Introduction
Melanoma is the most lethal form of skin cancer that
arises from melanocytes, and although it constitutes only ~4% of
all skin cancers it is responsible for >80% of mortalities in
patients with skin cancer (1). Since
the 1950s, the incidence of melanoma has been rapidly increasing in
non-Hispanic white populations worldwide (2). Although surgery may cure the majority of
patients in early stages of melanoma, patients with distant
metastasis have a poor prognosis with a median survival time of
only 6–8 months, and <15% of patients with distant metastatic
melanoma survive for 5 years (3).
Since melanoma has an extremely high metastatic potential, it is
crucial to discover factors that are involved in the progression
and metastasis of melanoma in an attempt to identify an effective
treatment regimen for this devastating disease.
Eukaryotic translation initiation factor (EIF) 5A is
best known for its cytoplasmic role in translation regulation as a
eukaryotic translation initiation factor (4,5). In
mammals, it is encoded by two highly-associated genes EIF5A1 and
EIF5A2. However, a number of studies have suggested that EIF5A1 may
also be active in the nucleus, particularly in mammals (6–8). EIF5A1
has been revealed to take part in the nucleocytoplasmic transport
of incompletely spliced and unspliced human immunodeficiency
virus-1 mRNAs (6) that are
translocated across the nuclear envelope via chromosomal
maintenance 1 (CRM1), which is a member of the importin β family of
transport receptors. The main role of CRM1 is to facilitate the
translocation of rRNA, U snRNA and ribosomal subunits across the
nuclear envelope (9). EIF5A1 has also
been revealed to be capable of interacting and colocalizing with
transport receptor exportin 4, which is another member of the
importin β family (8). In addition,
it has been suggested that EIF5A1 may recognize nitric oxide
synthase 2 mRNA in the nucleus and facilitate its transport to the
cytoplasm in a CRM1 dependent-manner (10). Furthermore in Saccharomyces
cerevisiae, EIF5A1 appears to be involved in mRNA
degradation/turnover, which is a process highly associated with
nucleocytoplasmic transport of mRNA (11,12). In
summary, these data demonstrate a nuclear activity for EIF5A1 that
is possibly associated with cellular mRNA metabolism.
EIF5A2, a phylogenetically conserved vertebrate
variant of EIF5A1, was first reported to be highly expressed in
testis and colorectal adenocarcinoma, and at moderate levels in the
brain (13). EIF5A2 shares 83% amino
acid identity with EIF5A1 (14) and
has been associated with various oncogenic roles, including
invasion and metastasis, and a poor prognosis in a variety of
cancers, such as ovarian cancer (15), gastric adenocarcinoma (16), colorectal cancer (17,18),
hepatocellular carcinoma (HCC) (19),
ovarian cancer (20), non-small cell
lung cancer (21) and bladder cancer
(22,23). However, no studies have investigated
the nuclear expression or activity of EIF5A2 in cancer, with the
exception of Zender et al (24), who addressed the importance of nuclear
EIF5A2 in HCC.
Previously, the present authors reported an increase
in the cytoplasmic expression of EIF5A2 in melanoma and its role in
melanoma progression and patient survival (25). The present study investigates, using
immunohistochemistry and tissue microarray (TMA), the status of
nuclear EIF5A2 expression in melanoma. The results revealed that
nuclear EIF5A2 is an independent prognostic marker in melanoma, and
its expression is significantly increased during melanoma
progression. In addition, upregulation of nuclear EIF5A2 was
determined to be associated with a significantly poorer 5-year
survival rate for all and primary melanoma patients. Furthermore,
simultaneous nuclear and cytoplasmic EIF5A2 expression, as well as
concurrent nuclear EIF5A2 and matrix metalloproteinase-2 (MMP-2)
expression, were associated with a poorer 5-year patient survival
rate.
Materials and methods
Patient specimens
In total, 459 formalin-fixed, paraffin-embedded
human tissues, consisting of 28 common acquired nevi, 49 dysplastic
nevi, 242 primary melanomas and 140 metastatic melanomas, were used
in the present study. The human skin tissues and the patients' data
were acquired from the 1990–1998 archives of the Department of
Pathology, Vancouver General Hospital (Vancouver, Canada), and
their use was approved by the Clinical Research Ethics Board of the
University of British Columbia (Vancouver, Canada; certificate
number H09-01321), in accordance with the Declaration of Helsinki
guidelines (26). Patient consent was
not required under the Canadian law, since the present report is a
retrospective study using anonymized data and several patients had
already succumbed to the disease.
TMA construction and
immunohistochemistry
TMA construction and immunohistochemical staining
were performed as previously described (27,28).
Briefly for immunohistochemical staining, the TMA slides were
dewaxed by heating at 55°C for 30 min followed by three 5 min
washes with xylene. Subsequently, the samples were rehydrated by
washing in 100, 95 and 80% ethanol, and distilled water for 5 min
each. For antigen retrieval, the samples were heated for 30 min at
95°C in 10 mmol/l sodium citrate (pH 6.0). The samples were
incubated with 3% hydrogen peroxide in order to block endogenous
peroxidase activity, and were next incubated for 30 min with
universal blocking serum (Dako Canada ULC, Mississauga, ON, Canada)
and then incubated with a primary rabbit anti-EIF5A2 antibody
(dilution, 1:100; catalog no. E9781; Sigma-Aldrich, St. Louis, MO,
USA) overnight at 4°C. Subsequently, the slides were incubated for
30 min with a non-diluted biotin-labelled secondary antibody raised
in swine (catalog no. K0690; Dako Canada ULC), and then incubated
with streptavidin-peroxidase (Dako Canada ULC). Subsequently, the
samples were developed with 3,3-diaminobenzidine [Vector
Laboratories (Canada), Inc., Burlington, ON, Canada] and
counterstained with hematoxylin. Dehydration of the sections was
performed using a standard procedure and the slides were sealed
with coverslips. For blocking experiments, the anti-EIF5A2 antibody
was incubated with a 10 times concentration of its synthetic
immunogenic peptide (dilution, 1:10; Biomatik Corporation,
Cambridge, ON, Canada) at 4°C the night prior to
immunohistochemical staining.
Evaluation of immunostaining
Three independent observers, including one
dermatopathologist, from the Departments of Dermatology and Skin
Science or Pathology of the University of British Columbia,
simultaneously evaluated and scored the nuclear EIF5A2 staining of
the TMA, one core at a time, and a consensus score was reached. The
intensity of nuclear EIF5A2 staining was scored as 0, 1+, 2+ and 3+
based on visual estimation. The percentage of nuclear
EIF5A2-positive cells was scored as follows: 1, 0–25% cells
stained; 2, 26–50% cells stained; 3, 51–75% cells stained; and 4,
76–100% cells stained. An immunoreactive score was used to
determine the level of nuclear EIF5A2 staining by multiplying the
scores of staining intensity and the percentage of positive cells.
On the basis of the immunoreactive score, the nuclear EIF5A2
staining pattern was defined as 0, negative; and 1–12, positive
(29,30).
Statistical analysis
Differences in demographics and clinicopathological
characteristics and nuclear EIF5A2 expression between patients was
evaluated by the χ2 test. Survival time was calculated
between the date of melanoma diagnosis and the date the patient
succumbed to the disease or last follow-up. Kaplan-Meier analysis
and log-rank test were used to assess the association between
nuclear EIF5A2 expression and patient survival times. The Cox
proportional hazards regression model was performed for univariate
and multivariate survival analysis. P<0.05 was considered to
indicate a statistically significant difference, and all tests were
two-sided. SPSS version 16.0 software (SPSS, Inc., Chicago, IL,
USA) was used for all analysis.
Results
Association between nuclear EIF5A2
expression and clinicopathologic characteristics of melanoma
patients
A total of 713 melanoma patients were enrolled for
TMA construction. Due to loss of biopsy cores, insufficient tumour
cells present in the cores or loss to follow-up, 382 melanoma
(primary melanoma, 242 cases; metastatic melanoma, 140 cases) and
77 cases of nevi (common acquired nevi, 28 cases; dysplastic nevi,
49 cases) were evaluated for nuclear EIF5A2 staining. A synthetic
immunogenic peptide against anti-EIF5A2 antibody was used in order
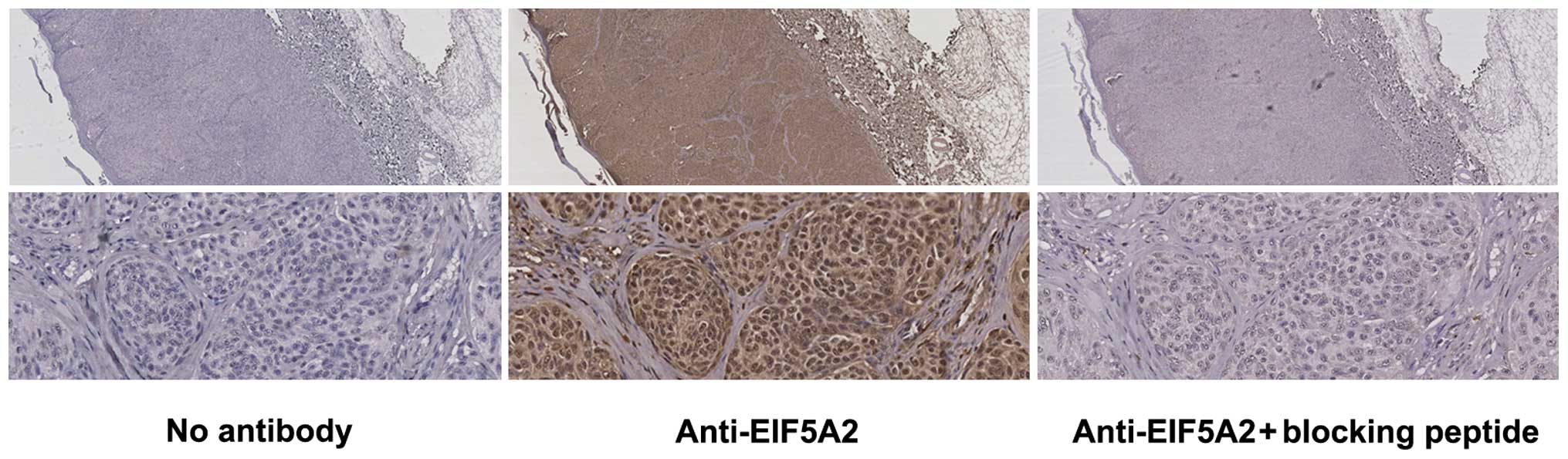
to validate the specificity of the antibody used for staining, and
the results revealed that this peptide considerably blocked nuclear
and cytoplasmic EIF5A2 staining (Fig.
1) (25). The clinical features
of the melanoma patients are listed in Table I.
 | Table I.Nuclear EIF5A2 staining and
clinicopathological characteristics of patients with melanoma. |
Table I.
Nuclear EIF5A2 staining and
clinicopathological characteristics of patients with melanoma.
|
|
| Nuclear EIF5A2
staining |
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Total, n | Positive, n
(%) | Negative, n
(%) | χ2
value | P-value |
|---|
| All melanoma
patients | 382 | 242 (63.4) | 140 (36.6) |
|
|
| Age,
years |
|
|
|
0.533 |
0.465 |
|
≤60 | 198 | 122 (61.6) | 76
(38.4) |
|
|
|
>60 | 184 | 120 (65.2) | 64
(34.8) |
|
|
|
Gender |
|
|
| 0.040 |
0.841 |
|
Male | 229 | 146 (63.8) | 83
(36.2) |
|
|
|
Female | 153 | 96
(62.7) | 57
(37.3) |
|
|
| AJCC
stage |
|
|
| 44.611 |
<0.001a |
|
I | 127 | 56
(44.1) | 71
(55.9) |
|
|
|
II | 115 | 67
(58.3) | 48
(41.7) |
|
|
|
III | 54 | 51
(94.4) | 3
(5.6) |
|
|
|
IV | 86 | 68
(79.1) | 18
(20.9) |
|
|
| Primary melanoma
patients | 242 | 123 (50.8) | 119 (49.2) |
|
|
| Age,
years |
|
|
|
1.349 |
0.245 |
|
≤61 | 123 | 58
(47.2) | 65
(52.8) |
|
|
|
>61 | 119 | 65
(54.6) | 54
(45.4) |
|
|
|
Gender |
|
|
|
1.413 |
0.235 |
|
Male | 133 | 63
(47.4) | 70
(52.6) |
|
|
|
Female | 109 | 60
(55.0) | 49
(45.0) |
|
|
| Tumor thickness,
mm |
|
|
|
4.398 |
0.036 |
|
≤2 | 134 | 60
(44.8) | 74
(55.2) |
|
|
|
>2 | 108 | 63
(58.3) | 45
(41.7) |
|
|
|
Ulceration |
|
|
|
0.705 |
0.401 |
|
Absent | 194 | 96
(49.5) | 98
(50.5) |
|
|
|
Present | 48 | 27
(56.2) | 21
(43.8) |
|
|
|
Subtype |
|
|
|
2.843 |
0.416 |
|
Lentigo
maligna | 37 | 21
(56.8) | 16
(43.2) |
|
|
|
Superficial
spreading | 89 | 39
(43.8) | 50
(56.2) |
|
|
|
Nodular | 41 | 22
(53.7) | 19
(46.3) |
|
|
|
Unspecified | 75 | 41
(54.7) | 34
(45.3) |
|
|
| Tumor
siteb |
|
|
|
0.029 |
0.865 |
|
Sun-protected | 188 | 95
(50.5) | 93
(49.5) |
|
|
|
Sun-exposed | 54 | 28
(51.9) | 26
(48.1) |
|
|
| Metastatic melanoma
patients | 140 | 119 (85.0) | 21
(15.0) |
|
|
| Age,
years |
|
|
|
0.248 |
0.618 |
|
≤59 | 73 | 61
(83.6) | 12
(16.4) |
|
|
|
>59 | 67 | 58
(86.6) | 9
(13.4) |
|
|
| Gender |
|
|
|
0.510 |
0.475 |
|
Male | 96 | 83
(86.5) | 13
(13.5) |
|
|
|
Female | 44 | 36
(81.8) | 8
(18.2) |
|
|
Thickness is one feature that is an extremely
important prognostic marker for primary melanoma patients (31). The present analysis demonstrated that
in primary melanoma patients, positive nuclear EIF5A2 expression
was exhibited by 58% of melanoma patients with a tumour thickness
of >2 mm, compared with 45% of melanoma patients with a tumour
thickness of ≤2 mm (P=0.036; Table I;
Fig. 2A). This suggests that in
primary melanoma, nuclear EIF5A2 expression may be induced during
the transition between thin and thick melanoma. In all melanoma
patients, the expression of nuclear EIF5A2 was detected in 85% of
advanced stage melanomas [American Joint Committee on Cancer (AJCC)
stages III and IV] compared with 51% of early stage melanomas (AJCC
stages I and II) (P<0.001; Table
I; Fig. 2B) (32). The association between positive
nuclear EIF5A2 expression and melanoma thickness and AJCC stages
may be an indication of the involvement of nuclear EIF5A2
expression in melanoma cell invasion.
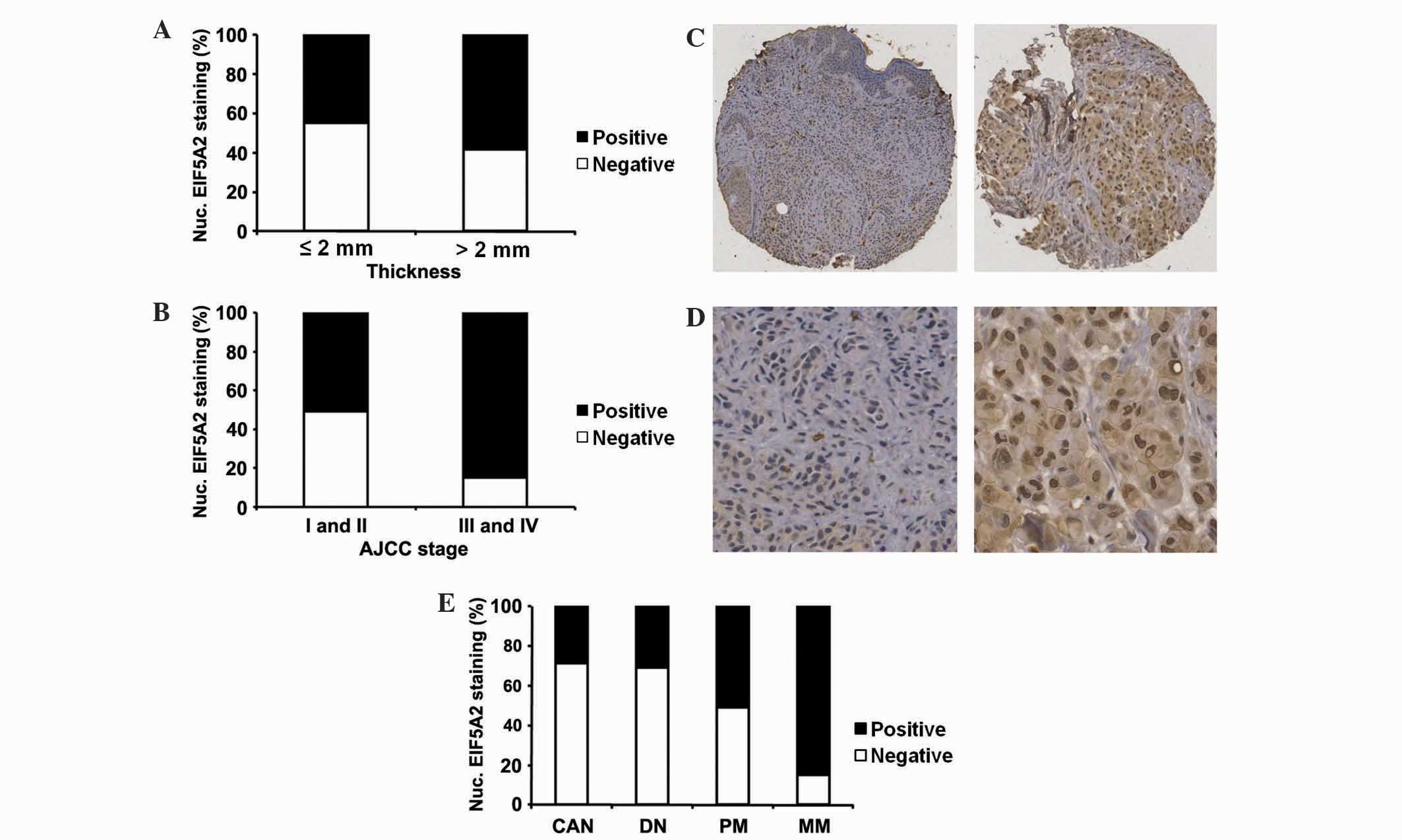 | Figure 2.Association between nuclear EIF5A2
expression and tumor thickness, AJCC stage and various stages of
melanoma progression. (A) Nuclear EIF5A2 expression was
significantly higher in melanoma patients with tumour thickness
>2 mm compared with melanoma patients with tumour thickness ≤2
mm (P=0.036; χ2 test). (B) Nuclear EIF5A2 expression was
significantly higher in advanced stage melanomas (AJCC stages III
and IV) compared with early stage melanomas (AJCC stages I and II)
(P<0.001; χ2 test). (C and D) Representative images
of nuclear EIF5A2 immunohistochemical staining in melanocytic
lesions at (C) ×100 and (D) ×400 magnification. Left panel,
negative nuclear EIF5A2 staining; right panel, positive nuclear
EIF5A2 staining. (E) Nuclear EIF5A2 expression was increased in MM
compared with CAN, DN, and PM (P<0.001; χ2 test).
Nuclear EIF5A2 expression was also increased in PM compared with
CAN and DN (P=0.010 and P=0.026, respectively; χ2 test).
EIF5A2, eukaryotic translation initiation factor 5A2; AJCC,
American Joint Committee on Cancer; CAN, common acquired nevi; DN,
dysplastic nevi; PM, primary melanoma; MM, metastatic melanoma;
Nuc., nuclear. |
Nuclear EIF5A2 expression increases
with melanoma progression
To study the alterations in the expression of
nuclear EIF5A2 with melanoma progression, immunohistochemical
staining was performed on TMA slides and samples were categorized
into negative and positive EIF5A2 staining groups (Fig. 2C and D). Positive nuclear EIF5A2
staining was observed in 29% of common acquired nevi, 31% of
dysplastic nevi, 51% of primary melanomas and 85% of metastatic
melanomas. Consequently, nuclear EIF5A2 expression was observed to
be significantly higher in primary melanomas compared with
dysplastic nevi and common acquired nevi (P=0.010 and P=0.026,
respectively; Fig. 2E) and in
metastatic melanomas compared with primary melanomas, dysplastic
nevi and common acquired nevi (P<0.001; Fig. 2E). This suggests the role of nuclear
EIF5A2 in the transformation between nevus and malignant tumors and
the development of melanoma metastasis. No difference in nuclear
EIF5A2 expression was observed between common acquired nevi and
dysplastic nevi (P=0.852; Fig.
2E).
Nuclear EIF5A2 expression is
positively associated with poor patient survival
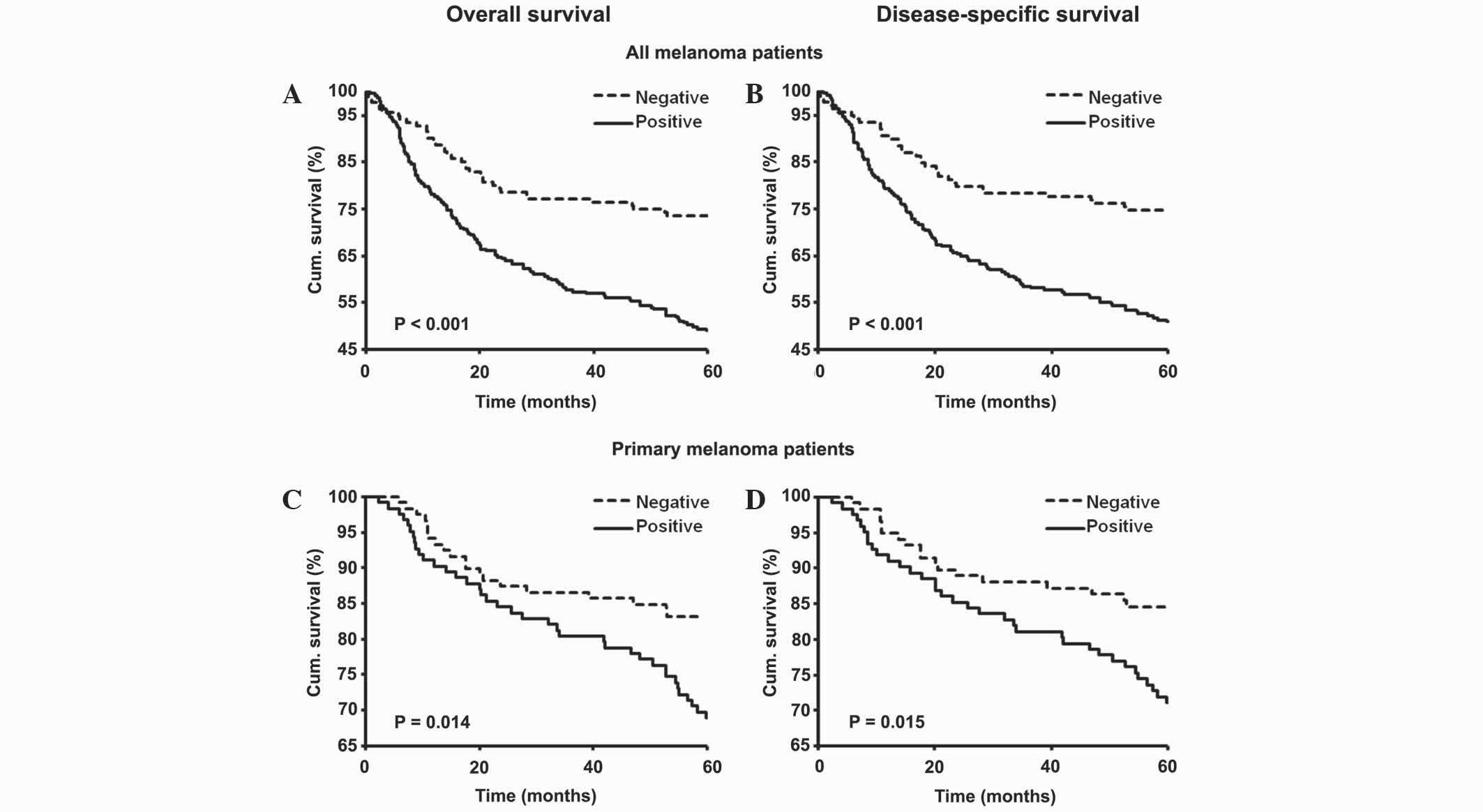
The present study evaluated the association between
nuclear EIF5A2 expression and the 5-year survival rate of primary
and metastatic melanoma patients by constructing Kaplan-Meier
survival curves. Overall and disease-specific 5-year survival rates
were poorer for all (P<0.001; Fig. 3A
and B) and primary (P=0.014 and P=0.015, respectively; Fig. 3C and D) melanoma patients with
positive staining for nuclear EIF5A2 compared with patients with
negative staining. The results from the Kaplan-Meier survival
analysis were further supported by univariate Cox proportional
hazard regression analysis, which indicated that nuclear EIF5A2
expression was a significant prognostic factor for the overall and
disease-specific 5-year survival rates of all melanoma patients
[hazards ratio (HR), 2.26 and 2.27; 95% confidence interval (CI),
1.57–3.27 and 1.56–3.31; P<0.001; Table II] and primary melanoma patients (HR,
1.95 and 2.00; 95% CI, 1.14–3.36 and 1.13–3.53; P=0.015 and
P=0.017; Table II).
 | Table II.Univariate Cox regression analysis on
5-year overall and disease-specific survival rates of 382 total
melanoma and 242 primary melanoma patients. |
Table II.
Univariate Cox regression analysis on
5-year overall and disease-specific survival rates of 382 total
melanoma and 242 primary melanoma patients.
|
|
| Overall
survival | Disease-specific
survival |
|---|
|
|
|
|
|
|---|
| Characteristics
P-valuea | Total, n (%) | Mortalities, n | Mortality rate,
% | HR (95% CI) |
P-valuea | Mortalities, n | Mortality rate,
% | HR (95% CI) |
|---|
| All melanoma
patients | 382
(100.0) | 160 |
|
|
| 152 |
|
|
|
| Nuclear
EIF5A2 expression |
|
|
Negative | 140 (36.6) | 37 | 26.4 | 1.00 | <0.001 | 35 | 25.0 | 1.00 | <0.001 |
|
Positive | 242 (63.4) | 123 | 50.8 | 2.26
(1.57–3.27) |
| 117 | 48.3 | 2.27
(1.56–3.31) |
|
| Age,
years |
|
|
≤60 | 198 (51.8) | 79 | 39.9 | 1.00 | 0.426 | 77 | 38.9 | 1.00 |
0.648 |
|
>60 | 184 (48.2) | 81 | 44.0 | 1.13
(0.83–1.55) |
| 75 | 40.1 | 1.08
(0.78–1.48) |
|
|
Gender |
|
|
Male | 229 (60.0) | 99 | 43.2 | 1.00 | 0.616 | 93 | 40.1 | 1.00 |
0.755 |
|
Female | 153 (40.0) | 61 | 39.9 | 0.92
(0.67–1.27) |
| 59 | 38.6 | 0.95
(0.68–1.32) |
|
| AJCC
stage |
|
|
I–II | 242 (63.4) | 58 | 24.0 | 1.00 | <0.001 | 53 | 22.0 | 1.00 | <0.001 |
|
III–IV | 140 (36.6) | 102 | 72.9 | 5.07
(3.66–7.03) |
| 99 | 70.7 | 5.37
(3.84–7.53) |
|
| Primary melanoma
patients | 242 (63.4) | 58 |
|
|
| 53 |
|
|
|
| Nuclear
EIF5A2 expression |
|
|
Negative | 119 (49.2) | 20 | 16.8 | 1.00 | 0.015 | 18 | 15.1 | 1.00 |
0.017 |
|
Positive | 123 (50.8) | 38 | 30.9 | 1.95
(1.14–3.36) |
| 35 | 28.5 | 2.00
(1.13–3.53) |
|
| Age,
years |
|
|
≤61 | 123 (50.8) | 19 | 15.4 | 1.00 | 0.002 | 19 | 15.4 | 1.00 |
0.010 |
|
>61 | 119 (49.2) | 39 | 32.8 | 2.41
(1.39–4.17) |
| 34 | 28.6 | 2.10
(1.20–3.69) |
|
| Gender |
|
|
Male | 133 (55.0) | 31 | 23.3 | 1.00 | 0.749 | 28 | 21.1 | 1.00 |
0.733 |
|
Female | 109 (45.0) | 27 | 24.8 | 1.07
(0.64–1.80) |
| 25 | 22.9 | 1.10
(0.64–1.88) |
|
|
Ulceration |
|
|
Absent | 194 (80.2) | 30 | 15.5 | 1.00 | <0.001 | 26 | 13.4 | 1.00 | <0.001 |
|
Present | 48
(19.8) | 28 | 58.3 | 5.38
(3.20–9.03) |
| 27 | 56.3 | 6.00
(3.49–10.31) |
|
| Tumor
thickness, mm |
|
|
≤2 | 134 (55.4) | 11 |
8.2 | 1.00 | <0.001 | 9 |
6.7 | 1.00 | <0.001 |
| Tumor
siteb |
|
|
Sun-protected | 188 (77.7) | 45 | 23.9 | 1.00 | 0.892 | 43 | 22.9 | 1.00 | 0.460 |
|
Sun-exposed | 54 (22.3) | 13 | 24.1 | 0.96
(0.52–1.78) |
| 10 | 18.5 | 0.77
(0.39–1.54) |
|
|
Subtype |
|
|
Others | 153 (63.2) | 42 | 27.5 | 1.00 | 0.129 | 38 | 24.8 | 1.00 | 0.177 |
|
Superficial
spreading | 89 (36.8) | 16 | 18.0 | 1.56
(0.88–2.78) |
| 15 | 16.9 | 1.51
(0.83–2.74) |
|
Nuclear EIF5A2 is an independent
prognostic marker for melanoma patients
Results from multivariate Cox regression analysis
revealed that nuclear EIF5A2 was an adverse independent prognostic
marker for overall and disease-specific 5-year survival rates of
all melanoma patients (HR, 1.78 and 1.77; 95% CI, 1.22–2.60 and
1.20–2.62; P=0.003 and P=0.004; Table
III) and primary melanoma patients (HR, 1.78 and 1.90; 95% CI,
1.02–3.12 and 1.06–3.43; P=0.043 and P=0.032; Table III). For the analysis, gender, age,
AJCC and EIF5A2 expression were included for all melanoma patients,
and gender, age, tumor thickness, ulceration status, tumor site,
histological subtype and EIF5A2 expression were included for
primary melanoma patients.
 | Table III.Multivariate Cox regression analysis
indicating that nuclear EIF5A2 expression is an adverse independent
prognostic marker for 5-year survival rates of melanoma
patients. |
Table III.
Multivariate Cox regression analysis
indicating that nuclear EIF5A2 expression is an adverse independent
prognostic marker for 5-year survival rates of melanoma
patients.
|
| Overall
survival | Disease-specific
survival |
|---|
|
|
|
|
|---|
|
Characteristics | β | SE | HR | 95% CI | P-value | β | SE | HR | 95% CI | P-value |
|---|
| All melanoma
(n=382) |
|
| Nuclear
EIF5A2 (neg vs. pos) |
0.576 | 0.194 | 1.778 | 1.22–2.60 |
0.003 |
0.575 | 0.199 | 1.777 | 1.20–2.62 |
0.004 |
| Age
(≤60 vs. >60 years) |
0.099 | 0.159 | 1.104 | 0.81–1.51 |
0.534 |
0.050 | 0.163 | 1.052 | 0.76–1.45 |
0.758 |
| Gender
(male vs. female) |
0.168 | 0.169 | 1.183 | 0.85–1.65 |
0.319 |
0.206 | 0.173 | 1.229 | 0.88–1.72 |
0.232 |
| AJCC
stage (1 + 2 + 3 vs. 4) |
1.174 | 0.173 | 3.236 | 2.31–4.54 | <0.001 |
1.215 | 0.177 | 3.372 | 2.38–4.77 | <0.001 |
| Primary melanoma
(n=242) |
|
| Nuclear
EIF5A2 (neg vs. pos) |
0.578 | 0.286 | 1.783 | 1.02–3.12 |
0.043 |
0.643 | 0.300 | 1.902 | 1.06–3.43 |
0.032 |
| Age
(≤61 vs. >61 years) |
0.269 | 0.298 | 1.309 |
0.73–2.35 |
0.367 |
0.080 | 0.307 | 1.083 | 0.59–1.98 |
0.795 |
| Gender
(male vs. female) | −0.107 | 0.276 | 0.899 |
0.52–1.55 |
0.699 | −0.110 | 0.288 | 0.896 | 0.51–1.58 |
0.704 |
|
Ulceration (absent vs.
present) |
1.134 | 0.297 | 3.109 |
1.74–5.57 | <0.001 |
1.269 | 0.310 | 3.559 | 1.94–6.54 | <0.001 |
|
Thickness (≤2 vs. >2
mm) |
1.410 | 0.362 | 4.095 |
2.01–8.33 | <0.001 |
1.563 | 0.394 | 4.772 |
2.20–10.34 | <0.001 |
| Site
(sun-protected vs. exposed) | −0.335 | 0.322 | 0.715 |
0.38–1.34 |
0.298 | −0.556 | 0.358 | 0.573 | 0.28–1.16 |
0.120 |
| Subtype
(superficial vs. others) |
0.052 | 0.301 | 1.054 |
0.58–1.90 |
0.862 |
0.012 | 0.313 | 1.012 | 0.55–1.87 |
0.969 |
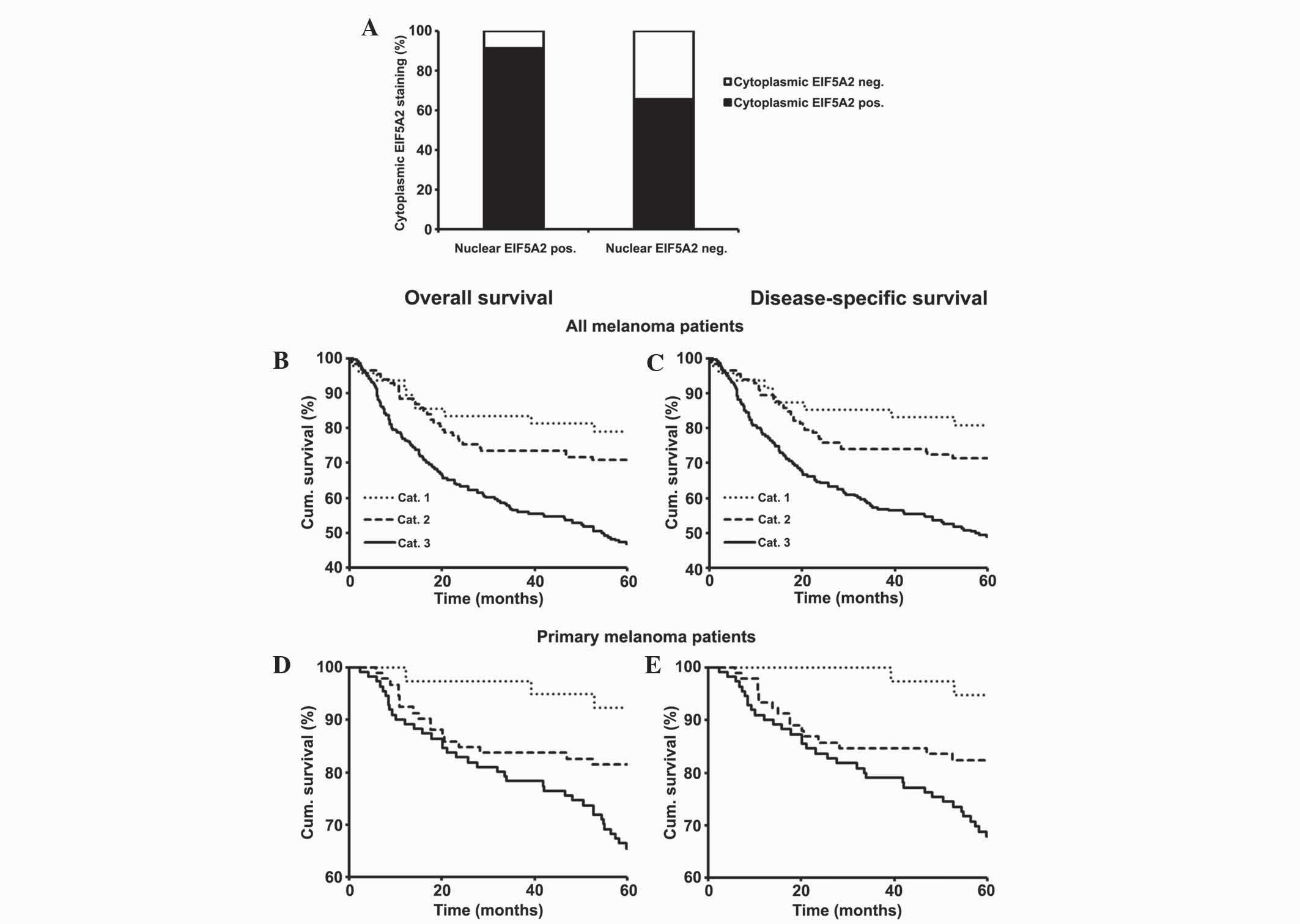
Concurrent cytoplasmic and nuclear
EIF5A2 expression is correlated with a poorer 5-year survival rate
for all and primary melanoma patients
Previously, the present authors investigated the
expression of cytoplasmic EIF5A2 in melanoma using TMA and revealed
that cytoplasmic EIF5A2 expression is a prognostic marker for
melanoma patients (25). The present
study analyzed the association between cytoplasmic and nuclear
expression of EIF5A2 using the 382 melanoma cases, and the results
revealed that there was a direct association between the positive
staining of cytoplasmic and nuclear EIF5A2 (P<0.001; Fig. 4A). To further examine this association
and its effect on patient survival, the melanoma samples were
classified into three groups according to their staining as
follows: Category 1, negative cytoplasmic and nuclear EIF5A2
staining; category 2, negative cytoplasmic and positive nuclear
EIF5A2 or positive cytoplasmic and negative nuclear EIF5A2
staining; category 3, positive cytoplasmic and nuclear EIF5A2
staining. Based on the results from Kaplan-Meier survival analysis,
overall and disease-specific 5-year survival rates for all
(P<0.001; Fig. 4B and C) and
primary (P=0.002; Fig. 4D and E)
melanoma patients were poorest for category 3 patients, best for
category 1 patients and intermediate for category 2 patients.
Furthermore, multivariate Cox regression analysis demonstrated that
the simultaneous positive expression of cytoplasmic and nuclear
EIF5A2 (category 3) was an independent prognostic factor for
overall and disease-specific 5-year survival for all (HR, 1.87 and
1.87; 95% CI, 1.31–2.68 and 1.28–2.67; P=0.001 and P=0.001;
Table IV) and primary (HR, 2.01 and
2.11; 95% CI, 1.15–3.51 and 1.17–3.78; P=0.014 and P=0.013;
Table IV) melanoma patients.
 | Table IV.Multivariate Cox regression analysis
indicating that the simultaneous expression of cytoplasmic and
nuclear EIF5A2 is an adverse independent prognostic marker for
5-year survival rates of melanoma patients. |
Table IV.
Multivariate Cox regression analysis
indicating that the simultaneous expression of cytoplasmic and
nuclear EIF5A2 is an adverse independent prognostic marker for
5-year survival rates of melanoma patients.
|
| Overall
survival | Disease-specific
survival |
|---|
|
|
|
|
|---|
|
Characteristics | β | SE | HR | 95% CI | P-value | β | SE | HR | 95% CI | P-value |
|---|
| All melanoma
(n=382) |
|
| EIF5A2
(cat. 1 + 2 vs. 3) |
0.626 | 0.184 | 1.871 | 1.31–2.68 |
0.001 |
0.614 | 0.188 | 1.848 | 1.28–2.67 |
0.001 |
| Age
(≤60 vs. >60 years) |
0.112 | 0.159 | 1.118 | 0.82–1.53 |
0.483 |
0.063 | 0.163 | 1.065 | 0.77–1.47 |
0.698 |
| Gender
(male vs. female) |
0.195 | 0.170 | 1.216 | 0.87–1.70 |
0.251 |
0.234 | 0.174 | 1.264 | 0.90–1.78 |
0.178 |
| AJCC
stage (1 + 2 + 3 vs. 4) |
1.169 | 0.174 | 3.220 | 2.29–4.52 | <0.001 |
1.214 | 0.178 | 3.365 | 2.38–4.77 | <0.001 |
| Primary melanoma
(n=242) |
|
| EIF5A2
(cat. 1 + 2 vs. 3) |
0.699 | 0.284 | 2.012 | 1.15–3.51 |
0.014 |
0.745 | 0.299 | 2.107 | 1.17–3.78 |
0.013 |
| Age
(≤61 vs. >61 years) |
0.328 | 0.299 | 1.388 |
0.77–2.50 |
0.273 |
0.132 | 0.309 | 1.141 | 0.62–2.09 |
0.670 |
| Gender
(male vs. female) | −0.128 | 0.277 | 0.880 |
0.51–1.51 |
0.644 | −0.125 | 0.289 | 0.882 | 0.50–1.55 |
0.665 |
|
Ulceration (absent vs.
present) |
1.107 | 0.296 | 3.026 |
1.69–5.41 | <0.001 |
1.245 | 0.310 | 3.472 | 1.89–6.37 | <0.001 |
|
Thickness (≤2 vs. >2
mm) |
1.355 | 0.363 | 3.876 |
1.90–7.89 | <0.001 |
1.505 | 0.395 | 4.506 | 2.08–9.77 | <0.001 |
| Site
(sun-protected vs. exposed) | −0.352 | 0.320 | 0.704 |
0.38–1.32 |
0.272 | −0.573 | 0.356 | 0.564 | 0.28–1.13 |
0.108 |
| Subtype
(superficial vs. others) |
0.054 | 0.301 | 1.056 |
0.59–1.90 |
0.857 |
0.015 | 0.312 | 1.015 | 0.55–1.87 |
0.962 |
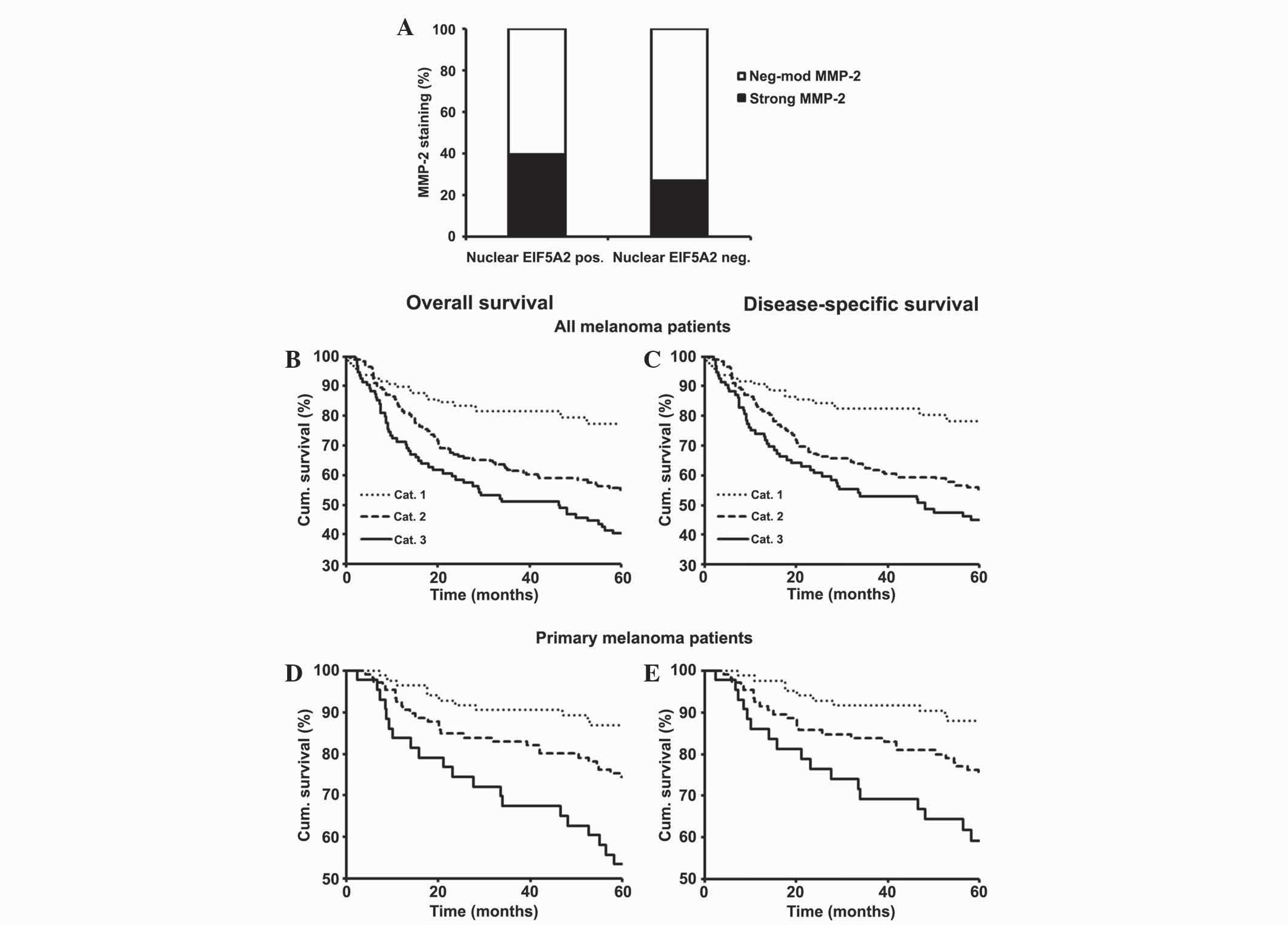
Simultaneous expression of nuclear
EIF5A2 and MMP-2 is associated with poorer 5-year survival rates
for all and primary melanoma patients
Results from the TMA study suggested the involvement
of nuclear EIF5A2 for developing melanoma invasion, metastasis and
poor patient survival. Since cell invasion is one of the hallmarks
of cancer that may lead to increased metastasis and poor patient
survival, the association between the expression of nuclear EIF5A2
and MMP-2, which is an important factor in the promotion of cancer
cell invasion, was evaluated (33). A
previous TMA study by the same group indicated that MMP-2
expression was a prognostic marker for melanoma (34). As a result, the present study examined
the association between the expression of nuclear EIF5A2 and MMP-2
using the same 369 melanoma cases that had been previously analyzed
in the aforementioned TMA study (34). The result revealed that positive
staining of nuclear EIF5A2 was directly associated with strong
MMP-2 expression (P=0.015; Fig. 5A).
To more extensively study the association between MMP-2 and nuclear
EIF5A2 expression and their effects on patient survival, the
samples were classified into three groups based on their staining
as follows: Category 1, negative nuclear EIF5A2 expression and
negative-moderate MMP-2 expression; category 2, negative nuclear
EIF5A2 expression and strong MMP-2 expression or positive nuclear
EIF5A2 expression and negative-moderate MMP-2 expression; category
3, positive EIF5A2 expression and strong MMP-2 expression.
Kaplan-Meier survival analyses revealed that overall and
disease-specific 5-year survival outcomes for all (P<0.001;
Fig. 5B and C) and primary melanoma
(P<0.001 and P=0.001, respectively; Fig. 5D and E) patients were most favorable
for category 1, least favorable for category 3 and moderate for
patients in category 2.
Discussion
Recently, the present authors demonstrated that
there was an increase in cytoplasmic EIF5A2 expression with
melanoma progression (25);
therefore, additional investigation of the nuclear expression of
EIF5A2 using TMA data consisting of 459 melanocytic lesions was
undertaken. The present study revealed that an increase in nuclear
EIF5A2 expression was significantly associated with melanoma
progression, thickness and AJCC stage. Nuclear EIF5A2 expression
was significantly associated with poorer overall and
disease-specific 5-year survival rates of all and primary melanoma
patients. This may be a result of the direct association identified
by the present study between nuclear EIF5A2 expression and melanoma
thickness and AJCC stage, which is also consistent with the
hypothesis that EIF5A2 is an oncogene in various cancers (35). Notably, nuclear expression of EIF5A2
and a combination of nuclear and cytoplasmic expression of EIF5A2
were identified as independent prognostic markers for overall and
disease specific 5-year survival of all and primary melanoma
patients.
Furthermore, the present study observed a direct
association between nuclear EIF5A2 expression and a strong
expression of MMP-2, which may demonstrate why nuclear EIF5A2
expression is directly correlated with melanoma thickness and a
poorer 5-year survival rate for melanoma patients. In addition,
simultaneous negative-moderate MMP-2 expression and loss of nuclear
EIF5A2 expression (category 3) was demonstrated to be associated
with an improved 5-year survival rate compared with either strong
MMP-2 expression and loss of nuclear EIF5A2 expression or positive
expression of nuclear EIF5A2 and negative-moderate expression of
MMP-2 (category 2). This may possibly be a rational for dual
therapeutic targeting of nuclear EIF5A2 and MMP-2 in melanoma
patients; however, this requires additional study. The importance
of MMP-2 in melanoma has also been demonstrated by other studies.
MT1-MMP was demonstrated to increase melanoma cell invasion and
motility by activating its target MMP-2 (36). Another study identified that
expression of activated MMP-2 in a melanoma xenograft model is
associated with increased malignancy, highlighting the role of
MMP-2 in melanoma invasion and metastasis (37).
The present study also compared the nuclear and
cytoplasmic EIF5A2 expression in the same cases of melanocytic
lesions and revealed a significant association between positive
staining of nuclear and cytoplasmic EIF5A2. However, this
association is not perfect, which may indicate the differential
regulation of nuclear and cytoplasmic EIF5A2 expression in
melanoma. Notably, the present results revealed that simultaneous
expression of nuclear and cytoplasmic EIF5A2 (category 3) was
associated with a poorer survival outcome compared with the
expression of only one form of EIF5A2 (category 2), which was
associated with an intermediate survival outcome. This suggests
that oncogenic properties of nuclear and cytoplasmic EIF5A2 may at
least partly differ from each other in melanoma. Therefore,
concurrent expression of the two forms (category 3) may have
synergistic or additive effects on melanoma progression, metastasis
and patient survival. Previous in vitro results by the
present authors, indicated that EIF5A2 promotes melanoma cell
invasion partly via increasing the activity of MMP-2 (25). However, further investigation is
required to determine which form of EIF5A2 functions this way, and
to what extent it is responsible for this role.
To the best of our knowledge, the present study is
the first to report the nuclear expression of EIF5A2 and its
importance in melanoma. However, the presence of EIF5A2 in the
nuclei of HCC cells has been previously demonstrated (24). Exportin 4 (Xpo4) is a tumour
suppressor that belongs to the importin-β family of nuclear
transporters, and EIF5A is known to be a substrate of Xpo4
(8,38). Knockdown of Xpo4 in murine hepatoma
cells leads to nuclear accumulation of EIF5A1 and EIF5A2, which
significantly increases the in vitro proliferation of murine
liver progenitor cells (24). In a
human HCC cell line, XPO4 inactivation was revealed to contribute
to tumour maintenance. In the same study, EIF5A2 was required for
efficient proliferation of cells lacking XPO4, suggesting the
importance of nuclear accumulation of EIF5A2 in mediating the
oncogenic effects associated with XPO4 loss (24). A similar phenomenon is observed when
an increase or decrease in the nuclear accumulation of β-catenin
and FOXO affects tumorigenesis as a result of deregulation of the
WNT and AKT signalling pathways, respectively (39). In addition, nuclear expression of
EIF5A2 has also been observed in the human bladder carcinoma 5637
cell line (23).
In conclusion, the present study examined the
expression profile of nuclear EIF5A2 and revealed that nuclear
EIF5A2 expression is increased during melanoma progression and is
associated with a poor 5-year survival rate in all melanoma and
primary melanoma patients. In addition, the present study
identified that nuclear EIF5A2 was an independent prognostic factor
for the 5-year survival of all and primary melanoma patients,
suggesting that nuclear EIF5A2 may be a potential target candidate
for melanoma therapy. Simultaneous expression of cytoplasmic and
nuclear EIF5A2 was associated with a poor survival outcome as well.
Similar to HCC, the presence of nuclear and cytoplasmic EIF5A2 may
be a reason for the existence of a shuttling mechanism between the
cytoplasm and nucleus in melanoma. Additional investigation is
required to further determine the mechanisms behind the subcellular
localization of EIF5A2, the biological functions of nuclear EIF5A2
and its role in the tumorigenesis of melanoma.
Acknowledgements
The authors would like to thank Dr Ladan Fazli from
the Vancouver Prostate Centre (Vancouver, Canada) for her advice in
pathology and Dr Anand Rotte from the Department of Dermatology and
Skin Science of the University of British Columbia (Vancouver,
Canada) for providing the MMP-2 database used in the present study
for the correlation analysis between the expression of nuclear
EIF5A2 and MMP-2. The present study was funded by grants from the
Canadian Institutes of Health Research (Ottawa, Canada; grant nos.
MOP-93810, MOP-110974 and CCI-117958), the Canadian Dermatology
Foundation (Ottawa, Canada; grant no. CDFYZ1315) and the Canadian
Cancer Society Research Institute (Toronto, Canada; grant no.
CCS-2-11-7007). Mr. Shahram Khosravi received a scholarship from
the Natural Sciences and Engineering Research Council of Canada
(Ottawa, Canada; grant no. NSERC IPS 9415).
References
|
1
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl
J Med. 355:51–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Linos E, Swetter SM, Cockburn MG, Colditz
GA and Clarke CA: Increasing burden of melanoma in the United
States. J Invest Dermatol. 129:1666–1674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cummins DL, Cummins JM, Pantle H,
Silverman MA, Leonard AL and Chanmugam A: Cutaneous malignant
melanoma. Mayo Clin Proc. 81:500–507. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saini P, Eyler DE, Green R and Dever TE:
Hypusine-containing protein eIF5A promotes translation elongation.
Nature. 459:118–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel PH, Costa-Mattioli M, Schulze KL and
Bellen HJ: The Drosophila deoxyhypusine hydroxylase homologue nero
and its target eIF5A are required for cell growth and the
regulation of autophagy. J Cell Biol. 185:1181–1194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hauber I, Bevec D, Heukeshoven J, Krätzer
F, Horn F, Choidas A, Harrer T and Hauber J: Identification of
cellular deoxyhypusine synthase as a novel target for
antiretroviral therapy. J Clin Invest. 115:76–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kruse M, Rosorius O, Kratzer F, Bevec D,
Kuhnt C, Steinkasserer A, Schuler G and Hauber J: Inhibition of
CD83 cell surface expression during dendritic cell maturation by
interference with nuclear export of CD83 mRNA. J Exp Med.
191:1581–1590. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lipowsky G, Bischoff FR, Schwarzmaier P,
Kraft R, Kostka S, Hartmann E, Kutay U and Görlich D: Exportin 4: A
mediator of a novel nuclear export pathway in higher eukaryotes.
EMBO J. 19:4362–4371. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hutten S and Kehlenbach RH: CRM1-mediated
nuclear export: To the pore and beyond. Trends Cell Biol.
17:193–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maier B, Ogihara T, Trace AP, Tersey SA,
Robbins RD, Chakrabarti SK, Nunemaker CS, Stull ND, Taylor CA,
Thompson JE, et al: The unique hypusine modification of eIF5A
promotes islet beta cell inflammation and dysfunction in mice. J
Clin Invest. 120:2156–2170. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuk D and Jacobson A: A single amino acid
substitution in yeast eIF-5A results in mRNA stabilization. EMBO J.
17:2914–2925. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schrader R, Young C, Kozian D, Hoffmann R
and Lottspeich F: Temperature-sensitive eIF5A mutant accumulates
transcripts targeted to the nonsense-mediated decay pathway. J Biol
Chem. 281:35336–35346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jenkins ZA, Hååg PG and Johansson HE:
Human eIF5A2 on chromosome 3q25-q27 is a phylogenetically conserved
vertebrate variant of eukaryotic translation initiation factor 5A
with tissue-specific expression. Genomics. 71:101–109. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clement PM, Henderson CA, Jenkins ZA,
Smit-McBride Z, Wolff EC, Hershey JW, Park MH and Johansson HE:
Identification and characterization of eukaryotic initiation factor
5A-2. Eur J Biochem. 270:4254–4263. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guan XY, Fung JM, Ma NF, Lau SH, Tai LS,
Xie D, Zhang Y, Hu L, Wu QL, Fang Y and Sham JS: Oncogenic role of
eIF-5A2 in the development of ovarian cancer. Cancer Res.
64:4197–4200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marchet A, Mocellin S, Belluco C, Ambrosi
A, DeMarchi F, Mammano E, Digito M, Leon A, D'Arrigo A, Lise M and
Nitti D: Gene expression profile of primary gastric cancer: Towards
the prediction of lymph node status. Ann Surg Oncol. 14:1058–1064.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie D, Ma NF, Pan ZZ, Wu HX, Liu YD, Wu
GQ, Kung HF and Guan XY: Overexpression of EIF-5A2 is associated
with metastasis of human colorectal carcinoma. Hum Pathol.
39:80–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu W, Cai MY, Tong ZT, Dong SS, Mai SJ,
Liao YJ, Bian XW, Lin MC, Kung HF, Zeng YX, et al: Overexpression
of EIF5A2 promotes colorectal carcinoma cell aggressiveness by
upregulating MTA1 through C-myc to induce
epithelial-mesenchymaltransition. Gut. 61:562–575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang DJ, Dong SS, Ma NF, Xie D, Chen L, Fu
L, Lau SH, Li Y, Li Y and Guan XY: Overexpression of eukaryotic
initiation factor 5A2 enhances cell motility and promotes tumor
metastasis in hepatocellular carcinoma. Hepatology. 51:1255–1263.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang GF, Xie D, Liu JH, Luo JH, Li LJ, Hua
WF, Wu HM, Kung HF, Zeng YX and Guan XY: Expression and
amplification of eIF-5A2 in human epithelial ovarian tumors and
overexpression of EIF-5A2 is a new independent predictor of outcome
in patients with ovarian carcinoma. Gynecol Oncol. 112:314–318.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He LR, Zhao HY, Li BK, Liu YH, Liu MZ,
Guan XY, Bian XW, Zeng YX and Xie D: Overexpression of eIF5A-2 is
an adverse prognostic marker of survival in stage I non-small cell
lung cancer patients. Int J Cancer. 129:143–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen W, Luo JH, Hua WF, Zhou FJ, Lin MC,
Kung HF, Zeng YX, Guan XY and Xie D: Overexpression of EIF-5A2 is
an independent predictor of outcome in patients of urothelial
carcinoma of the bladder treated with radical cystectomy. Cancer
Epidemiol Biomarkers Prev. 18:400–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei JH, Cao JZ, Zhang D, Liao B, Zhong WM,
Lu J, Zhao HW, Zhang JX, Tong ZT, Fan S, et al: EIF5A2 predicts
outcome in localised invasive bladder cancer and promotes bladder
cancer cell aggressiveness in vitro and in vivo. Br J Cancer.
110:1767–1777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zender L, Xue W, Zuber J, Semighini CP,
Krasnitz A, Ma B, Zender P, Kubicka S, Luk JM, Schirmacher P, et
al: An oncogenomics-based in vivo RNAi screen identifies tumor
suppressors in liver cancer. Cell. 135:852–864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khosravi S, Wong RP, Ardekani GS, Zhang G,
Martinka M, Ong CJ and Li G: Role of EIF5A2, a downstream target of
Akt, in promoting melanoma cell invasion. Br J Cancer. 110:399–408.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
World Medical Association, . World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dai DL, Martinka M and Li G: Prognostic
significance of activated Akt expression in melanoma: A
clinicopathologic study of 292 cases. J Clin Oncol. 23:1473–1482.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Z, Chen G, Cheng Y, Martinka M and
Li G: Prognostic significance of RUNX3 expression in human
melanoma. Cancer. 117:2719–2727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen G, Cheng Y, Tang Y, Martinka M and Li
G: Role of Tip60 in human melanoma cell migration, metastasis, and
patient survival. J Invest Dermatol. 132:2632–2641. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jafarnejad SM, Ardekani GS, Ghaffari M,
Martinka M and Li G: Sox4-mediated Dicer expression is critical for
suppression of melanoma cell invasion. Oncogene. 32:2131–2139.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soong SJ, Shaw HM, Balch CM, McCarthy WH,
Urist MM and Lee JY: Predicting survival and recurrence in
localized melanoma: A multivariate approach. World J Surg.
16:191–195. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rotte A, Martinka M and Li G: MMP2
expression is a prognostic marker for primary melanoma patients.
Cell Oncol (Dordr). 35:207–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang FW, Guan XY and Xie D: Roles of
eukaryotic initiation factor 5A2 in human cancer. Int J Biol Sci.
9:1013–1020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shaverdashvili K, Wong P, Ma J, Zhang K,
Osman I and Bedogni B: MT1-MMP modulates melanoma cell
dissemination and metastasis through activation of MMP2 and RAC1.
Pigment Cell Melanoma Res. 27:287–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hofmann UB, Westphal JR, Waas ET, Zendman
AJ, Cornelissen IM, Ruiter DJ and van Muijen GN: Matrix
metalloproteinases in human melanoma cell lines and xenografts:
Increased expression of activated matrix metalloproteinase-2
(MMP-2) correlates with melanoma progression. Br J Cancer.
81:774–782. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kurisaki A, Kurisaki K, Kowanetz M, Sugino
H, Yoneda Y, Heldin CH and Moustakas A: The mechanism of nuclear
export of Smad3 involves exportin 4 and Ran. Mol Cell Biol.
26:1318–1332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kau TR, Way JC and Silver PA: Nuclear
transport and cancer: From mechanism to intervention. Nat Rev
Cancer. 4:106–117. 2004. View
Article : Google Scholar : PubMed/NCBI
|