Introduction
Cancer is a significant public health problem
worldwide. According to the World Cancer Research Fund
International, ~12.7 million cancer-associated mortalities (13% of
all mortalities) occurred worldwide in 2008, with males accounting
for 6.6 million mortalities and females accounting for 6 million
(1). In developed countries,
colorectal cancer is one of the most commonly observed types of
cancer, ranking 2nd and 3rd in women and men, respectively
(1). The lifetime risk for colorectal
cancer development in the general population is ~6%, and colorectal
cancer is responsible for ~8% of all cancer-associated mortalities
worldwide (1). Among the Chinese
population, the incidence of colorectal cancer is increasing
(2). At present, no optimal adjuvant
chemotherapy exists for clinical use; therefore, developing
rationally designed, novel adjuvant therapeutic tools for the
treatment of colon cancer is a constant requirement (2). Previously, the use of natural
substances, including curcumin, eicosapentaenoic acid, apple
polyphenols, capsaicin and thymoquinone (TQ), for cancer
chemoprevention has been investigated (3–5). TQ is the
primary active ingredient of volatile Nigella sativa (black
cumin) seed oil, which is used as a spice in countries with a low
incidence of colorectal cancer, including Egypt, Pakistan and India
(6). Traditional medicine has taken
advantage of the anti-inflammatory, antioxidant and
anticarcinogenic properties associated with TQ, which supports the
hypothesis of TQ being a promising dietary chemopreventive agent
(6). In the previous decade, the
antitumor activity of TQ has been investigated in a number of
studies (6–8). TQ was observed to induce antitumor
effects in several types of cancer, including breast (9), lung (10),
multiple myeloma (11), pancreatic
(12), cervical (13), colon (14) and prostate cancer (15), as well as squamous (16) and hepatocellular carcinoma (17), acute lymphoblastic leukemia (18), glioblastoma (19), osteosarcoma (20), neuroblastoma (21), bladder (22), gastric (23) and ovarian cancer (24). Although the effect of TQ has been
investigated in numerous types of cancer, the molecular mechanisms
underlying its action remain to be elucidated. The present study
investigated the effect of TQ on colon cancer cell growth and the
underlying molecular mechanisms. In addition, the present study
identified the effect and molecular mechanism of TQ action on the
chemosensitivity of colon cancer cells to cisplatin (CisPt).
Materials and methods
Cell culture and materials
TQ, PDTC, Tris, glycine, NaCl, sodium dodecyl
sulfate (SDS), bovine serum albumin (BSA) and β-actin antibody were
obtained from Sigma-Aldrich (St. Louis, MO, USA). TQ was dissolved
in dimethyl sulfoxide (DMSO; Merck Millipore, Darmstadt, Germany)
to make a 50 mM stock solution and stored at −20°C until use in
subsequent experiments. Additional dilutions were performed in cell
culture medium (RPMI-1640; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) so that the final DMSO concentration was
<0.1%. Primary antibodies, including rabbit polyclonal
anti-human p65 (catalog no., sc-101749; 1:500), rabbit polyclonal
anti-human B-cell lymphoma 2 (Bcl-2; catalog no., sc-492; 1:2,000),
rabbit polyclonal anti-human vascular endothelial growth factor
(VEGF; catalog no., sc-507; 1:1,000), mouse polyclonal anti-human
c-Myc (catalog no., sc-40: 1:500), and secondary antibodies,
including goat anti-rabbit horseradish peroxidase (HRP)-conjugated
(catalog no., sc-2054: 1:10,000) and goat anti-mouse HRP-conjugated
(catalog no., sc-2005 1:10,000) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Primary rabbit polyclonal
anti-human β-actin (catalog no., sc-7210: 1:200) antibody was
purchased from Sigma-Aldrich. The COLO205 and HCT116 colon cancer
cell lines were purchased from American Type Culture Collection
(Manassas, VA, USA). The cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.), supplemented with 10%
heat-inactivated fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 2% penicillin/streptomycin (6 mg/ml
penicillin, 10 mg/ml streptomycin; Sigma-Aldrich). The cells were
cultured in tissue culture flasks (75 cm2; Corning
Incorporated, New York, NY, USA) and incubated at 37°C in a
humidified chamber containing 5% CO2.
Cell viability assay
Cell viability was determined using a Cell Counting
Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). Briefly,
2×104 cells per well were seeded into a 96-well plate
(Corning Incorporated). Subsequent to 24 h culturing, cells were
treated with cisplatin and TQ for 24 h or TQ alone for 48 h.
Finally, 20 µl CCK-8 solution was added to each well, and was
incubated for 2 h at 37°C. The optical density (OD) of each well at
450 nm was measured using a VICTOR™ X multi-label reader
(PerkinElmer, Inc., Waltham, MA, USA). The percentage cell
viability was calculated as follows: (ODdrug /
ODcontrol) × 100. To analyze the role of nuclear
factor-κB (NF-κB) in TQ activity, cells (2×104 cells per
well) were treated with 50 µm pyrrolidine dithiocarbamate (PDTC) in
combination with TQ for 12, 24 and 48 h. The percentage cell growth
inhibition was calculated as follows:
(ODcontrol-ODdrug) × 100.
Preparation of nuclear extract
Nuclear extracts were prepared as previously
described (25). Briefly, cells were
harvested, washed twice with ice-cold phosphate buffered saline
(PBS; Hyclone, Beijing, China) for 1 min and resuspended in 1 ml of
ice-cold PBS. Cells were pelleted by centrifugation at 12,000 × g
for 5 min, suspended in ice-cold buffer [10 mmol/l
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1.5
mmol/l MgCl2, 0.2 mmol/l KCl, 0.2 mmol/l
phenylmethylsulphonyl fluoride, 0.5 mmol/l dithothreitol], mixed by
vortexing for 10 sec and centrifuged at 12,000 × g for 5 min. The
nuclear pellet was washed in 1 ml buffer (20 mmol/l HEPES, 25%
glycerol, 0.42 mol/l NaCl, 1.5 mmol/l MgCl2, 0.2 mmol/l
ethylenediaminetetraacetic acid), resuspended in 30 ml buffer,
mixed by rotation for 30 min at 4°C and centrifuged at 12,000 × g
for 20 min. Finally, the supernatants were used as nuclear
extracts.
Western blot analysis
Using 12% SDS-polyacrylamide gel electrophoresis
(SDS-PAGE), whole cell lysates or nuclear extracts were
electrophoresed, as previously described (26). The proteins were transferred onto a
0.4-µm polyvinylidene difluoride membrane (EMD Millipore, Bedford,
MA, USA) in transfer buffer (25 mM Tris, pH 8.5, 0.2 M glycine and
20% methanol). The membranes were blocked by 5% BSA in
Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h,
washed twice with TBST for 10 min each and incubated with primary
β-actin, p65, Bcl-2, VEGF and c-Myc antibodies at 4°C overnight.
Subsequent to three washes with TBST for 10 min each, the membranes
were probed with secondary peroxidase-conjugated antibody
(dilution, 1:10,000; Sigma-Aldrich). Finally, the immunoreactive
bands were visualized using an enhanced chemiluminescence detection
kit (Immobilon WBKLS0500; Merck Millipore). β-actin was used as an
internal control for all western blot analyses.
Statistical analysis
SPSS version 17.0 software (SPSS Inc., Chicago, IL,
USA) was used for statistical analysis. Values were expressed as
the mean ± standard deviation. Numeric variables were compared by
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effect of TQ on the viability of colon
cancer cells
Previous studies have shown the antitumor and
anticarcinogenic activities of TQ in numerous types of cancer,
including breast cancer, glioblastoma and lymphoma (27–29). In
order to elucidate the molecular mechanisms underlying the function
of TQ, the effect of TQ on colon cancer cells was examined in the
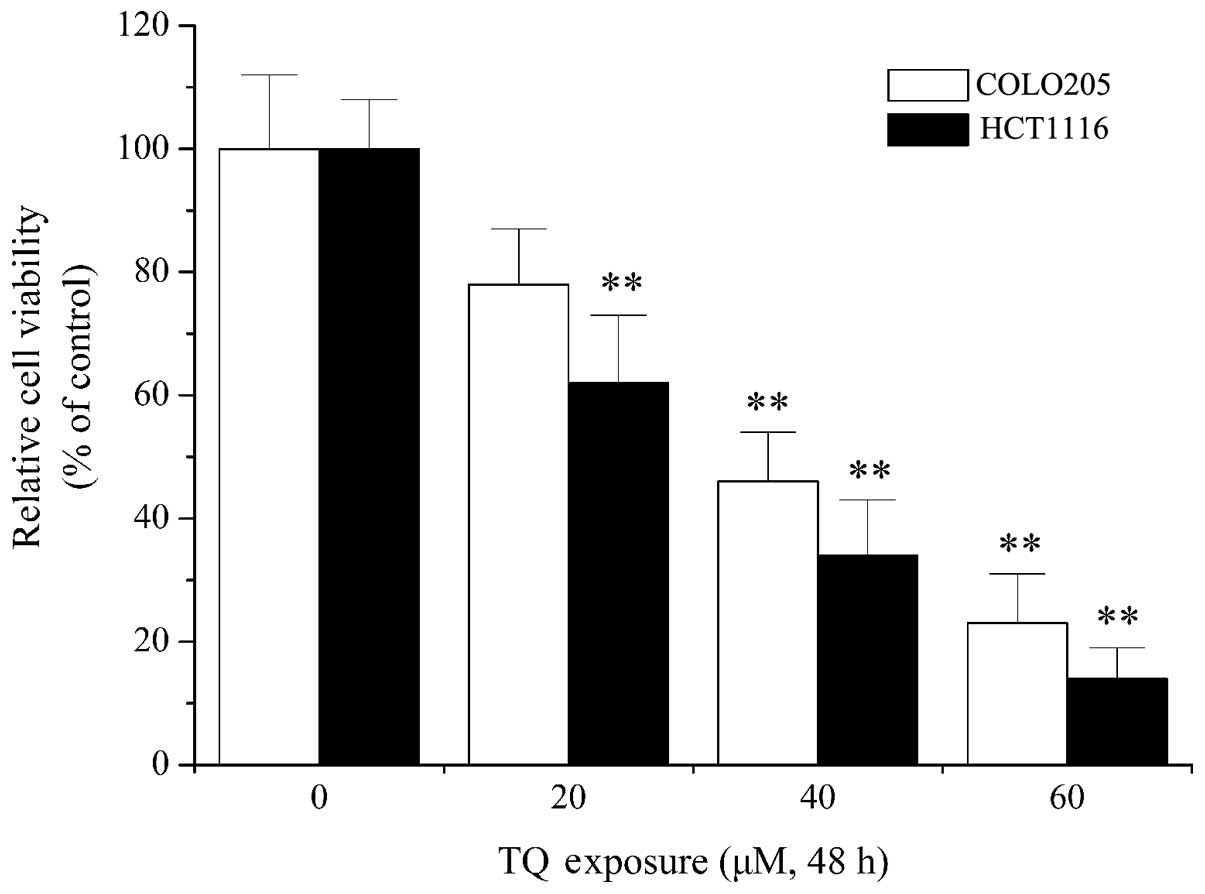
present study. As shown in Fig. 1, 20
µM TQ treatment resulted in a significant decrease in cell
viability in HCT1116 cells when compared with the control
(P=0.013). Furthermore, 40 µM TQ treatment significantly decreased
call viability in COLO205 cells compared with the control
(P=0.007). Cytotoxicity assays indicated that TQ dose-dependently
decreased cell viability of the COLO205 and HCT116 colon cancer
cell lines (Fig. 1), which confirmed
the antitumor activity of TQ, as previously reported (30,31).
TQ chemosensitizes colon cancer
cells
The present study aimed to investigate whether TQ
affected the sensitivity of colon cancer cells to chemotherapy.
Therefore, TQ and CisPt were combined to treat COLO205 and HCT116
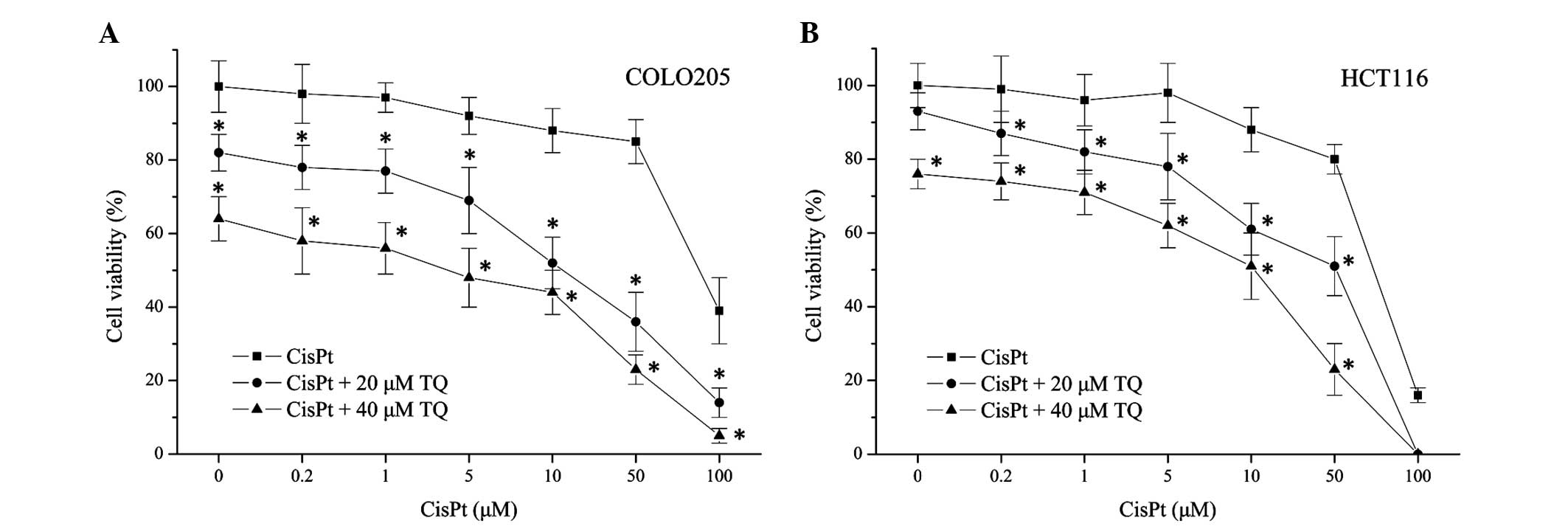
colon cancer cell lines. As shown in Fig.
2, treatment with cisplatin alone induced COLO205 and HCT1116
cell death in a dose-dependent manner. Combined treatment with
cisplatin (0.2 µM) and TQ (20 or 40 µM) significantly decreased the
cell viability of COLO205 (P=0.021, 20 µM TQ; P=0.003, 40 µM TQ)
and HCT116 cells (P=0.038, 20 µM TQ; P=0.004, 40 µM TQ) when
compared with 0.2 µM cisplatin treatment alone. These results
revealed that cell death induced by CisPt was enhanced by TQ in a
concentration-dependent manner (Fig.
2), which indicated that TQ may potentiate the chemosensitivity
of colon cancer cells.
Role of NF-κB in TQ activity
As demonstrated in the present results, TQ treatment
may result in cell death and chemosensitizing of colon cancer
cells. Therefore, the present study aimed to investigate the
underlying mechanisms of TQ action, including the role of NF-κB in
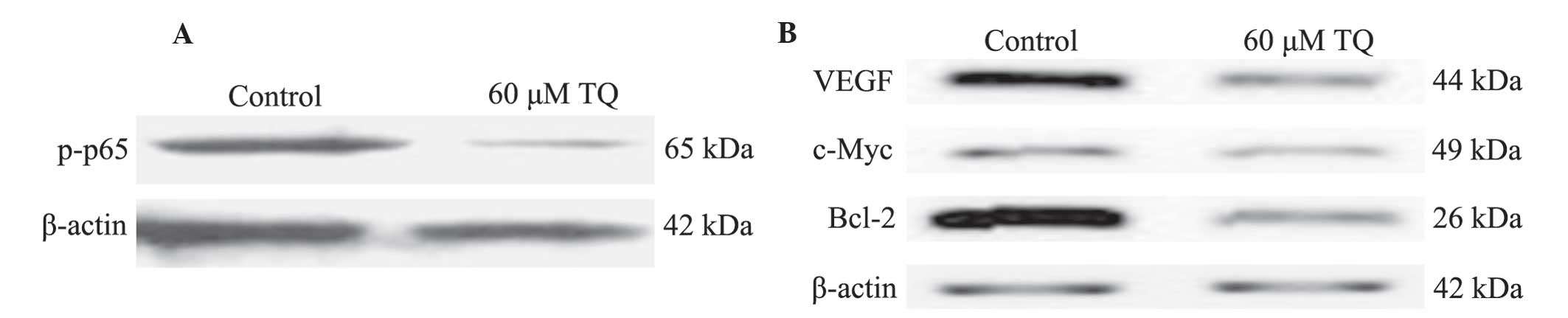
the process. Western blot analysis was used to determine the effect
of TQ on NF-κB activation. The results demonstrated that 60 µM TQ
significantly inhibited the phosphorylation of p65 protein, subunit
of NF-κB (Fig. 3A). Furthermore, the
expression levels of NF-κB-regulated genes that are involved in
tumor angiogenesis, survival and apoptosis were measured. As
demonstrated in Fig. 3B, protein
levels of VEGF, c-Myc and Bcl-2 were markedly downregulated
following 60 µM TQ treatment.
NF-κB inhibitor, PDTC, potentiates the
activity of TQ
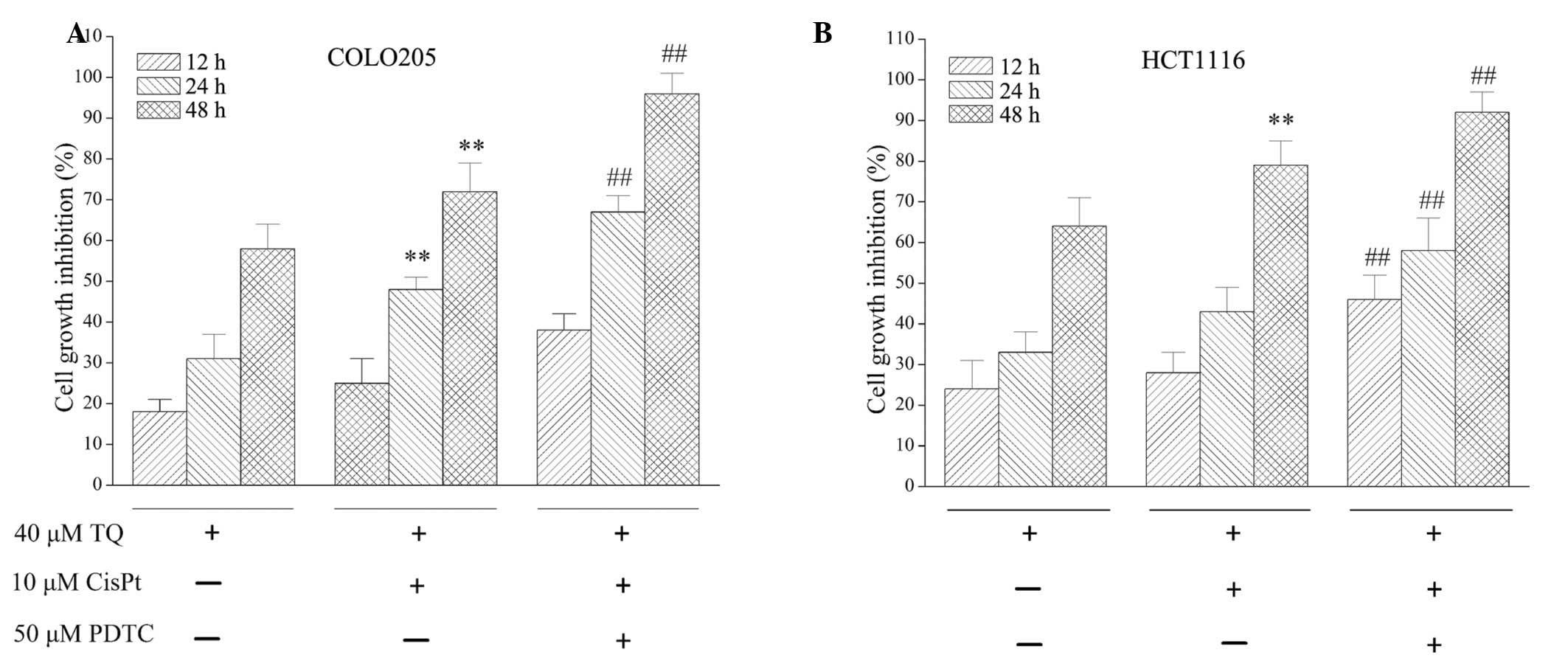
As TQ inhibited NF-κB activation, the effect of
NF-κB on TQ function was assessed. The NF-κB inhibitor, PDTC, was
used to investigate the activity of TQ. As demonstrated in Fig. 4, combined treatment with 10 µM
cisplatin and 40 µM TQ treatment for 24 or 48 h significantly
inhibited cell growth inhibition when compared with 40 µM TQ
treatment alone (P=0.018 and P=0.009, respectively) in COLO205
cells. Treatment with 50 µM PDTC further potentiated the growth
inhibition observed following 24 h (P=0.002) and 48 h (P=0.031)
treatment when compared with 10 µM cisplatin and 40 µM TQ combined
treatment. For HCT1116 cells, following 12, 24 and 48 h treatment
with 50 µM PDTC, 10 µM cisplatin and 40 µM TQ significantly
inhibited cell growth inhibition (P=0.014, P=0.043 and P=0.027,
respectively) when compared with combined 10 µM cisplatin and 40 µM
TQ treatment. Furthermore, a chemosensitization effect of TQ on
colon cancer cells was enhanced by 50 µM PDTC treatment, which was
demonstrated by increased cell growth inhibition (Fig. 4).
Discussion
Nigella sativa is an annual herbaceous plant
belonging to the Ranunculaceae family, which has been commonly used
in traditional Middle Eastern folk medicine as a natural remedy for
various ailments for >2,000 years (8). Nigella sativa is additionally
used as a food additive and flavoring in numerous countries
(8). TQ, or
2-isopropyl-5-methyl-1,4-benzoquinone, is a Nigella sativa
essential oil that is known to be the principal active compound of
the seed, and is responsible for a number of its antioxidant and
anti-inflammatory effects (6,8).
In 2003, Shoieb et al (32) reported in vitro experimental
results that revealed TQ was able to inhibit growth and induce
apoptosis in cancer cell lines. Since then, increasing numbers of
studies have focused on TQ in cancer therapy. Numerous in
vitro and in vivo studies have investigated the
antitumor activity of TQ in several types of cancer (6,14,19,27–29,33).
In tumor protein p53-null myeloblastic leukemia HL-60 cells, TQ
induced apoptosis through an intrinsic signaling pathway (34). In human multiple myeloma cells, TQ
inhibits proliferation, induces apoptosis and exerts a
chemosensitization effect through suppressing signal transducer and
activator of transcription 3 (acute-phase response factor)
activation (35), and decreases
F-actin polymerization and Bcl-2/Bcl-2 like 1 expression (36). TQ-induced apoptosis has been indicated
to be mediated by reactive oxygen species generation (29,33,37) and
the mitogen-activated protein kinase 14 signaling pathway (33). In 2004, Gali-Muhtasib et al
(38) reported that TQ triggered
apoptotic cell death in human colorectal cancer cells via a
p53-dependent mechanism, and in 2008, Gali-Muhtasib et al
(31) demonstrated that TQ triggered
inactivation of the stress response pathway sensor checkpoint
kinase 1 and contributed to apoptosis in colorectal cancer cells.
Gali-Muhtasib et al (30)
additionally indicated that TQ may inhibit colon tumor cell
invasion (30), which was confirmed
by additional studies (39–41).
A recent study reported that TQ exerted an antitumor
effect through the interruption of pro-survival mitogen-activated
protein kinase kinase 7-mitogen-activated protein kinase 1
signaling in colorectal cancer (14).
TQ exerted a direct antitumor effect, and also sensitized cancer
cells to other therapies (23,42–44).
In general, cancer cells may be initially susceptible to
chemotherapy; however, over time they may develop resistance
through certain mechanisms, including DNA mutations and metabolic
changes that promote drug inhibition and degradation. Drug
resistance has been a challenge for clinical cancer treatment
(45). Velho-Pereira et al
(42) reported that TQ may
radiosensitize human breast carcinoma cells. Jafri et al
(43) indicated that in lung cancer,
TQ treatment may overcome resistance and sensitize lung cancer
cells to CisPt. Previous studies have revealed the
chemosensitization and radiosensitization effect of TQ in
pancreatic (44), lung (43), gastric (23) and breast cancer (42). However, the mechanism by which TQ
affects the sensitivity of colon cancer to chemotherapy has not
been investigated. In the present study, the results confirmed the
antitumor activity of TQ in colon cancer cells and provided novel
evidence that TQ sensitizes colon cancer cells to CisPt by
suppressing NF-κB activation.
NF-κB is an ubiquitous transcription factor,
consisting of p50, p65 and nuclear factor-κB inhibitor α (IκBα),
which is present in the cytoplasm and is activated in response to
certain inflammatory stimuli, environmental pollutants,
prooxidants, carcinogens, stress and growth factors (46). Following activation, NF-κB
translocates from the cytoplasm to the nucleus, binds DNA and
induces gene transcription. A number of kinases have been
associated with the activation of NF-κB, including IκBα kinase.
This activation has been shown to result in the expression of a
number of gene products that regulate apoptosis, proliferation,
chemoresistance, radioresistance, invasion, angiogenesis,
metastasis and inflammation (47,48). In
numerous human cancers NF-κB is constitutively activated (49–51). NF-κB
activation has been associated with various aspects of oncogenesis,
including control of apoptosis, cell cycle, differentiation and
cell migration (52,53). In addition, the activation of NF-κB in
cancer cells by chemotherapy or radiation may hinder the ability of
cancer therapy to induce cell death; therefore, NF-κB has been used
as a target for tumor therapies (52,53). In
colon cancer, via the regulation of numerous genes differentially
expressed and implicated in tumorigenesis, NF-κB activation
participates in the promotion and progression steps of colon cancer
(54). The present study investigated
the effect of TQ on NF-κB activation, and additionally examined the
effect of TQ on NF-κB-regulated gene products. The results of the
present study revealed that TQ treatment inhibited the
phosphorylation of p65 protein in the nucleus of colon cancer cells
and decreased the expression of NF-κB-regulated genes, including
VEGF, c-Myc and Bcl-2. In addition, the inhibition of NF-κB with a
specific inhibitor, PDTC, may potentiate the cell death induction
and chemosensitization effect of TQ in colon cancer cells.
In conclusion, the present study demonstrated that
TQ may result in cell death in colon cancer cells and sensitize
colon cancer cells to CisPt therapy by suppressing NF-κB
activation. TQ may be a positive option for adjuvant chemotherapy
in the treatment of colon cancer.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin P, Wu ZT, Li SR, Li SJ, Wang JH, Wang
ZH, Lu JG, Cui XJ, Han Y, Rao J and Sheng JQ: Colorectal cancer
screening with fecal occult blood test: A 22-year cohort study.
Oncol Lett. 6:576–582. 2013.PubMed/NCBI
|
|
3
|
Kim B and Giardiello FM: Chemoprevention
in familial adenomatous polyposis. Best Pract Res Clin
Gastroenterol. 25:607–622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fini L, Piazzi G, Daoud Y, Selgrad M,
Maegawa S, Garcia M, Fogliano V, Romano M, Graziani G, Vitaglione
P, et al: Chemoprevention of intestinal polyps in ApcMin/+ mice fed
with western or balanced diets by drinking annurca apple polyphenol
extract. Cancer Prev Res (Phila). 4:907–915. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rajput S and Mandal M: Antitumor promoting
potential of selected phytochemicals derived from spices: A review.
Eur J Cancer Prev. 21:205–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woo CC, Kumar AP, Sethi G and Tan KH:
Thymoquinone: Potential cure for inflammatory disorders and cancer.
Biochem Pharmacol. 83:443–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abukhader MM: Thymoquinone in the clinical
treatment of cancer: Fact or fiction? Pharmacogn Rev. 7:117–120.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schneider-Stock R, Fakhoury IH, Zaki AM,
El-Baba CO and Gali-Muhtasib HU: Thymoquinone: Fifty years of
success in the battle against cancer models. Drug Discov Today.
19:18–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sutton KM, Greenshields AL and Hoskin DW:
Thymoquinone, a bioactive component of black caraway seeds, causes
G1 phase cell cycle arrest and apoptosis in triple-negative breast
cancer cells with mutant p53. Nutr Cancer. 66:408–418. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Kuang XR, Lv PT and Yan XX:
Thymoquinone inhibits proliferation and invasion of human
nonsmall-cell lung cancer cells via ERK pathway. Tumour Biol.
36:259–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siveen KS, Mustafa N, Li F, Kannaiyan R,
Ahn KS, Kumar AP, Chng WJ and Sethi G: Thymoquinone overcomes
chemoresistance and enhances the anticancer effects of bortezomib
through abrogation of NF-κB regulated gene products in multiple
myeloma xenograft mouse model. Oncotarget. 5:634–648. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mu GG, Zhang LL, Li HY, Liao Y and Yu HG:
Thymoquinone pretreatment overcomes the insensitivity and
potentiates the antitumor effect of gemcitabine through abrogation
of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic
cancer. Dig Dis Sci. 60:1067–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ichwan SJ, Al-Ani IM, Bilal HG, Suriyah
WH, Taher M and Ikeda MA: Apoptotic activities of thymoquinone, an
active ingredient of black seed (Nigella sativa), in cervical
cancer cell lines. Chin J Physiol. 57:249–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
El-Baba C, Mahadevan V, Fahlbusch FB, S
SM, Rau TT, Gali-Muhtasib H and Schneider-Stock R:
Thymoquinone-induced conformational changes of PAK1 interrupt
prosurvival MEK-ERK signaling in colorectal cancer. Mol Cancer.
13:2012014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dirican A, Atmaca H, Bozkurt E, Erten C,
Karaca B and Uslu R: Novel combination of docetaxel and
thymoquinone induces synergistic cytotoxicity and apoptosis in
DU-145 human prostate cancer cells by modulating PI3K-AKT pathway.
Clin Transl Oncol. 17:145–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chu SC, Hsieh YS, Yu CC, Lai YY and Chen
PN: Thymoquinone induces cell death in human squamous carcinoma
cells via caspase activation-dependent apoptosis and LC3-II
activation-dependent autophagy. PLoS One. 9:e1015792014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ashour AE, Abd-Allah AR, Korashy HM, Attia
SM, Alzahrani AZ, Saquib Q, Bakheet SA, Abdel-Hamied HE, Jamal S
and Rishi AK: Thymoquinone suppression of the human hepatocellular
carcinoma cell growth involves inhibition of IL-8 expression,
elevated levels of TRAIL receptors, oxidative stress and apoptosis.
Mol Cell Biochem. 389:85–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salim LZ, Mohan S, Othman R, Abdelwahab
SI, Kamalidehghan B, Sheikh BY and Ibrahim MY: Thymoquinone induces
mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in
vitro. Molecules. 18:11219–11240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Racoma IO, Meisen WH, Wang QE, Kaur B and
Wani AA: Thymoquinone inhibits autophagy and induces
cathepsin-mediated, caspase-independent cell death in glioblastoma
cells. PLoS One. 8:e728822013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng L, Liu A, Shen Y, Xu HZ, Yang SZ,
Ying XZ, Liao W, Liu HX, Lin ZQ, Chen QY, et al: Antitumor and
anti-angiogenesis effects of thymoquinone on osteosarcoma through
the NF-κB pathway. Oncol Rep. 29:571–578. 2013.PubMed/NCBI
|
|
21
|
Paramasivam A, Sambantham S, Shabnam J, et
al: Anti-cancer effects of thymoquinone in mouse neuroblastoma
(Neuro-2a) cells through caspase-3 activation with down-regulation
of XIAP. Toxicol Lett. 213:151–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mu HQ, Yang S, Wang YJ and Chen YH: Role
of NF-κB in the anti-tumor effect of thymoquinone on bladder
cancer. Zhonghua Yi Xue Za Zhi. 92:392–396. 2012.(In Chinese).
PubMed/NCBI
|
|
23
|
Lei X, Lv X, Liu M, Yang Z, Ji M, Guo X
and Dong W: Thymoquinone inhibits growth and augments
5-fluorouracil-induced apoptosis in gastric cancer cells both in
vitro and in vivo. Biochem Biophys Res Commun. 417:864–868. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nessa MU, Beale P, Chan C, Yu JQ and Huq
F: Synergism from combinations of cisplatin and oxaliplatin with
quercetin and thymoquinone in human ovarian tumour models.
Anticancer Res. 31:3789–3797. 2011.PubMed/NCBI
|
|
25
|
Luo P, Tan Z, Zhang Z, Li H and Mo Z:
Inhibitory effects of salvianolic acid B on the high
glucose-induced mesangial proliferation via NF-κB-dependent
pathway. Biol Pharm Bull. 31:1381–1386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lui VW, Boehm AL, Koppikar P, Leeman RJ,
Johnson D, Ogagan M, Childs E, Freilino M and Grandis JR:
Antiproliferative mechanisms of a transcription factor decoy
targeting signal transducer and activator of transcription (STAT)
3: The role of STAT1. Mol Pharmacol. 71:1435–1443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rajput S, Kumar BN, Sarkar S, Das S, Azab
B, Santhekadur PK, Das SK, Emdad L, Sarkar D, Fisher PB and Mandal
M: Targeted apoptotic effects of thymoquinone and tamoxifen on XIAP
mediated Akt regulation in breast cancer. PLoS One. 8:e613422013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kolli-Bouhafs K, Boukhari A, Abusnina A,
Velot E, Gies JP, Lugnier C and Rondé P: Thymoquinone reduces
migration and invasion of human glioblastoma cells associated with
FAK, MMP-2 and MMP-9 down-regulation. Invest New Drugs.
30:2121–2131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hussain AR, Ahmed M, Ahmed S, Manogaran P,
Platanias LC, Alvi SN, Al-Kuraya KS and Uddin S: Thymoquinone
suppresses growth and induces apoptosis via generation of reactive
oxygen species in primary effusion lymphoma. Free Radic Biol Med.
50:978–987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gali-Muhtasib H, Ocker M, Kuester D,
Krueger S, El-Hajj Z, Diestel A, Evert M, El-Najjar N, Peters B,
Jurjus A, et al: Thymoquinone reduces mouse colon tumor cell
invasion and inhibits tumor growth in murine colon cancer models. J
Cell Mol Med. 12:330–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gali-Muhtasib H, Kuester D, Mawrin C,
Bajbouj K, Diestel A, Ocker M, Habold C, Foltzer-Jourdainne C,
Schoenfeld P, Peters B, et al: Thymoquinone triggers inactivation
of the stress response pathway sensor CHEK1 and contributes to
apoptosis in colorectal cancer cells. Cancer Res. 68:5609–5618.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shoieb AM, Elgayyar M, Dudrick PS, Bell JL
and Tithof PK: In vitro inhibition of growth and induction of
apoptosis in cancer cell lines by thymoquinone. Int J Oncol.
22:107–113. 2003.PubMed/NCBI
|
|
33
|
Woo CC, Hsu A, Kumar AP, Sethi G and Tan
KH: Thymoquinone inhibits tumor growth and induces apoptosis in a
breast cancer xenograft mouse model: The role of p38 MAPK and ROS.
PLoS One. 8:e753562013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
El-Mahdy MA, Zhu Q, Wang QE, Wani G and
Wani AA: Thymoquinone induces apoptosis through activation of
caspase-8 and mitochondrial events in p53-null myeloblastic
leukemia HL-60 cells. Int J Cancer. 117:409–417. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li F, Rajendran P and Sethi G:
Thymoquinone inhibits proliferation, induces apoptosis and
chemosensitizes human multiple myeloma cells through suppression of
signal transducer and activator of transcription 3 activation
pathway. Br J Pharmacol. 161:541–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Badr G, Mohany M and Abu-Tarboush F:
Thymoquinone decreases F-actin polymerization and the proliferation
of human multiple myeloma cells by suppressing STAT3
phosphorylation and Bcl2/Bcl-XL expression. Lipids Health Dis.
10:2362011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
El-Najjar N, Chatila M, Moukadem H,
Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R and
Gali-Muhtasib H: Reactive oxygen species mediate
thymoquinone-induced apoptosis and activate ERK and JNK signaling.
Apoptosis. 15:183–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gali-Muhtasib H, Diab-Assaf M, Boltze C,
Al-Hmaira J, Hartig R, Roessner A and Schneider-Stock R:
Thymoquinone extracted from black seed triggers apoptotic cell
death in human colorectal cancer cells via a p53-dependent
mechanism. Int J Oncol. 25:857–866. 2004.PubMed/NCBI
|
|
39
|
Lang M, Borgmann M, Oberhuber G, Evstatiev
R, Jimenez K, Dammann KW, Jambrich M, Khare V, Campregher C, Ristl
R and Gasche C: Thymoquinone attenuates tumor growth in ApcMin mice
by interference with Wnt-signaling. Mol Cancer. 12:412013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jrah-Harzallah H, Ben-Hadj-Khalifa S,
Almawi WY, Maaloul A, Houas Z and Mahjoub T: Effect of Thymoquinone
on 1,2-dimethyl-hydrazine-induced oxidative stress during
initiation and promotion of colon carcinogenesis. Eur J Cancer.
49:1127–1135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Asfour W, Almadi S and Haffar L:
Thymoquinone suppresses cellular proliferation, inhibits VEGF
production and obstructs tumor progression and invasion in the rat
model of DMH-induced colon carcinogenesis. Pharmacol Pharm. 4:7–17.
2013. View Article : Google Scholar
|
|
42
|
Velho-Pereira R, Kumar A, Pandey BN,
Jagtap AG and Mishra KP: Radiosensitization in human breast
carcinoma cells by thymoquinone: Role of cell cycle and apoptosis.
Cell Biol Int. 35:1025–1029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jafri SH, Glass J, Shi R, Zhang S, Prince
M and Kleiner-Hancock H: Thymoquinone and cisplatin as a
therapeutic combination in lung cancer: In vitro and in vivo. J Exp
Clin Cancer Res. 29:872010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Banerjee S, Kaseb AO, Wang Z, Kong D,
Mohammad M, Padhye S, Sarkar FH and Mohammad RM: Antitumor activity
of gemcitabine and oxaliplatin is augmented by thymoquinone in
pancreatic cancer. Cancer Res. 69:5575–5583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Housman G, Byler S, Heerboth S, Lapinska
K, Longacre M, Snyder N and Sarkar S: Drug resistance in cancer: An
overview. Cancers (Basel). 6:1769–1792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shishodia S and Aggarwal BB: Nuclear
factor-kappaB activation mediates cellular transformation,
proliferation, invasion angiogenesis and metastasis of cancer.
Cancer Treat Res. 119:139–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ahn KS and Aggarwal BB: Transcription
factor NF-kappaB: A sensor for smoke and stress signals. Ann NY
Acad Sci. 1056:218–233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nair A, Venkatraman M, Maliekal TT, Nair B
and Karunagaran D: NF-kappaB is constitutively activated in
high-grade squamous intraepithelial lesions and squamous cell
carcinomas of the human uterine cervix. Oncogene. 22:50–58. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li W, Tan D, Zenali MJ and Brown RE:
Constitutive activation of nuclear factor-kappaB (NF-κB) signaling
pathway in fibrolamellar hepatocellular carcinoma. Int J Clin Exp
Pathol. 3:238–243. 2009.PubMed/NCBI
|
|
51
|
Nagel D, Vincendeau M, Eitelhuber AC and
Krappmann D: Mechanisms and consequences of constitutive NF-κB
activation in B-cell lymphoid malignancies. Oncogene. 33:5655–5665.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shen HM and Tergaonkar V: NFkappaB
signaling in carcinogenesis and as a potential molecular target for
cancer therapy. Apoptosis. 14:348–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang S, Liu Z, Wang L and Zhang X:
NF-kappaB signaling pathway, inflammation and colorectal cancer.
Cell Mol Immunol. 6:327–334. 2009. View Article : Google Scholar : PubMed/NCBI
|