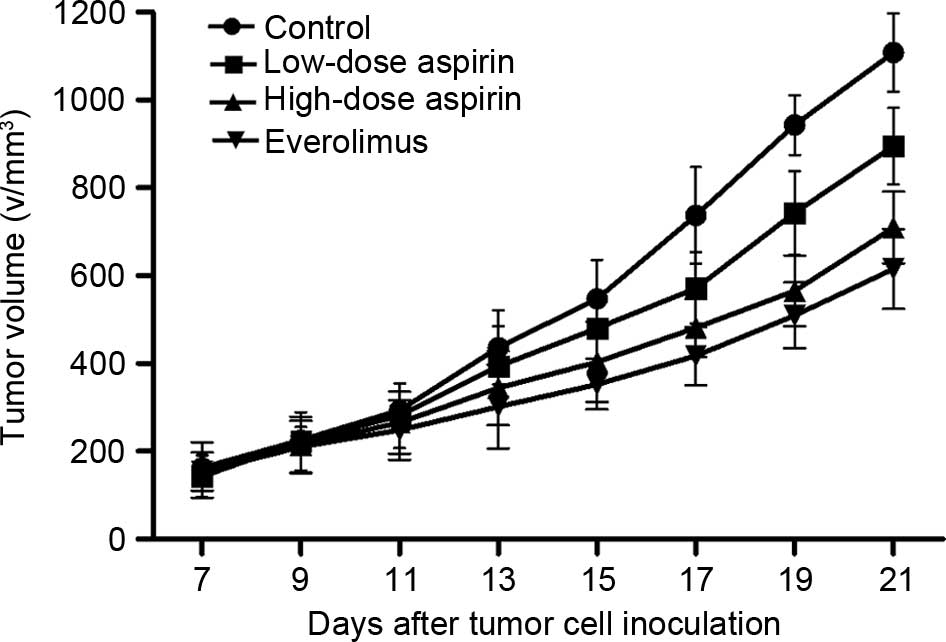
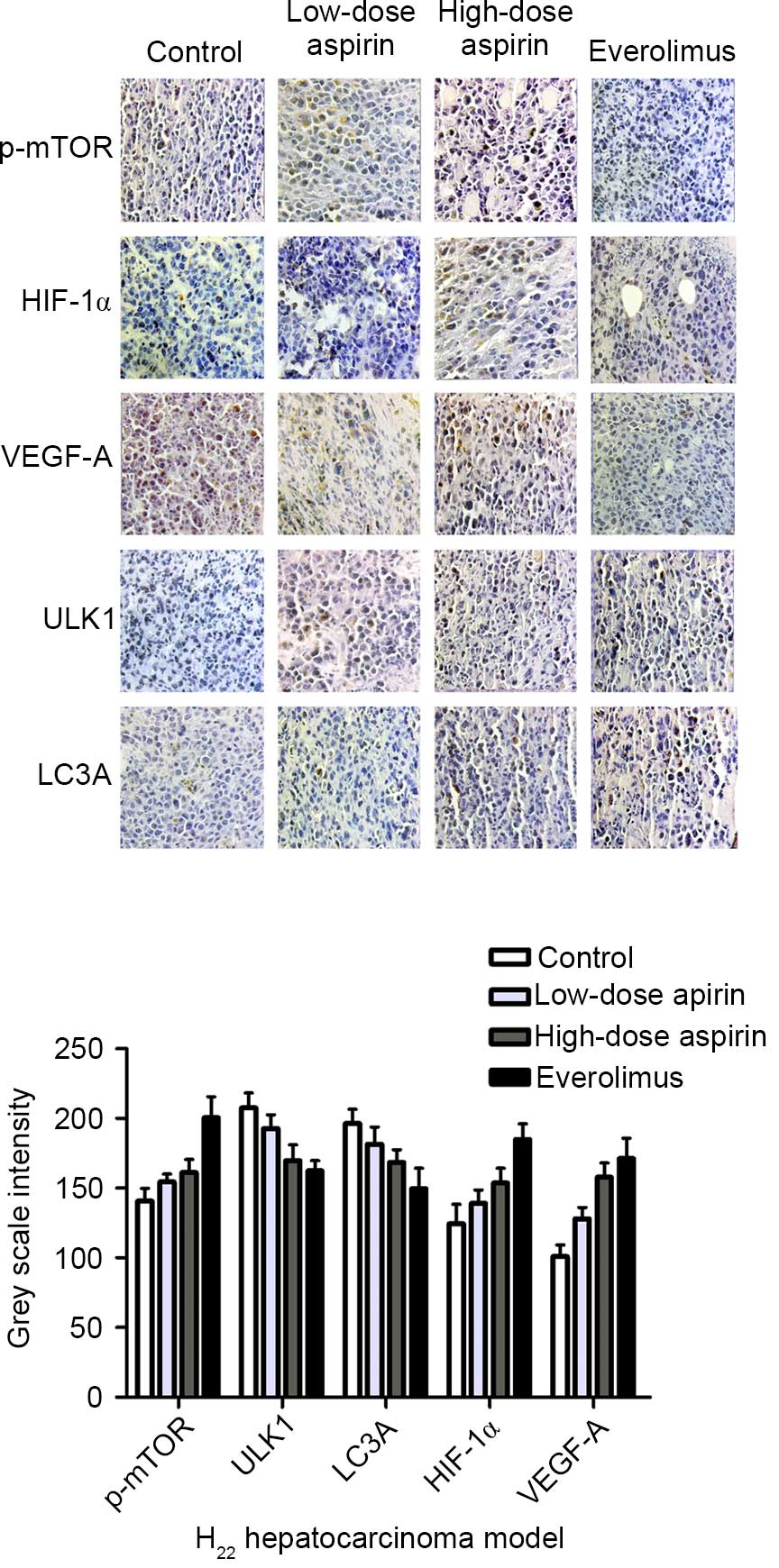
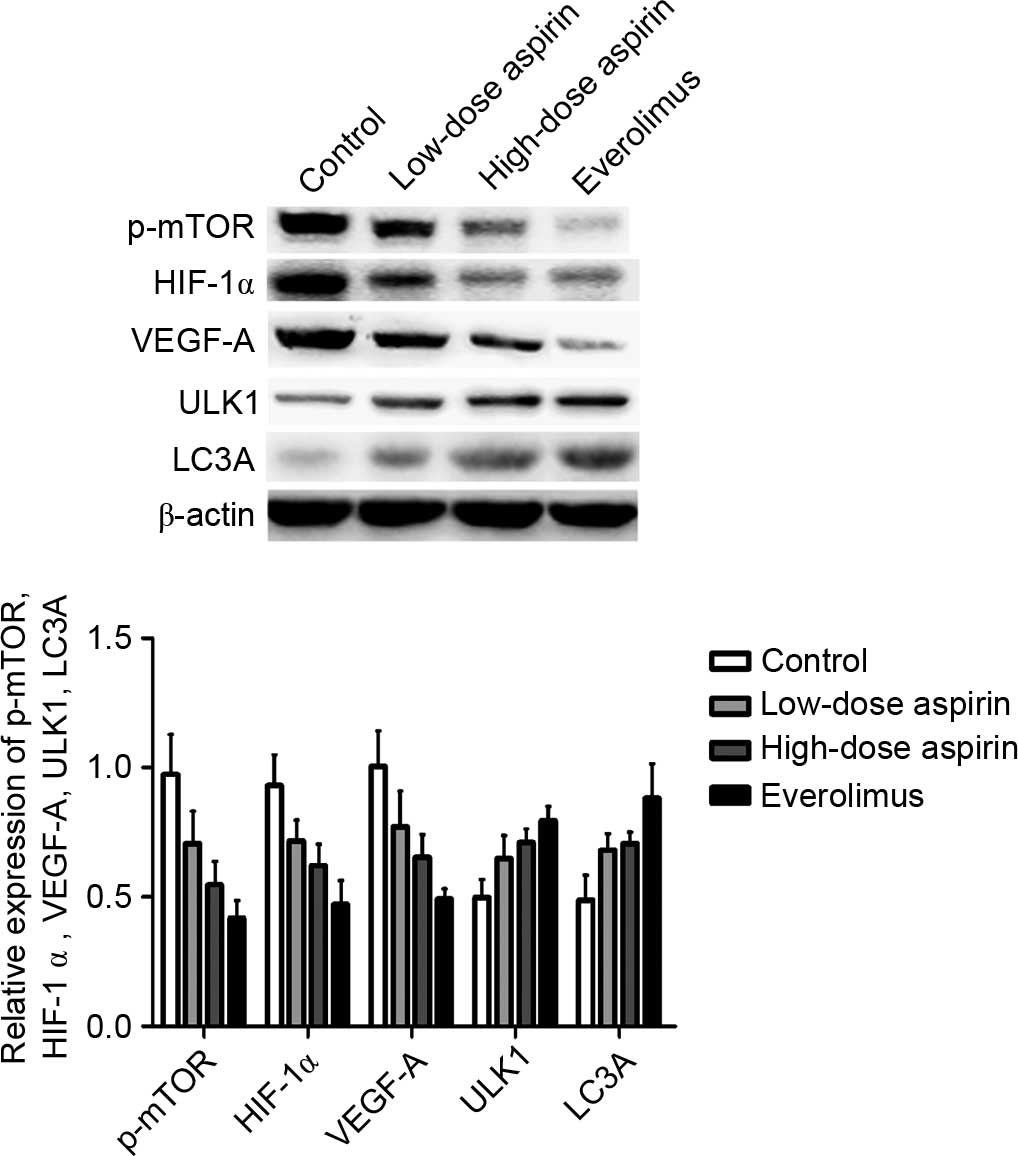
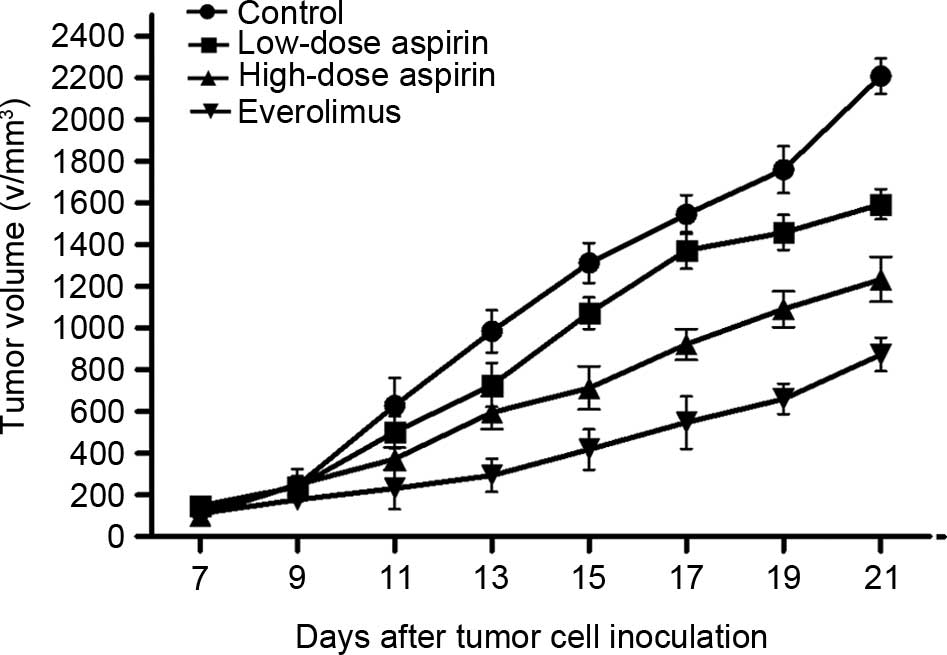
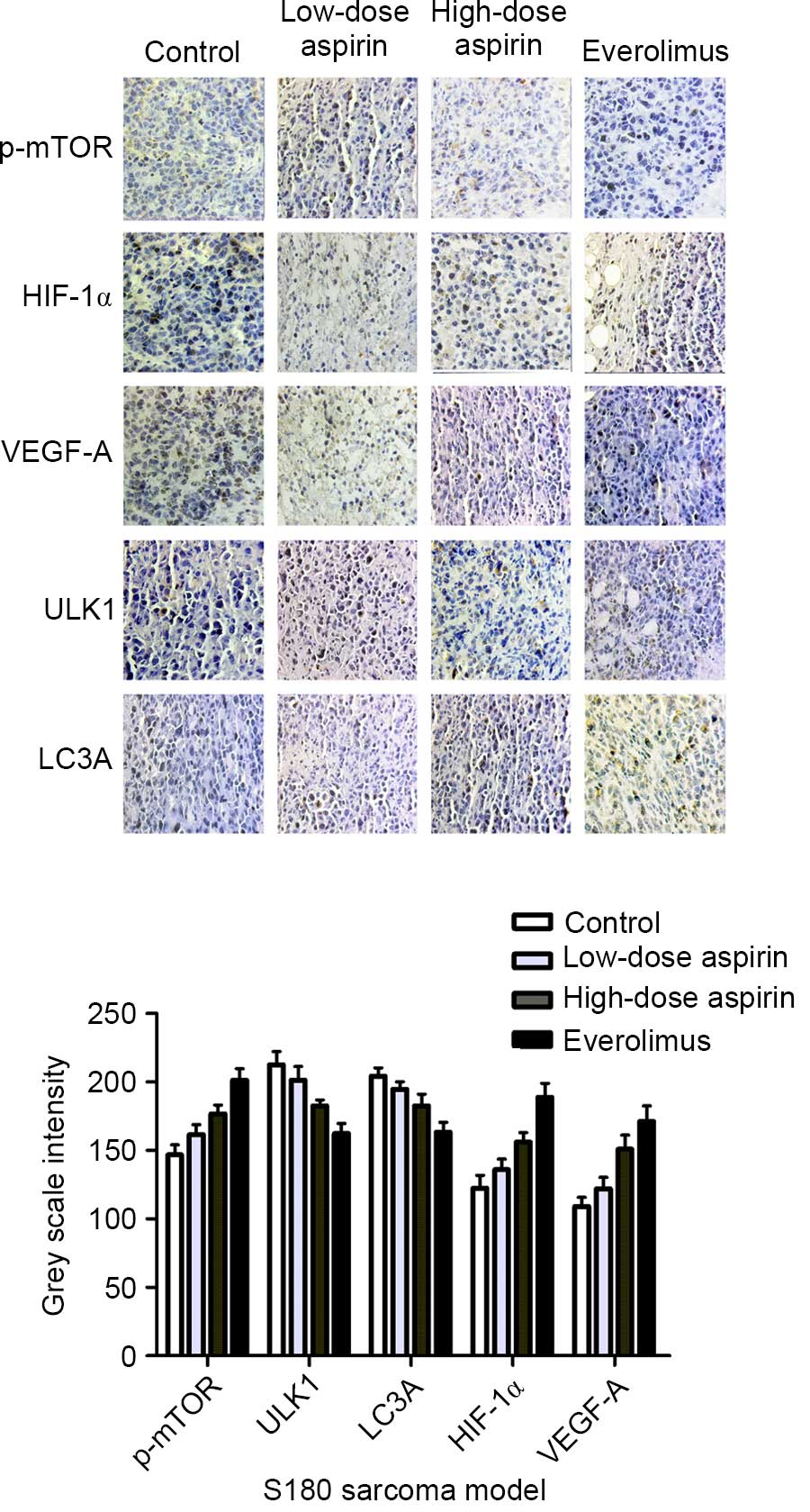
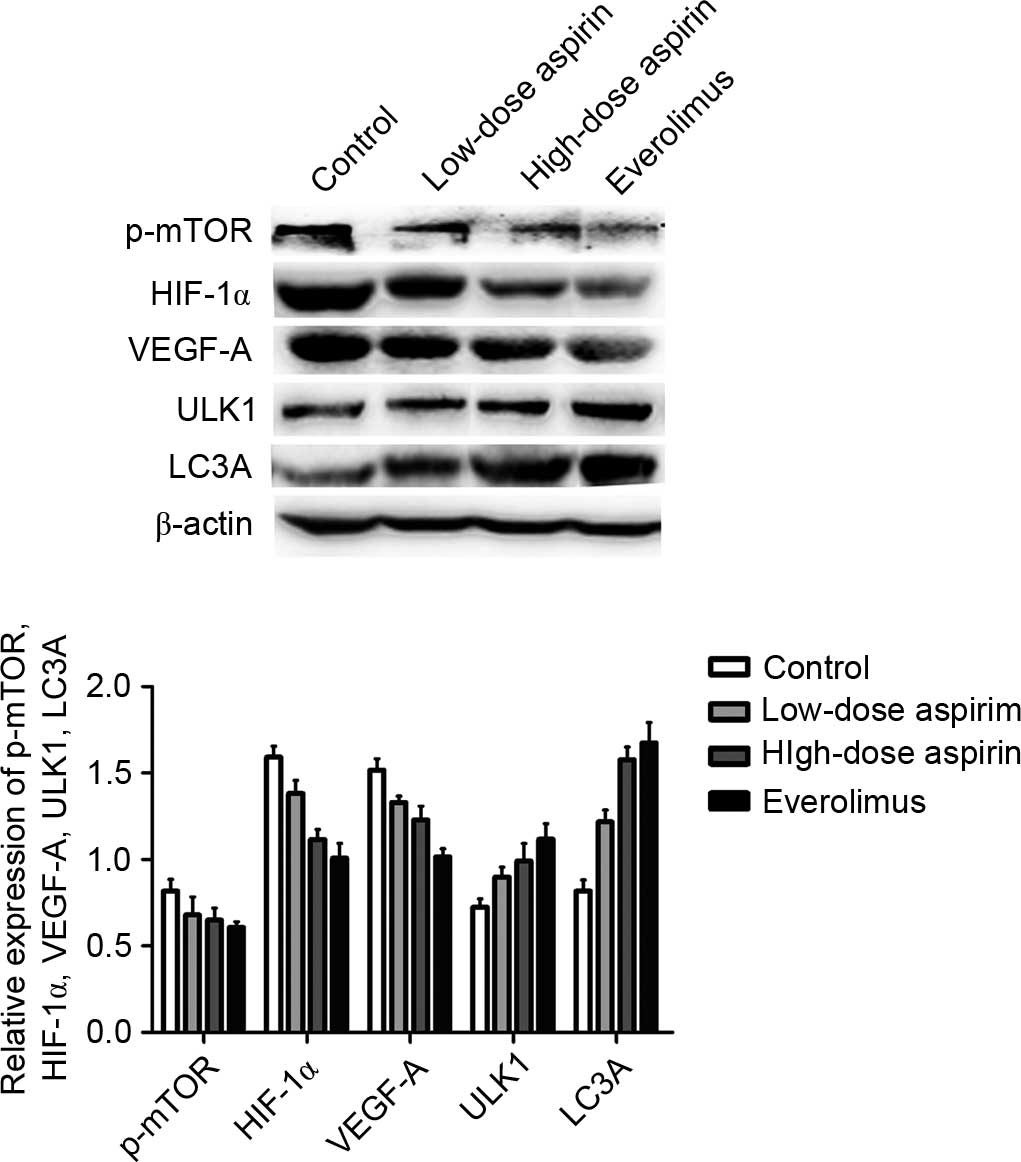
|
1
|
Gee JR, Jarrard DF, Bruskewitz RC, Moon
TD, Hedican SP, Leverson GE, Nakada SY and Messing EM: Reduced
bladder cancer recurrence rate with cardioprotective aspirin after
intravesical bacille Calmette-Guérin. BJU Int. 103:736–739. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alfonso LF, Srivenugopal KS, Arumugam TV,
Abbruscato TJ, Weidanz JA and Bhat GJ: Aspirin inhibits
camptothecin-induced p21CIP1 levels and potentiates apoptosis in
human breast cancer cells. Int J Oncol. 34:597–608. 2009.PubMed/NCBI
|
|
3
|
Iwama T: NSAIDs and colorectal cancer
prevention. J Gastroenterol. 44(Suppl 19): S72–S76. 2009.
View Article : Google Scholar
|
|
4
|
Din FV, Theodoratou E, Farrington SM,
Tenesa A, Barnetson RA, Cetnarskyj R, Stark L, Porteous ME,
Campbell H and Dunlop MG: Effect of aspirin and NSAIDs on risk and
survival from colorectal cancer. Gut. 59:1670–1679. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rothwell PM, Wilson M, Elwin CE, Norrving
B, Algra A, Warlow CP and Meade TW: Long-term effect of aspirin on
colorectal cancer incidence and mortality: 20-year follow-up of
five randomised trials. Lancet. 376:1741–1750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Din FV, Valanciute A, Houde VP, Zibrova D,
Green KA, Sakamoto K, Alessi DR and Dunlop MG: Aspirin inhibits
mTOR signaling, activates AMP-activatedprotein kinase, and induces
autophagy in colorectal cancer cells. Gastroenterology.
142:1504–1515.e3. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
from growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alayev A and Holz MK: mTOR signaling for
biological control and cancer. J Cell Physiol. 228:1658–1664. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Foster KG, Acosta-Jaquez HA, Romeo Y, Ekim
B, Soliman GA, Carriere A, Roux PP, Ballif BA and Fingar DC:
Regulation of mTOR complex l (mTORCI) by raptor Ser863 and
multisite phosphorylation. J Biol Chem. 285:80–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carbajo-Pescador S, Ordoñez R, Benet M,
Jover R, GarcíaPalomo A, Mauriz JL and González-Gallego J:
Inhibition of VEGF expression through blockade of Hif1α and STAT3
signalling mediates the anti-angiogenic effect of melatonin in
HepG2 liver cancer cells. Br J Cancer. 109:83–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Francesco EM, Lappano R, Santolla MF,
Marsico S, Caruso A and Maggiolini M: HIF-1α/GPER signaling
mediates the expression of VEGF induced by hypoxia in breast cancer
associated fibroblasts (CAFs). Breast Cancer Res. 15:R642013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie SR, Wang Y, Liu CW, Luo K and Cai YQ:
Liquiritigenin inhibits serum-induced HIF-1α and VEGF expression
via the AKT/mTOR-p70S6K signalling pathway in HeLa cells. Phytother
Res. 26:1133–1141. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruan GX and Kazlauskas A: Axl is essential
for VEGF-A-dependent activation of PI3K/Akt. EMBO J. 31:1692–1703.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wouters BG and Koritzinsky M: Hypoxia
signalling through mTOR and the unfolded protein response in
cancer. Nat Rev Cancer. 8:851–864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sengupta S, Peterson TR and Sabatini DM:
Regulation of the mTOR complex 1 pathway by nutrients, growth
factors, and stress. Mol Cell. 40:310–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jain K, Paranandi KS, Sridharan S and Basu
A: Autophagy in breast cancer and its implications for therapy. Am
J Cancer Res. 3:251–265. 2013.PubMed/NCBI
|
|
18
|
Morselli E, Galluzzi L, Kepp O, Vicencio
JM, Criollo A, Maiuri MC and Kroemer G: Anti- and pro-tumor
functions of autophagy. Biochim Biophys Acta. 1793:1524–1532. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan EY, Longatti A, McKnight NC and Tooze
SA: Kinase-inactivated ULK proteins inhibit autophagy via their
conserved C-terminal domains using an Atg13-independent mechanism.
Mol Cell Biol. 29:157–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hosokawa N, Sasaki T, Iemura S, Natsume T,
Hara T and Mizushima N: Atg101, a novel mammalian autophagy protein
interacting with Atg13. Autophagy. 5:973–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamada Y, Yoshino K, Kondo C, Kawamata T,
Oshiro N, Yonezawa K and Ohsumi Y: Tor directly controls the Atg1
kinase complex to regulate autophagy. Mol Cell Biol. 30:1049–1058.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Behrends C, Sowa ME, Gygi SP and Harper
JW: Network organization of the human autophagy system. Nature.
466:68–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wirawan E, Berghe T Vanden, Lippens S,
Agostinis P and Vandenabeele P: Autophagy: for better or for worse.
Cell Res. 22:43–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moretti L, Yang ES, Kim KW and Lu B:
Autophagy signaling in cancer and its potential as novel target to
improve anticancer therapy. Drug Resist Updat. 10:135–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goel S, Duda DG, Xu L, Munn LL, Boucher Y,
Fukumura D and Jain RK: Normalization of the vasculature for
treatment of cancer and other diseases. Physiol Rev. 91:1071–1121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Wang Z, Wang Z, Zhang Y, Jia Q,
Wu L and Zhang W: Impact of acetylsalicylic acid on tumor
angiogenesis and lymphangiogenesis through inhibition of VEGF
signaling in a murine sarcoma model. Oncol Rep. 29:1907–1913.
2013.PubMed/NCBI
|
|
27
|
Dazert E and Hall MN: mTOR signaling in
disease. Curr Opin Cell Biol. 23:744–755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
López-Knowles E, O'Toole SA, McNeil CM,
Millar EK, Qiu MR, Crea P, Daly RJ, Musgrove EA and Sutherland RL:
PI3K pathway activation in breast cancer is associated with the
basal-like phenotype and cancer-specific mortality. Int J Cancer.
126:1121–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hydbring P and Larsson LG: Cdk2: a key
regulator of the senescence control function of Myc. Aging (Albany
NY). 2:244–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cavazzoni A, Bonelli MA, Fumarola C, La
Monica S, Airoud K, Bertoni R, Alfieri RR, Galetti M, Tramonti S,
Galvani E, et al: Overcoming acquired resistance to letrozole by
targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones.
Cancer Lett. 323:77–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang FZ, Peng-Jiao, Yang NN, Chuang-Yuan,
Zhao YL, Liu QQ, Fei HR and Zhang JG: PF-04691502 triggers cell
cycle arrest, apoptosis and inhibits the angiogenesis in
hepatocellular carcinoma cells. Toxicol Lett. 220:150–156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chaachouay H, Ohneseit P, Toulany M,
Kehlbach R, Multhoff G and Rodemann HP: Autophagy contributes to
resistance of tumor cells to ionizing radiation. Radiother Oncol.
99:287–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O'Donovan TR, O'Sullivan GC and McKenna
SL: Induction of autophagy by drug-resistant esophageal cancer
cells promotes their survival and recovery following treatment with
chemotherapeutics. Autophagy. 7:509–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anbalagan S, Pires IM, Blick C, Hill MA,
Ferguson DJ, Chan DA and Hammond EM: Radiosensitization of renal
cell carcinoma in vitro through the induction of autophagy.
Radiother Oncol. 103:388–393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hao J, Pei Y, Ji G, Li W, Feng S and Qiu
S: Autophagy is induced by 3β-Osuccinyl-lupeol (LD9-4) in A549
cells via up-regulation of Beclin 1 and down-regulation mTOR
pathway. Eur J Pharmacol. 670:29–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He Z, Mangala LS, Theriot CA, Rohde LH, Wu
H and Zhang Y: Cell killing and radiosensitizing effects of
atorvastatin in PC3 prostate cancer cells. J Radiat Res.
53:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|