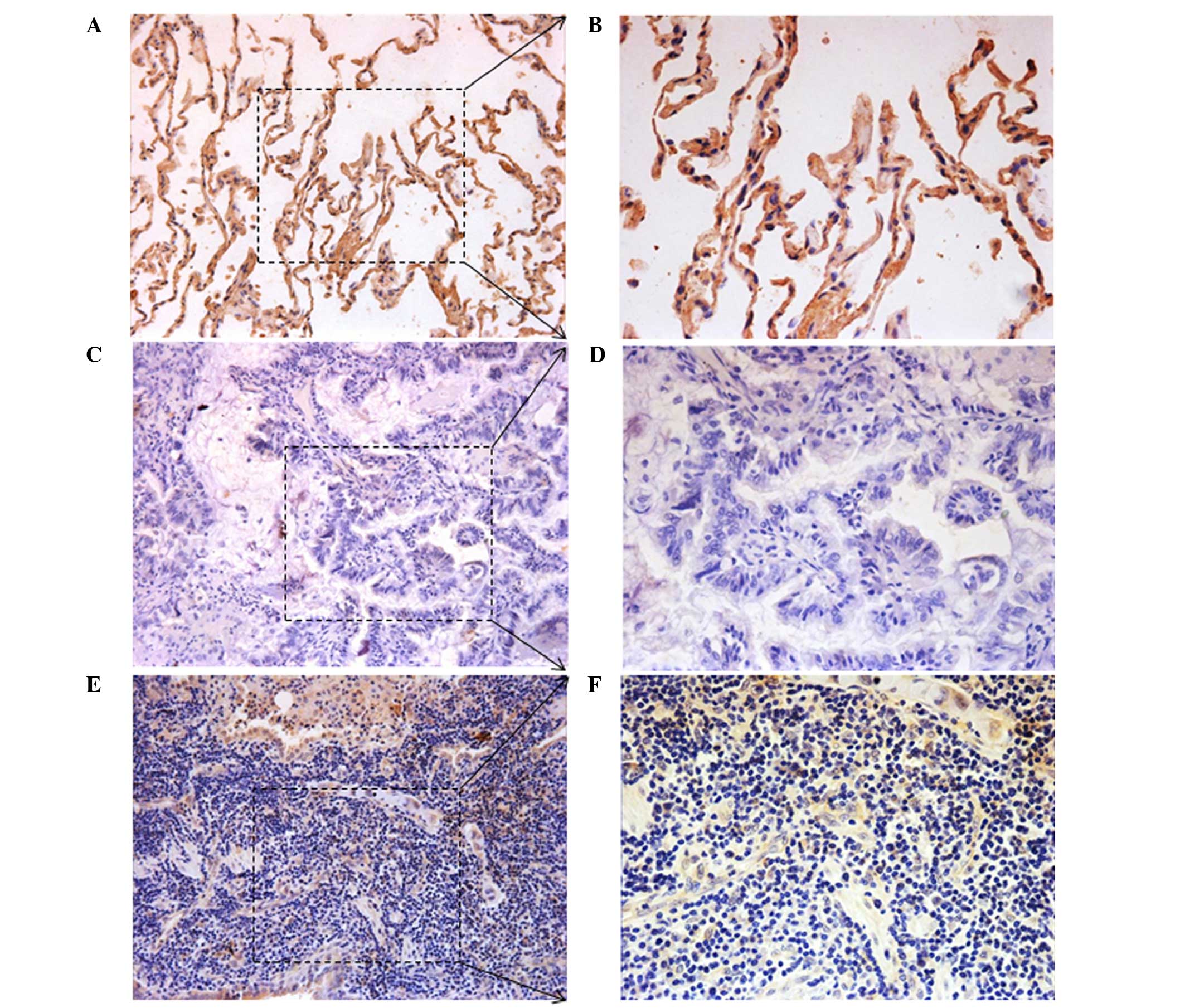
To additionally investigate the expression of TIG2
protein in cancer and corresponding adjacent noncancerous tissues,
immunohistochemical analysis was performed on NSCLC specimens. TIG2
expression was detected at various levels, primarily in the
cytoplasm of cells. High TIG2 expression was detected in 41.83%
(41/98) of NSCLC samples, whereas the remaining 58.17% (57/98)
displayed low TIG2 expression (Fig.
2). The above results indicated that TIG2 may have a tumor
suppressor role in NSCLC.
Correlation of clinicopathological
features and TIG2 expression
To additionally investigate the significance of TIG2
expression in NSCLC, the present study evaluated the correlation of
TIG2 expression with clinicopathological data. The expression
levels of TIG2 in NSCLC tissues were categorized as low or high
depending on the mean score. As shown in Table I, the χ2 analysis revealed
that the TIG2 level was associated with lymph node metastasis
(P=0.006), Tumor-Node-Metastasis (TNM) stage (P=0.021) and
differentiation (P=0.025). However, there was no significant
correlation between TIG2 expression and other clinicopathological
features, including age, gender, tumor size, smoking history,
histological classification or distant metastasis (P>0.05 in all
cases).
 | Table I.Correlation of TIG2 expression in
tumor tissues with clinicopathological characteristics in non-small
cell lung cancer patients. |
Table I.
Correlation of TIG2 expression in
tumor tissues with clinicopathological characteristics in non-small
cell lung cancer patients.
|
|
| TIG2 expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Total, n | High (n=41) | Low (n=57) | P-value |
|---|
| Age, years |
|
|
| 0.258 |
|
<60 | 46 | 22 | 24 |
|
| ≥60 | 52 | 19 | 33 |
|
| Gender |
|
|
| 0.182 |
| Male | 72 | 33 | 39 |
|
|
Female | 26 | 8 | 18 |
|
| Tumor size, cm |
|
|
| 0.342 |
|
<5 | 65 | 25 | 40 |
|
| ≥5 | 33 | 16 | 17 |
|
| Smoking history |
|
|
| 0.104 |
|
Smoker | 64 | 23 | 41 |
|
|
Non-smoker | 34 | 18 | 16 |
|
| Lymph node
metastasis |
|
|
| 0.006a |
|
Negative | 61 | 32 | 29 |
|
|
Positive | 37 | 9 | 28 |
|
| Histological
classification |
|
|
| 0.657 |
| Squamous
cell carcinoma | 39 | 18 | 21 |
|
|
Adenocarcinoma | 55 | 22 | 33 |
|
|
Other | 4 | 1 | 3 |
|
| Tumor-Node-Metastasis
stage |
|
|
| 0.021a |
| I/II | 69 | 34 | 35 |
|
|
III/IV | 29 | 7 | 22 |
|
| Differentiation |
|
|
| 0.025a |
|
Well/moderate | 42 | 23 | 19 |
|
| Poor | 56 | 18 | 38 |
|
| Distant
metastasis |
|
|
| 0.211 |
| M0 | 89 | 39 | 50 |
|
| M1 | 9 | 2 | 7 |
|
Downregulated expression level of TIG2
predicts poor prognosis in NSCLC patients
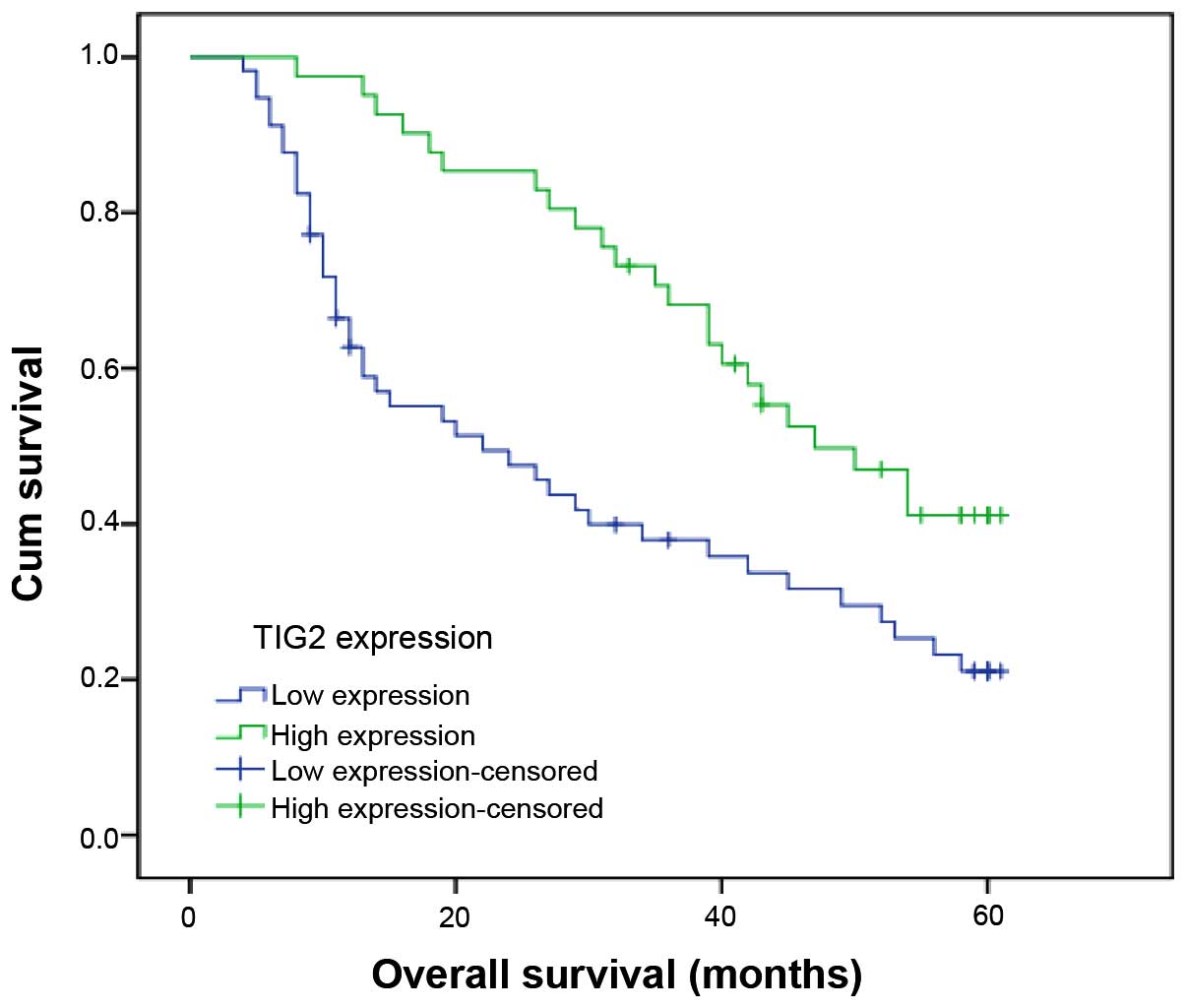
To investigate the prognostic value of TIG2
expression in NSCLC patients, survival analysis was performed to
assess the effect of TIG2 on survival time of NSCLC patients.
Kaplan-Meier curves for overall survival are presented in Fig. 3. Log-rank test was utilized for
statistical analysis. Patients with low TIG2 expression possessed
shorter overall survival times compared with those with high TIG2
expression (P=0.003).
Furthermore, multivariate analysis revealed that
TIG2 expression, TNM stage and differentiation were independent
prognostic markers for NSCLC (Table
II; P<0.05 in all cases). In conclusion, the results of the
present study suggested that patients with lower expression of TIG2
in NSCLC tissues demonstrated poorer overall survival compared with
patients with higher expression, providing evidence that decreased
expression of TIG2 in NSCLC may facilitate increased malignancy and
a poorer prognostic phenotype.
 | Table II.Multivariate analysis of prognostic
factors in non-small cell lung cancer for overall survival by Cox
regression model. |
Table II.
Multivariate analysis of prognostic
factors in non-small cell lung cancer for overall survival by Cox
regression model.
|
| Multivariate
analysis |
|---|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value |
|---|
| Tumor size, cm (≥5
vs. <5) | 1.242 | 0.346–2.864 | 0.565 |
| Histology (squamous
cell carcinoma vs. adenocarcinoma) | 1.051 | 0.672–2.162 | 0.354 |
| Smoking history
(smoker vs. non-smoker) | 1.429 | 0.799–2.786 | 0.448 |
| Lymph node metastasis
(positive vs. negative) | 0.894 | 0.562–1.876 | 0.189 |
| Tumor-Node-Metastasis
stage (III–IV vs. I–II) | 2.397 | 1.426–3.048 | 0.038a |
| Differentiation
(Well/moderate vs. poor) | 1.873 | 1.270–3.392 | 0.012a |
| Distant metastasis
(M1 vs. M0) | 2.674 | 1.632–3.893 | 0.206 |
| Tazarotene-induced
gene 2 expression (high vs. low) | 2.225 | 1.332–4.291 | 0.008a |
Discussion
Tazarotene upregulates the expression of TIG1 TIG2,
and TIG3 (10). TIG1 may function as
a cell adhesion molecule, whose expression on the cell surface may
lead to improved cell-cell contact and a reduction in
proliferation, which may additionally have a tumor suppressive role
by acting as a retinoid mediator of the cells (15). TIG3, also known as a class II tumor
suppressor via interaction with retinoids, has been demonstrated to
inhibit cellular proliferation when expressed and is associated
with retinoid responsiveness in malignant cell lines in
vitro (16). TIG2, also known as
chemerin, has been characterized as a chemotactic agent and was
identified as the ligand for an orphan G protein-coupled receptor,
ChemR23, which is expressed by immature dendritic cells and
macrophages (17). Previously, it was
reported that chemerin recruits ChemR23 receptor
(chemerinR)-expressing dendritic cells and macrophages, suggesting
a regulatory function in the development of the immune response
(18). A high expression level of
chemerinR transcript was additionally detected in the spleen, lymph
nodes and lung. Simultaneous expression of receptor and chemerin
mRNA transcript was observed in the spleen, lymph node and lung
(17). However, to the best of our
knowledge, the prognostic significance of TIG2 in NSCLC has not
been investigated. In the present study, the expression of TIG2 was
evaluated in NSCLC by RT-qPCR, western blotting and
immunohistochemistry. The present study additionally analyzed the
clinicopathological and prognostic significance of TIG2 in a large
number of human samples. It was demonstrated that TIG2 was
expressed at reduced levels in terms of mRNA and protein in NSCLC
tissues compared with corresponding adjacent noncancerous tissues.
Immunohistochemical staining analysis additionally demonstrated
that TIG2 expression was decreased in 57/98 NSCLC tissues. However,
the mechanism of downregulation of TIG2 expression during the
occurrence and development of NSCLC remains to be elucidated, and
requires additional investigation in future studies.
Furthermore, the present study observed that reduced
expression of TIG2 was significantly associated with lymph node
metastasis, TNM stage and level of differentiation, suggesting that
aberrant expression of TIG2 may participate in NSCLC tumor
development and progression. In Kaplan-Meier curve analysis of the
overall survival of 98 NSCLC patients, the overall survival rate in
patients with low TIG2 expression was significantly reduced
compared with that in patients with high TIG2 expression. In
addition, Cox multivariate analysis revealed that TIG2 expression
was an independent prognostic factor for overall survival in NSCLC
tissues. The results of the present study suggested that TIG2
expression may be a potential molecular marker for predicting
outcomes in NSCLC patients.
In conclusion, the present study demonstrated that
the expression of TIG2 was significantly decreased in NSCLC
tissues. Downregulated expression of TIG2 was significantly
associated with tumor progression and decreased survival in
patients with NSCLC, indicating that TIG2 may act as a novel
prognostic marker for the diagnosis and treatment of NSCLC
patients.
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heighway J and Betticher DC: Lung tumors:
an overview. Atlas Genet Cytogenet Oncol Haematol. 8:139–141.
2004.
|
|
3
|
Ramalingam SS, Owonikoko TK and Khuri FR:
Lung cancer: New biological insights and recent therapeutic
advances. CA Cancer J Clin. 61:91–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morgensztern D, Ng SH, Gao F and Govindan
R: Trends in stage distribution for patients with non-small cell
lung cancer: A National Cancer Database survey. J Thorac Oncol.
5:29–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seewaldt VL, Johnson BS, Parker MB,
Collins SJ and Swisshelm K: Expression of retinoic acid receptor
beta mediates retinoic acid-induced growth arrest and apoptosis in
breast cancer cells. Cell Growth Differ. 6:1077–1088.
1995.PubMed/NCBI
|
|
7
|
Huang P, Chandra V and Rastinejad F:
Structural overview of the nuclear receptor superfamily: Insights
into physiology and therapeutics. Annu Rev Physiol. 72:247–272.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boehm MF, Heyman RA, Patel S, Stein RB and
Nagpal S: Section Review: Retinoids: Biological function and use in
the treatment of dermatological diseases: Pulmonary-allergy,
dermatological, gastrointestinal & arthritis. Expert Opin
Investig Drugs. 4:593–612. 2008. View Article : Google Scholar
|
|
9
|
Chandraratna RA: Tazarotene: The first
receptor-selective topical retinoid for the treatment of psoriasis.
J Am Acad Dermatol. 37(Suppl): S12–S17. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duvic M, Nagpal S, Asano AT and
Chandraratna RA: Molecular mechanisms of tazarotene action in
psoriasis. J Am Acad Dermatol. 37(Suppl): S18–S24. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagpal S, Patel S, Jacobe H, DiSepio D,
Ghosn C, Malhotra M, Teng M, Duvic M and Chandraratna RA:
Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene
in skin. J Invest Dermatol. 109:91–95. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adams AE, Abu-Amer Y, Chappel J, Stueckle
S, Ross FP, Teitelbaum SL and Suva LJ: 1,25 dihydroxyvitamin D3 and
dexamethasone induce the cyclooxygenase 1 gene in
osteoclast-supporting stromal cells. J Cell Biochem. 74:587–595.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng Y, Luo S, Wang G, Peng Z, Zeng W,
Tan S, Xi Y and Fan J: Downregulation of tazarotene induced gene-2
(TIG2) in skin squamous cell carcinoma. Eur J Dermatol. 18:638–641.
2008.PubMed/NCBI
|
|
14
|
Livak and Schmittgen. Analysis of relative
gene expression data using real-time quantitative PCR and the
2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jing C, El-Ghany MA, Beesley C, Foster CS,
Rudland PS, Smith P and Ke Y: Tazarotene-induced gene 1 (TIG1)
expression in prostate carcinomas and its relationship to
tumorigenicity. J Natl Cancer Inst. 94:482–490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DiSepio D, Ghosn C, Eckert RL, Deucher A,
Robinson N, Duvic M, Chandraratna RA and Nagpal S: Identification
and characterization of a retinoid-induced class II tumor
suppressor/growth regulatory gene. Proc Natl Acad Sci USA.
95:14811–14815. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wittamer V, Franssen JD, Vulcano M,
Mirjolet JF, Le Poul E, Migeotte I, Brézillon S, Tyldesley R,
Blanpain C, Detheux M, et al: Specific recruitment of
antigen-presenting cells by chemerin, a novel processed ligand from
human inflammatory fluids. J Exp Med. 198:977–985. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luangsay S, Wittamer V, Bondue B, De Henau
O, Rouger L, Brait M, Franssen JD, de Nadai P, Huaux F and
Parmentier M: Mouse ChemR23 is expressed in dendritic cell subsets
and macrophages, and mediates an anti-inflammatory activity of
chemerin in a lung disease model. J Immunol. 183:6489–6499. 2009.
View Article : Google Scholar : PubMed/NCBI
|