Introduction
Worldwide, ~320,000 women are diagnosed with
endometrial cancer each year and there are 76,000 mortalities
associated with endometrial cancer, which results in it being the
sixth most common cancer in women. Compared with an incidence rate
of 0.6% in developing countries, in developed countries the
incidence rate of endometrial cancer is 1.6% (1). The incidence of endometrial cancer is
increasing, which has been hypothesized to be associated with an
increased life expectancy and increasing incidence of obesity
(2).
Endometrioid endometrial cancer (EEC) accounts for
~80% of endometrial cancers. EEC tumors are usually
estrogen-dependent, often low-grade, diagnosed at an early stage
and are associated with an improved prognosis (3,4). However,
a previous study has revealed that certain survival outcomes,
including recurrence-free survival (RFS) and disease-specific
survival (DSS), are similar among malignant mixed mullerian tumors,
high-grade EEC, clear cell (CC) and uterine serous carcinoma (USC)
subtypes of endometrial cancer (5).
Del Carmen et al (6) also
reported that patients with grade 3 EEC (G3EEC) have a higher
relapse rate compared with patients with low-grade endometrioid
adenocarcinoma of the uterus. Therefore, G3EEC is considered as a
high-risk endometrial cancer, along with USC and CC; however,
factors associated with behavior and recurrence of G3EEC remain
unclear.
The Gynecologic Oncology Group (GOG) divided
endometrial cancer into three subgroups based on
surgical-pathological factors and age in order to evaluate the risk
of relapse accurately (7,8). A risk stratification algorithm,
published in GOG99 (8), has been
increasingly used to guide adjuvant therapy for patients with
intermediate and high-risk endometrial adenocarcinoma (9,10).
However, it is unknown whether this risk stratification algorithm
is suitable for G3EEC, and it is unclear if there is an association
between conventional prognostic indicators and prognosis among
G3EEC patients.
The present study reviewed patients with G3EEC in
order to clarify the risk factors for relapse and mortality in
these patients. The aim of the present study was to assess the
association of GOG99 risk stratification and various
clinicopathological features documented at the time of diagnosis,
including depth of myometrial invasion (MI), lymph vascular space
invasion (LVSI), International Federation of Gynecology and
Obstetrics (FIGO) stage, depth of cervical mucosa (stromal)
involvement (CMI), adnexal involvement, pelvic lymph node
metastasis (PLNM) and tumor diameter, with the relapse and survival
rates of G3EEC patients, and to determine independent factors
affecting the recurrence and survival status of patients with
G3EEC.
Materials and methods
Patients
A retrospective review of 117 patients diagnosed
with G3EEC at the Obstetrics and Gynecology Hospital of Fudan
University (Shanghai, China) between January 2000 and December 2011
was performed. Information on patient demographics, clinical
presentation, pathological characteristics, recurrent and survival
outcomes was extracted from patient medical records. The
institutional review board of the Obstetrics and Gynecology
Hospital of Fudan University approved the study. The inclusion
criteria for the present study was patients that had undergone
comprehensive surgical staging with G3EEC during final pathology
evaluation; all paraffin-embedded pathology specimens underwent a
pathology review by experienced gynecological pathologists. The
exclusion criteria for the present study included patients that had
the following characteristics: Non-endometrioid histology; grade
(G) 1 or G2 disease; and presence of synchronous cancers. All of
the present patients underwent a total hysterectomy and bilateral
salpingo-oophorectomy and/or partial omentectomy with or without
pelvic and/or para-aortic lymphadenectomy according to the disease
stage of the patient. Post-operative adjuvant treatment was
determined based on FIGO stage, patient preference and physician
suggestion. Standard chemotherapy consisted of paclitaxel and
carboplatin for 4–6 cycles and was used in 51 patients. Pelvic
radiotherapy and/or vaginal brachytherapy were administered in 6
patients. In total, 39 patients were treated with standard
chemotherapy and pelvic radiotherapy and/or vaginal brachytherapy.
The post-surgery treatment of 3 patients was unknown, and the
remaining 18 patients underwent observation with no treatment
following surgery.
Clinicopathological
characteristics
Clinicopathological characteristics included age,
menopausal status, FIGO stage, depth of MI, tumor diameter, LVSI,
depth of CMI, adnexal involvement, para-aortic lymphadenectomy,
PLNM and GOG99 risk stratification. Risk stratification was
determined by the standards set forth by the GOG99 protocol
(8), and patients were either
classified as low-risk, low-intermediate risk (LIR),
high-intermediate risk (HIR) or high-risk (HR). Low-risk patients
were classified as stage IA, G1 and LVSI negative. LIR patients
were classified as stages IA, IB or II that did not otherwise meet
low-risk or high-intermediate risk criteria. HIR patients were
classified as stages IA, IB and II with the following criteria: G2
or G3; LVSI positive; outer half MI (MI ≥50%); ≥70 years of age
with one other risk factor, ≥50 years of age with two risk factors
or any age with three risk factors. HR patients were classified as
stage III or IV. The patients in the present study were G3, and
therefore were LIR, HIR or HR. FIGO 2009 staging (11) was used, since staging changes were
developed in 2014. Recurrent disease was categorized as regional
recurrence (intra-pelvic cavity or vagina) and distal recurrence
(extra-pelvic only or intra- and extra pelvic). Para-aortic lymph
node recurrence was regarded as an extra-pelvic lesion.
Follow-up
Follow-up evaluations, including the date of last
follow up, recurrence and the cause of patient mortalities, were
collected from the medical records of the patients. All patients
were observed until November 2014 or the date that the patient
succumbed to a disease. The status of the patients at the end of
the follow-up period was defined as no evidence of disease, alive
with disease or succumbed to disease. Patients that succumbed to
disease were divided into G3EEC-associated mortality, other
disease-associated mortality and succumbed while lost to follow-up.
The overall survival (OS) rate was defined as the time between
surgery and the date of mortality or last follow-up recorded. RFS
was defined as the time between surgery and physical or
radiographical evidence of disease recurrence. DSS was defined as
the time between diagnosis or treatment and G3EEC-associated
mortality; patients that succumbed to other causes were not
included.
Statistical analysis
The χ2 test and Fisher's exact test were
used to analyze categorical variables. Univariate and multivariate
Cox proportional hazard regression model were used to assess the
effect of the various clinicopathological characteristics on OS,
RFS and DSS. Kaplan-Meier method was used to compare categorical
predictors within sub-groups of patients. SPSS version 13.0
software (SPSS, Inc., Chicago, IL, USA) was used for statistical
analysis. All P-values were two-sided and P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinicopathological characteristics of
patients
The clinicopathological characteristics of the 117
patients are listed in Table I. The
mean age was 55.6 years (range, 21–80 years) and the mean follow-up
time for all patients was 63 months (range, 2–153 months).
According to the 2009 FIGO staging criteria, 84 patients (71.8%)
were classified as stage I, 12 (10.3%) as stage II, 18 (15.4%) as
stage III and 3 (2.6%) as stage IV. In total, 51 patients (43.6%)
had MI ≥50%, and LVSI was observed in 35.9% of patients. Out of the
117 patients, 53 (45.3%) were classified as LIR, 43 (36.8%) as HIR
and the remaining 21 patients (17.9%) as HR. In total, 72 of the
patients (61.5%) were menopausal. There was no difference
identified in age between recurrent and not-recurrent patients
(57.5 vs. 55.3 years; P=0.384).
 | Table I.Clinicopathological characteristics of
117 patients with grade 3 endometrioid endometrial cancer. |
Table I.
Clinicopathological characteristics of
117 patients with grade 3 endometrioid endometrial cancer.
|
|
| Recurrence | Mortality |
|---|
|
|
|
|
|
|---|
| Characteristic | n (%) | n | P-value | n | P-value |
|---|
| Age, years |
|
| 0.788 |
|
0.866 |
|
<50 | 29
(24.8) | 3 |
| 4 |
|
|
50–70 | 78
(66.7) | 12 |
| 8 |
|
|
>70 | 10 (8.5) | 1 |
| 1 |
|
| Menopausal
status |
|
| 0.277 |
|
1.000 |
|
Pre-menopausal | 45
(38.5) | 4 |
| 5 |
|
|
Post-menopausal | 72
(61.5) | 12 |
| 8 |
|
| FIGO stage |
|
|
0.002a |
|
<0.001a |
| I | 84
(71.8) | 6 |
| 4 |
|
| II | 12
(10.3) | 4 |
| 1 |
|
|
III | 18
(15.4) | 4 |
| 6 |
|
| IV | 3
(2.6) | 2 |
| 2 |
|
| Depth of myometrial
invasion |
|
|
0.005a |
|
0.006a |
|
Negative | 9
(7.7) | 1 |
| 1 |
|
|
<50% | 57
(48.7) | 2 |
| 1 |
|
|
≥50% | 51
(43.6) | 13 |
| 11 |
|
| Tumor diameter,
cm |
|
| 0.553 |
| 0.511 |
| ≤2 | 32
(27.4) | 3 |
| 2 |
|
|
>2 | 83
(70.9) | 12 |
| 11 |
|
|
Unknown | 2
(1.7) | 1 |
| 0 |
|
| Cervical
involvement |
|
| 0.066 |
|
0.408 |
|
Negative | 88
(75.2) | 9 |
| 8 |
|
|
Mucosal | 9
(7.7) | 2 |
| 2 |
|
|
Stromal | 20
(17.1) | 5 |
| 3 |
|
| LVSI |
|
| 0.092 |
|
0.013a |
|
Negative | 75
(64.1) | 7 |
| 4 |
|
|
Positive | 42
(35.9) | 9 |
| 9 |
|
| PLNM |
|
| 0.056 |
|
<0.001a |
|
Negative | 93
(79.5) | 11 |
| 6 |
|
|
Positive | 17
(14.5) | 5 |
| 7 |
|
|
Unknown | 7
(6.0) | 0 |
| 0 |
|
| Adnexal
involvement |
|
| 0.043 |
|
0.020a |
|
Negative | 106 (90.6) | 12 |
| 4 |
|
|
Positive | 11 (9.4) | 4 |
| 9 |
|
| Risk
stratification |
|
|
0.010a |
|
<0.001a |
|
LIR | 53
(45.3) | 2 |
| 1 |
|
|
HIR | 43
(36.8) | 8 |
| 4 |
|
| HR | 21
(17.9) | 6 |
| 8 |
|
| Para-aortic
lymphadenectomy |
|
| 1.000 |
|
0.356 |
|
Negative | 104 (88.9) | 14 |
| 13 |
|
|
Positive | 13
(11.1) | 2 |
| 0 |
|
| Adjuvant
therapy |
|
| 0.712 |
|
0.868 |
|
Observation | 18
(15.4) | 1 |
| 1 |
|
|
Chemotherapy | 51
(43.6) | 7 |
| 6 |
|
|
Radiotherapy | 6
(5.1) | 1 |
| 1 |
|
|
Chemoradiotherapy | 39
(33.3) | 7 |
| 5 |
|
|
Unknown | 3
(2.6) | 0 |
| 0 |
|
Association between recurrence and
clinicopathological characteristics
The overall relapse rate of the present patients
with G3EEC was ~13.7% (16/117). Of the 16 patients that relapsed,
14 (87.5%) developed recurrence within 3 years of primary
treatment. Of all recurrence cases, there were 6 (37.5%) regional
recurrent patients, including 5 patients in whom recurrence was
restricted to the pelvic cavity and 1 patient that had recurrence
restricted to the vagina, and 10 (55.6%) distal recurrence
patients, including 9 patients with extra-pelvic disease and 1
patient with intra- and extra-pelvic recurrence. Patterns of distal
recurrence included lung (n=4), abdomen (n=1), bone (n=2), liver
(n=2) and bowel (n=1). The mean time to regional recurrence
(intra-pelvic only) and distal recurrence (intra- and extra-pelvic)
was not significantly different (12.5 vs. 32.6 months; P=0.068). Of
16 recurred cases, 10 patients (62.5%) succumbed to G3EEC, 5
patients were (31.3%) alive with disease and 1 patient succumbed to
cerebral hemorrhage. Recurrence and mortality of patients with
G3EEC were additionally evaluated for the 117 patients in
association with GOG99 risk categories.
Association between risk factors and
patient outcome
Table II provides the
results of the Cox univariate analysis with corresponding hazard
ratio (HZR) and 95% confidence intervals (CI) for predicting
relapse and survival status. MI, FIGO stages, LVSI, adnexal
involvement, PLNM, CMI and risk stratification were significantly
associated with an increased risk of recurrence using univariate
analysis (P<0.05). DSS and OS rates were also associated with
these characteristics, with the exception of CMI and risk
stratification when divided into LIR and HIR groups.
 | Table II.Univariate Cox proportional hazards
analysis for the risk of tumor recurrence, disease-specific
survival and overall survival rates associated with various
clinicopathological characteristics. |
Table II.
Univariate Cox proportional hazards
analysis for the risk of tumor recurrence, disease-specific
survival and overall survival rates associated with various
clinicopathological characteristics.
|
| Recurrence-free
survival | Disease-specific
survival | Overall
survival |
|---|
|
|
|
|
|
|---|
| Characteristic | HZR (95% CI) | P-value | HZR (95% CI) | P-value | HZR (95% CI) | P-value |
|---|
| Age, years |
|
| ≤60 vs.
>60 | 1.57
(0.56–4.41) | 0.394 | 1.54
(0.57–4.18) | 0.396 | 1.74
(0.62–4.84) | 0.291 |
| Menopausal
status |
|
| Pre vs.
post | 1.82
(0.58–5.72) | 0.305 | 1.87
(0.60–5.82) | 0.277 | 1.25
(0.43–3.66) | 0.686 |
| Depth of MI |
|
|
Negative/<50% vs. ≥50% | 6.59
(1.88–23.15) |
0.003a | 8.06
(1.79–36.43) | 0.007a | 6.70
(1.92–23.33) |
0.003a |
| LVSI |
|
|
Negative vs. positive | 2.70
(1.01–7.25) |
0.049a | 4.48
(1.38–14.55) | 0.013a | 3.60
(1.33–9.73) |
0.012a |
| Adnexal
involvement |
|
|
Negative vs. positive | 4.74
(1.50–14.92) |
0.008a | 6.04
(1.81–20.14) | 0.003a | 4.30
(1.36–13.57) |
0.013a |
| Cervical
involvement |
|
|
Negative/mucosal vs.
interstitial | 3.25
(1.18–8.95) |
0.023a | 1.64
(0.45–5.96) | 0.456 | 1.70
(0.55–5.24) | 0.353 |
| PLNM |
|
|
Negative vs. positive | 3.90
(1.34–11.36) |
0.013a | 9.67
(3.21–29.11) |
<0.001a | 5.57
(2.10–14.77) |
0.001a |
| Tumor diameter,
cm |
|
| <2
vs. ≥2 | 1.69
(0.47–6.09) | 0.421 | 1.83
(0.52–6.51) | 0.348 | 1.66
(0.46–5.97) | 0.437 |
| Para-aortic
lymphadenectomy |
|
|
Negative vs. positive | 1.39
(0.31–6.25) | 0.667 | 1.38
(0.31–6.20) | 0.674 | 0.04
(0.00–90.54) | 0.417 |
Multivariate analysis (Table III) identified MI ≥50% (HZR, 4.91;
95% CI, 1.02–23.57; P=0.047) and adnexal involvement (HZR, 4.29;
95% CI, 1.07–17.15; P=0.040) were associated with RFS. For DSS, MI
≥50% (HR, 4.83; 95% CI, 1.06–22.04; P=0.042) and adnexal
involvement (HR, 4.04; 95% CI, 1.04–15.60; P=0.043) were also
independent prognostic factors.
 | Table III.Multivariate Cox proportional hazards
analysis for the risk of tumor recurrence, disease-specific
survival and overall survival rates associated with various
clinicopathological characteristics. |
Table III.
Multivariate Cox proportional hazards
analysis for the risk of tumor recurrence, disease-specific
survival and overall survival rates associated with various
clinicopathological characteristics.
|
| Recurrence-free
survival | Disease-specific
survival | Overall
survival |
|---|
|
|
|
|
|
|---|
| Characteristic | HZR (95% CI) | P-value | HZR (95% CI) | P-value | HZR (95% CI) | P-value |
|---|
| Age, years |
|
| ≤60 vs.
>60 | 0.76
(0.19–3.05) | 0.699 | 0.72
(0.18–2.84) | 0.640 | 1.00
(0.20–4.85) | 0.996 |
| Menopausal
status |
|
| Pre vs.
post | 2.41
(0.59–9.88) | 0.220 | 2.93
(0.71–12.03) | 0.136 | 1.50
(0.32–6.94) | 0.604 |
| Depth of MI |
|
|
Negative/<50% vs. ≥50% | 4.91
(1.02–23.57) | 0.047a | 4.83
(1.06–22.04) | 0.042a | 3.87
(0.77–19.35) | 0.099 |
| LVSI |
|
|
Negative vs. positive | 1.92
(0.53–6.90) | 0.318 | 2.08
(0.59–7.33) | 0.254 | 2.17
(0.61–7.77) | 0.234 |
| Adnexal
involvement |
|
|
Negative vs. positive | 4.29
(1.07–17.15) | 0.040a | 4.04
(1.04–15.60) | 0.043a | 2.62
(0.61–11.21) | 0.196 |
| Cervical
involvement |
|
|
Negative/mucosal vs.
interstitial | 1.15
(0.29–4.51) | 0.847 | 1.05
(0.27–4.05) | 0.943 | 0.51
(0.09–2.72) | 0.428 |
| PLNM |
|
|
Negative vs. positive | 1.77
(0.42–7.56) | 0.440 | 2.89
(0.78–10.66) | 0.111 | 2.21
(0.58–8.41) | 0.246 |
| Tumor diameter,
cm |
|
| <2
vs. ≥2 | 0.45
(0.10–2.13) | 0.317 | 0.50
(0.11–2.26) | 0.370 | 0.72
(0.17–3.03) | 0.653 |
| Para-aortic
lymphadenectomy |
|
|
Negative vs. positive | 1.18
(0.24–5.88) | 0.840 | 0.98
(0.20–4.87) | 0.982 | 0.83
(0.27–4.16) | 0.983 |
For all G3EEC patients, the OS, RFS and DSS were
70.8, 80.5 and 81.9%, respectively. When survival curves were
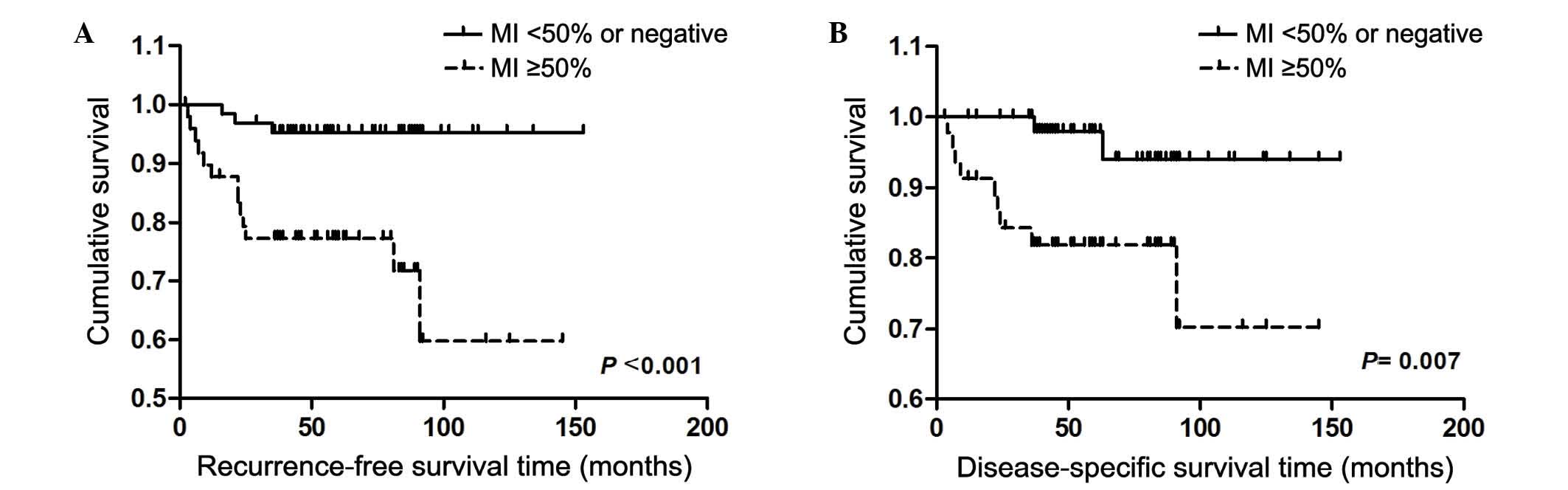
stratified by MI, there was a significant increase in RFS and DSS
rates for patients with tumors with MI <50% compared with those
with MI ≥50% (Fig. 1). In addition,
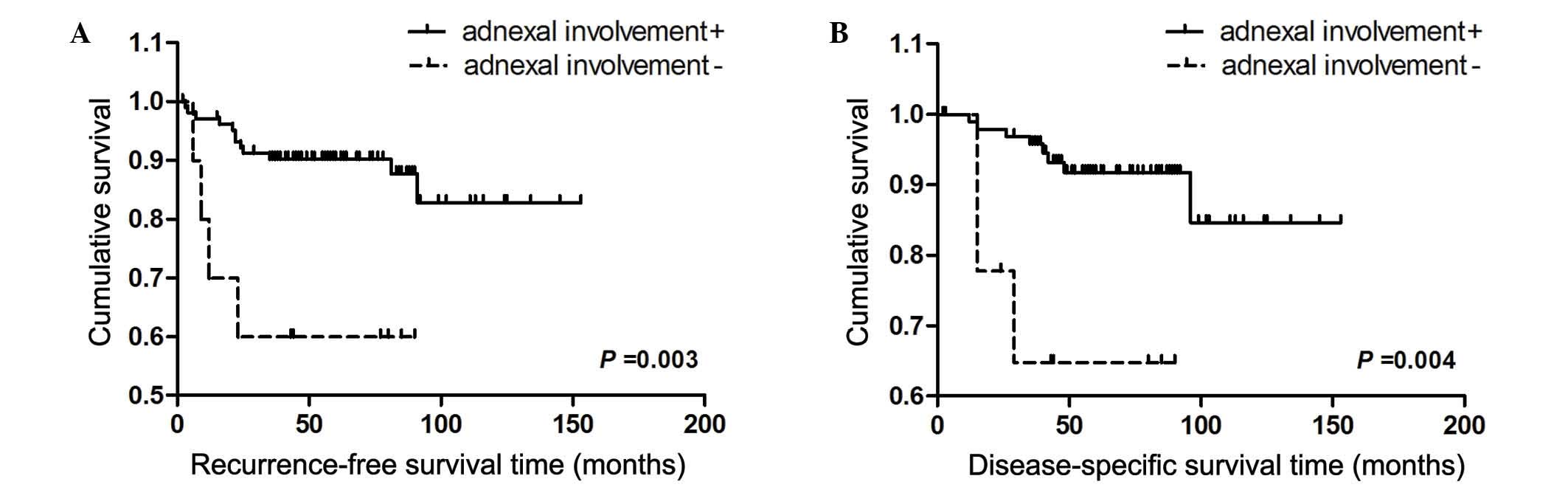
adnexal involvement was a negative prognostic factor for RFS and
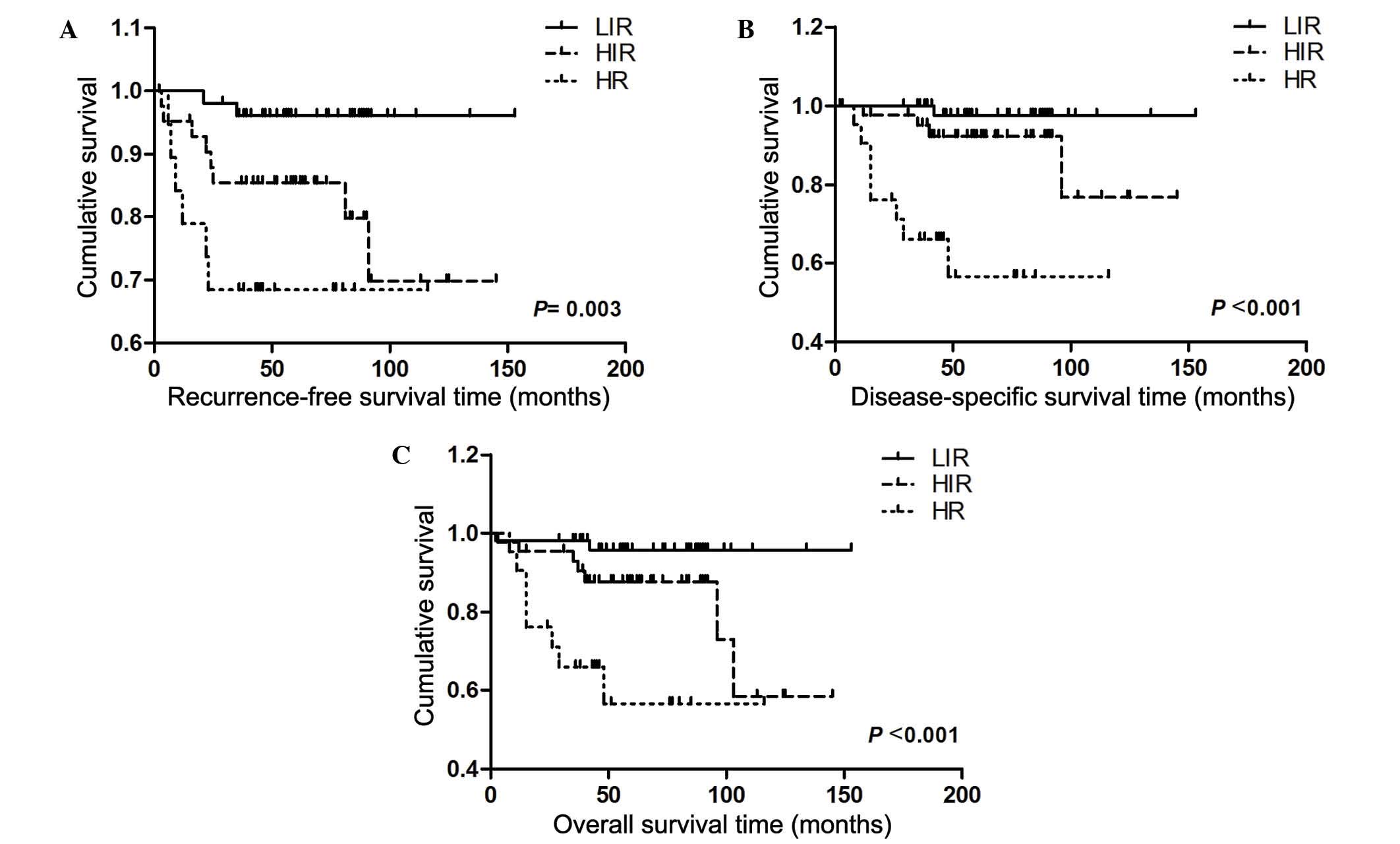
DSS (Fig. 2). Furthermore, OS rate,
RFS and DSS clearly differed between the LIR, HIR and HR groups, as
shown in Fig. 3.
Discussion
The present retrospective review evaluated a group
of 117 G3EEC patients treated in a single institution, and examined
the prognostic variables associated with recurrence and survival
status. The present study identified a recurrence rate of ~13.7%,
which is lower than the 19.6% (10/51) reported by a previous study
by Kim et al (12). This
difference may be due to the FIGO stages identified. In the present
study, early stage (I and II) tumors accounted for 82.1% of the
patients, which is increased compared with 80.4% observed by Kim
et al (12). However, Gayar
et al (13) reported a
recurrence rate of 23.6% (26/110) in G3 patients with early-stage
tumors (FIGO stage I–II), which is increased compared with 13.7%
(16/117 patients) observed in the present study. The OS rate in the
present study was 70.8%, which was lower compared with 81.0%
identified in a previous study including patients of all grades
(14). This difference supports the
hypothesis that G3 patients have a worse prognosis.
Although the vaginal cuff is the most common site
for tumor recurrence following a hysterectomy in early-stage
endometrial carcinoma patients, with locoregional recurrence rates
of 4–8% (8,15), Rasool et al (16) reported a high rate of distant
recurrence in patients with stage I G3EEC and a risk of
extra-pelvic recurrence of 80%, which is higher compared with local
regional recurrence. In a previous study of 28 cases with stage IV
G3EEC, recurrence was observed in 6 cases, and all of these were
distant recurrence (17). With a
relatively large sample size, the present study suggests that both
regional and distal recurrence occurs in patients with G3EEC. Of
the 16 patients in the present study with G3 tumors that had
recurrence, 10 patients (55.6%) had a component of distant failure,
which is similar to the results demonstrated by Kim et al
(12), who revealed 1 local regional,
3 lymphatic and 6 hematogenous distant failure recurrences in 10
G3EEC patients. Therefore, the majority of G3 patients have a
tendency for distant recurrence and may succumb to disease,
suggesting that current loco-regional adjuvant treatment
strategies, including brachytherapy and pelvic radiotherapy, are
not beneficial and additional systemic therapies are required.
In agreement with other studies (18–20), which
demonstrated that MI is associated with an increasing risk of tumor
failure and has a negative affect on survival, the present study
suggests that MI is a clear adverse predictor of tumor recurrence
and survival end points. Depth of MI has been recognized as an
independent prognostic factor for patients with stage I and II
endometrial adenocarcinoma (21) and
is the clearest predictor of distant failure and mortality from
disease in stage I endometrial cancer (18). MI ≥66% was identified as the only
independent predictor of disease-free survival (DFS) and RFS in 229
patients with stage I epithelial endometrial cancer of all subtypes
(18). Additionally, in a previous
study of 213 patients with endometrial cancer, MI >50% is clear
risk for extra-uterine metastases (22). Furthermore, patients with MI >50%
had a >6-fold higher prevalence of pelvic lymph node metastases
compared with patients with MI <50% (22). Depth of MI has also been revealed to
be an independent predictor of pelvic relapse in a multivariate
analysis (23). Hematogenous
dissemination is defined as tumor spread to the lung, liver or
other site via hematogenous routes. Mariani et al (24) reported that the presence of deep MI
was the clearest predictor of hematogenous dissemination in corpus
cancer. The present study also revealed that MI ≥50% was a
statistically significant characteristic associated with a decrease
in DSS and RFS rates. In the current study, the RFS and DSS rates
were 59.8 and 70.2% for patients with deep MI (≥50%), compared to
95.3 and 94.0% for patients with superficial MI (<50%),
respectively. In conclusion, MI ≥50% is the most important
prognostic factor for G3EEC patients, and patients with deep MI
require a more aggressive therapy and more frequent observation to
monitor the presence or absence of recurrence.
However, there are contrary views concerning the
importance of MI. Certain studies have demonstrated that in
patients with early stage endometrial cancer, MI does not appear to
be associated with patient outcomes, including RFS (25), DSS (25), OS (25,26) and
DFS (26). Chattopadhyay et al
(20) reported that tumor size was
the only independent predictor for distant recurrence and mortality
from disease in stage I EEC, while MI only predicted distant
failure. In the present study, distant failure was the main G3EEC
recurrence site; therefore, the study by Chattopadhyay et al
supports the present results to a certain extent. Overall, the
inconsistencies observed with MI may be due to differences in stage
or grade amongst selected patients and the various definitions of
MI; depths of >1/3 (26), >50%
(22) and ≥66% (18) are all regarded as cut-off values for
deep MI in various studies, indicating that in endometrial cancer,
the depth of MI has various roles according to the severity of the
disease. In previous studies MI has been associated with certain
biochemical indicators, including levels of free insulin-like
growth factor-1 plasma (27),
nucleoporin (88kDa)-mRNA expression (28) and endoplasmic reticulum-β (29). An association was also demonstrated
between tumor-associated macrophages and depth of MI (30).
The present study demonstrated that, in G3EEC
patients, adnexal involvement is also a risk factor for recurrence
and disease-related mortality. Adnexal metastasis is associated
with omental metastasis (31) and
para-aortic lymph node metastasis (32). The risk of tumor recurrence in
patients with adnexal involvement is 4.29-fold higher compared with
patients without adnexal involvement, which is consistent with the
results demonstrated by Hétu et al (33), who reported that patients with
metastasis to the adnexa have a higher risk of recurrence. With
regard to DSS, Jobsen et al (34) reported that the 7-year DSS rate was
71.8% for patients with adnexal involvement in stage IIIA
endometrial carcinoma. In the present study, patients with positive
adnexal involvement had a 4.04-fold higher risk of disease-related
mortality compared with patients without adnexal involvement.
The GOG99 trial was a randomized controlled trial
comparing postoperative pelvic radiotherapy to no additional
treatment in stage IB, IC or IIA endometrial carcinoma of any
histological grade (8). GOG99
revealed that age, grade, LVSI and MI are factors that influence
the risk of relapse. In the present study, risk stratification was
revealed to have a negative association with the outcome of
patients using univariate cox proportional hazards analysis. In a
previous study, HIR patients had a decreased OS rate compared with
patients that were not classified as HIR (35). The present results revealed that the
prognosis of patients clearly differed between the LIR, HIR and HR
groups in G3EEC. DSS, OS rate and RFS were 97.6, 95.7 and 96.1% for
the LIR group, 76.9, 58.4 and 69.8% in the HIR group, and 56.6,
56.6 and 68.4% for the HR group, respectively. However, in the
univariate cox proportional hazards analysis, compared with the LIR
group, patients in the HIR and HR groups demonstrated 5.30 and
11.04-fold decrease in RFS, 5.01 and 27.41-fold decrease in DSS and
4.20 and 13.14-fold decrease in OS rates, respectively. In the
multivariate Cox proportional hazards analysis, risk classification
was not an independent factor, which may be due to risk
stratification being associated with conventional prognostic
indicators (8,36). Therefore, the present study
hypothesizes that the criteria of risk stratification is associated
with the prognostic status of G3EEC patients, but is not an
independent factor.
In conclusion, the present results suggest that in
patients with G3EEC MI ≥50% and adnexal involvement are significant
risk factors affecting RFS and DSS. The present study has also
demonstrated that in patients with G3EEC, GOG99 risk stratification
had an association with the patient outcome of relapse and
survival. Based on these findings, the risk factors of G3EEC
evaluated by the present study may be used to evaluate the outcome
of patients. Patients with MI ≥50% or positive adnexal involvement
should undergo more aggressive therapy and a more rigorous
follow-up plan. The limitations of the present study include the
inherent biases of a retrospective single institution study design.
Furthermore, the study population was relatively small due to the
strict selection criteria. Additional larger prospective studies
examining the same type of patient population are required to
verify the present findings.
Acknowledgement
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant no.
30973185).
Glossary
Abbreviations
Abbreviations:
|
G3EEC
|
grade 3 endometrioid endometrial
carcinoma
|
|
RFS
|
recurrence-free survival
|
|
DSS
|
disease-specific survival
|
|
OS
|
overall survival
|
|
FIGO
|
international federation of gynecology
and obstetrics
|
|
PLNM
|
positive lymph nodes metastasis
|
|
GOG
|
the gynecologic oncology group
|
|
MI
|
myometrial invasion
|
|
LVSI
|
lymph vascular space invasion
|
|
LIR
|
low-intermediate risk
|
|
HIR
|
high-intermediate risk
|
|
HR
|
high-risk
|
References
|
1
|
Galaal K, Al Moundhri M, Bryant A, Lopes
AD and Lawrie TA: Adjuvant chemotherapy for advanced endometrial
cancer. Cochrane Database Syst Rev. 5:CD0106812014.PubMed/NCBI
|
|
2
|
Emily K, Clark L, Franasiak J, Bae-Jump V
and Gehrig P: Obesity portends a significantly higher risk of
recurrence in women with grade 3 endometrial carcinoma: A call to
action. Gynecolo Oncol. 125(Supp 1): S1582012. View Article : Google Scholar
|
|
3
|
Sorosky JI: Endometrial cancer. Obstetr
Gynecol. 120:383–397. 2012. View Article : Google Scholar
|
|
4
|
Tangjitgamol S1, Anderson BO, See HT,
Lertbutsayanukul C, Sirisabya N, Manchana T, Ilancheran A, Lee KM,
Lim SE, Chia YN, et al: Asian Oncology Summit: Management of
endometrial cancer in Asia: consensus statement from the Asian
Oncology Summit 2009. Lancet Oncol. 10:1119–1127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Felix AS, Stone RA, Bowser R, Chivukula M,
Edwards RP, Weissfeld JL and Linkov F: Comparison of survival
outcomes between patients with malignant mixed mullerian tumors and
high-grade endometrioid, clear cell, and papillary serous
endometrial cancers. Int J Gynecol Cancer. 21:877–884. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Del Carmen MG, Boruta DM II and Schorge
JO: Recurrent endometrial cancer. Clin Obstet Gynecol. 54:266–277.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morrow CP, Bundy BN, Kurman RJ, Creasman
WT, Heller P, Homesley HD and Graham JE: Relationship between
surgical-pathological risk factors and outcome in clinical stage I
and II carcinoma of the endometrium: A gynecologic oncology group
study. Gynecol Oncol. 40:55–65. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keys HM, Roberts JA, Brunetto VL, Zaino
RJ, Spirtos NM, Bloss JD, Pearlman A, Maiman MA and Bell JG:
Gynecologic Oncology Group: A phase III trial of surgery with or
without adjunctive external pelvic radiation therapy in
intermediate risk endometrial adenocarcinoma: A gynecologic
oncology group study. Gynecol Oncol. 92:744–751. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ioffe Y, Delic L, Amneus M, Leuchter R,
Karlan B, Li A, Walsh C, Lentz S, Farias-Eisner R and Cass I:
Before and after GOG 99: Did our practice patterns for treatment of
intermediate risk endometrial adenocarcinoma change? Gynecol Oncol.
116:5952010. View Article : Google Scholar
|
|
10
|
Rankins NC, Secord AA, Jewell E,
Havrilesky LJ, Soper JT and Myers E: Cost-effectiveness of adjuvant
radiotherapy in intermediate risk endometrial cancer. Gynecol
Oncol. 106:388–393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: Gynecologic Sites - Corpus Uteri. AJCC
Cancer Staging Manual (7th). Springer. (New York). 4032010.
|
|
12
|
Kim HJ, Kim TJ, Lee YY, Choi CH, Lee JW,
Bae DS and Kim BG: A comparison of uterine papillary serous, clear
cell carcinomas, and grade 3 endometrioid corpus cancers using 2009
FIGO staging system. J Gynecol Oncol. 24:120–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gayar OH, Patel S, Schultz D, Mahan M,
Rasool N and Elshaikh MA: The impact of tumor grade on survival end
points and patterns of recurrence of 949 patients with early-stage
endometrioid carcinoma: A single institution study. Int J Gynecol
Cancer. 24:97–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tejerizo-García A, Jiménez-López JS,
Muñoz-González JL, Bartolomé-Sotillos S, Marqueta-Marqués L,
López-González G and Gómez JF: Overall survival and disease-free
survival in endometrial cancer: Prognostic factors in 276 patients.
Onco Targets Ther. 9:1305–1313. 2013.PubMed/NCBI
|
|
15
|
Creutzberg CL, van Putten WL, Koper PC,
Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, De Winter KA, Lutgens
LC, van den Bergh AC, van de Steen-Banasik E, et al: Surgery and
postoperative radiotherapy versus surgery alone for patients with
stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC
study group. Post operative radiation therapy in endometrial
carcinoma. Lancet. 355:1404–1411. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rasool N, Fader AN, Seamon L, Neubauer NL,
Shahin FA, Alexander HA, Moore K, Moxley K, Secord AA, Kunos C, et
al: Stage I, grade 3 endometrioid adenocarcinoma of the
endometrium: An analysis of clinical outcomes and patterns of
recurrence. Gynecol Oncol. 116:10–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Long KC, Zhou Q, Hensley ML, Alektiar KM,
Gomez J, Gardner GJ, Chi DS, Barakat RR and Abu-Rustum NR: Patterns
of recurrence in 1988 FIGO stage IC endometrioid endometrial
cancer. Gynecol Oncol. 125:99–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mariani A, Webb MJ, Keeney GL, Lesnick TG
and Podratz KC: Surgical stage I endometrial cancer: Predictors of
distant failure and death. Gynecol Oncol. 87:274–280. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lindauer J, Fowler JM, Manolitsas TP,
Copeland LJ, Eaton LA, Ramirez NC and Cohn DE: Is there a
prognostic difference between depth of myometrial invasion and the
tumor-free distance from the uterine serosa in endometrial cancer?
Gynecol Oncol. 91:547–551. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chattopadhyay S, Cross P, Nayar A, Galaal
K and Naik R: Tumor size: A better independent predictor of distant
failure and death than depth of myometrial invasion in
international federation of gynecology and obstetrics stage I
endometrioid endometrial cancer. Int J Gynecol Cancer. 23:690–697.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zaino RJ, Kurman RJ, Diana KL and Morrow
CP: Pathologic models to predict outcome for women with endometrial
adenocarcinoma: The importance of the distinction between surgical
stage and clinical stage-a gynecologic oncology group study.
Cancer. 77:1115–1121. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Larson DM, Connor GP, Broste SK, Krawisz
BR and Johnson KK: Prognostic significance of gross myometrial
invasion with endometrial cancer. Obstet Gynecol. 88:394–398. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patel S, Portelance L, Gilbert L, Tan L,
Stanimir G, Duclos M and Souhami L: Analysis of prognostic factors
and patterns of recurrence in patients with pathologic stage III
endometrial cancer. Int J Radiat Oncol Biol Phys. 68:1438–1445.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariani A, Webb MJ, Keeney GL, Calori G
and Podratz KC: Hematogenous dissemination in corpus cancer.
Gynecol Oncol. 80:233–238. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gemer O, Uriev L, Harkovsky T, Peled R,
Ben-Dor D, Barak F and Seqal S: The significance of the degree of
myometrial invasion in patients with stage IB endometrial cancer.
Eur J Gynaecol Oncol. 25:336–338. 2004.PubMed/NCBI
|
|
26
|
Alektiar KM, McKee A, Lin O, Venkatraman
E, Zelefsky MJ, Mychalczak BR, Mckee B, Hoskins WJ and Barakat RR:
The significance of the amount of myometrial invasion in patients
with Stage IB endometrial carcinoma. Cancer. 95:316–321. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baloglu A, Bezircioglu I, Cetinkaya B and
Hicyilmaz L: Prospective clinical study of the association between
plasma level of free IGF-1 and myometrial invasion min patients
with endometrial adenocarcinoma. Ginekol Pol. 81:501–505.
2010.PubMed/NCBI
|
|
28
|
Schneider J, Martinez-Arribas F and
Torrejón R: Nup88 expression is associated with myometrial invasion
in endometrial carcinoma. Int J Gynecol Cancer. 20:804–808. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takama F, Kanuma T, Wang D, Kagami I and
Mizunuma H: Oestrogen receptor beta expression and depth of
myometrial invasion in human endometrial cancer. Br J Cancer.
84:545–549. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soeda S, Nakamura N, Ozeki T, Nishiyama H,
Hojo H, Yamada H, Abe M and Sato A: Tumor-associated macrophages
correlate with vascular space invasion and myometrial invasion in
endometrial carcinoma. Gynecol Oncol. 109:122–128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sakai K, Yamagami W, Susumu N, Nomura H,
Kataoka F, Banno K, Tsuda H and Aoki D: Pathological factors
associated with omental metastases in endometrial cancer. Eur J
Gynaecol Oncol. 36:397–401. 2015.PubMed/NCBI
|
|
32
|
Turan T, Hizli D, Sarici S, Boran N,
Gundogdu B, Karadag B, Tulunay G and Kose MF: Is it possible to
predict para-aortic lymph node metastasis in endometrial cancer?
Eur J Obstet Gynecol Reprod Biol. 158:274–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hétu V, Petignat P, Wu Y, Drouin P,
Sauthier P, Provencher D and Gauthier P: Positive adnexal or
uterine serosal involvement in stage IIIC endometrial cancer is an
adverse factor for recurrence. Gynecol Obstet Invest. 67:173–177.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jobsen JJ, Ten Cate L Naudin, Lybeert ML,
Scholten A, van der Steen-Banasik EM, van der Palen J, Kroese MC
Stenfert, Slot A, Schutter EM and Siesling S: Outcome of
Endometrial Cancer Stage IIIA with Adnexa or Serosal Involvement
Only. Obstet Gynecol Int. 2011:9625182011.PubMed/NCBI
|
|
35
|
Guntupalli SR, Zighelboim I, Kizer NT,
Zhang Q, Powell MA, Thaker PH, Goodfellow PJ and Mutch DG:
Lymphovascular space invasion is an independent risk factor for
nodal disease and poor outcomes in endometrioid endometrial cancer.
Gynecol Oncol. 124:31–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maggi R, Lissoni A, Spina F, et al:
Adjuvant chemotherapy vs radiotherapy in high-risk endometrial
carcinoma: results of a randomised trial. British journal of
cancer. 95:266–271. 2006. View Article : Google Scholar : PubMed/NCBI
|