Introduction
Worldwide, ~1.2 million patients are diagnosed with
colorectal cancer each year (1). Up
to one-quarter of these patients present with advanced disease at
diagnosis, and ~25% of patients with early stage disease relapse at
some point during the disease course (2). Although prognosis has greatly improved
over the past decades, due to significant surgical and medical
advances, once the tumour has progressed beyond surgical
resectability the disease is essentially incurable and the median
survival is ~2 years with the best available systemic therapy
(3). Treatment selection for patients
is affected by the treatment objective, overall condition and
comorbidity of the patient, and mutational state of the RAS
gene (4). It has recently been
identified that patients that are not only selected by mutation
status of the KRAS gene, but also the NRAS gene,
improve when a drug targeting the epidermal growth factor receptor
(EGFR) is incorporated into the treatment plan (5,6). However,
patients that present with a mutation in one of these genes may
experience a detrimental effect on survival by combining an
anti-EGFR drug with a chemotherapy regimen (5). Therefore, in patients with wild-type
RAS tumours (absence of mutations in exons 2, 3 and 4 of the
KRAS gene, and in mutations in exons 2, 3 and 4 of the
NRAS gene), it is currently possible to combine
chemotherapy, including fluoropyrimidine, oxaliplatin and
irinotecan-based therapies, with anti-EGFR therapies, including
cetuximab and panitumumab, or with antiangiogenic agents, such as
bevacizumab and aflibercept (2). In
addition to their predictive value, KRAS mutations have
prognostic significance, since patients with mutated KRAS
appear to have lower survival rates compared with patients with
wild-type KRAS (34.9 vs. 37.8 months, respectively)
(7). Survival is even lower (21.2
months) in cases of metastatic colorectal cancer (mCRC), where
mutations are expressed in the BRAF gene (7). In addition to a poorer prognosis, this
means that cases of mCRC with mutated RAS (KRAS and
NRAS), which is ~50% of the mCRC population, have fewer
treatment options available (6). In
this group of patients the only biological agents that have
demonstrated efficacy with chemotherapy are anti-vascular
endothelial growth factor (VEGF) drugs, including bevacizumab and
aflibercept.
Bevacizumab is a humanised monoclonal antibody that
binds to VEGF-A and prevents interaction between VEGF-A and VEGF
receptor (VEGFR) (8,9). Bevacizumab is the only anti-VEGF agent
approved for use in first-line treatment of mCRC and its efficacy
is independent of RAS family mutation status (10). By contrast, aflibercept is a
genetically engineered fusion protein that contains VEGFR-2
receptor domain 1 and VEGFR-1 receptor domain 2, with the ability
to ‘sequester’ all isoforms of VEGF, including VEGF-A, VEGF-B and
placental growth factor (PIGF) (11).
Aflibercept blocks the main VEGF factors that are involved in
tumoural angiogenesis, unlike other antiangiogenic drugs that act
against a single factor of the VEGF family (e.g. bevacizumab and
VEGF-A). This allows the control of escape routes that are
activated when only one of these factors is blocked. For example,
increased levels of PIGF have been associated with resistance to
bevacizumab (12). Aflibercept is
approved in combination with irinotecan, 5-fluorouracil (5FU) and
folinic acid treatment (FOLFIRI) in patients with mCRC, who have
disease progression following oxaliplatin treatment (13). Additionally, it is approved for use
independent of the patient's RAS gene family mutation
status, Eastern Cooperative Oncology Group (ECOG) performance
status (PS) or whether the patient has received bevacizumab
(14). Subsequent to the granting of
the Marketing Authorization in Europe (February 2012), a
Compassionate Use Program was approved, which provided early access
to aflibercept until it was marketed in Spain (September 2013),thus
increasing the amount of safety data on the general population
outside of randomised clinical trials.
The aim of the present study is to report the
experience of patients that have been prescribed with aflibercept
within the Compassionate Use Program at two centres: San Cecilio
Clinical Hospital (Granada, Spain) and Costa del Sol Hospital
(Marbella, Spain).
Case report
General patient information
All patients were characterised as having previously
received one line of treatment, including another antiangiogenic
agent, as follows: Bevacizumab combined with modified FOLFOX6
(FOLFOX6m) (oxaliplatin, 85 mg/m2 on day 1; leucovorin,
200 mg/m2 on day 1; 5FU bolus, 400 mg/m2 on
day 1; 5FU continuous infusion, 2,400 mg/m2 on day 1;
bevacizumab, 5 mg/kg on day 1; 14 day cycle), or XELOX
(capecitabine, 1,000 mg/m2 every 12 h on days 1–14;
oxaliplatin, 130 mg/m2 on day 1; bevacizumab 7.5 mg/kg
on day 1; 21 day cycle). At the progression of disease, the
patients received FOLFIRI in combination with aflibercept
(irinotecan, 180 mg/m2 on day 1; leucovorin, 200
mg/m2 on day 1; 5FU bolus, 400 mg/m2 on day
1; 5FU continuous infusion, 2,400 mg/m2 on day 1;
aflibercept, 4 mg/kg on day 1; 14 day cycle) for a specific number
of cycles, which in many cases was greater than the median number
of cycles received in the VELOUR study (11).
Table I summarizes the
main clinical characteristics of the patients included in the
present study, as well as the efficacy of the treatment
administered in each of the cases. All patients presented with
mCRC, as well as mutated KRAS in codon 12, except for case 1
and 5, whose mutations were localised in codon 13, specifically a
G13D/13ASP mutation. Patients were informed about their inclusion
in the compassionate use program by their treating physician and
all gave their informed consent in writing prior to inclusion.
 | Table I.Characteristics of five patients
included in the present study and their treatment response. |
Table I.
Characteristics of five patients
included in the present study and their treatment response.
| Case no. | 1 | 2 | 3 | 4 | 5 |
|---|
| Age, years | 40 | 54 | 66 | 57 | 60 |
| Gender | Male | Female | Female | Female | Male |
| PS, second
line | 0 | 0 | 1 | 0 | 1 |
| KRAS
mutation | C 13 | C 12 | C 12 | C 12 | C 13 |
|
| (13Asp/G13D) | (12Asp/G12D) | (12Arg/G12R) | (12Val/G12V) | (13Asp/G13D) |
| Primary tumour
location | Rectum | Rectum | Transverse
colon | Splenic angle of
the colon | Hepatic angle of
the colon |
| Metastasis
location | Liver and lung | Liver and lung | Liver | Liver and lymph
node | Liver, lymph node,
lung and bone |
| No. of cycles,
second line | 12 | 29 | 14 | 19 | 8 |
| No. of cycles,
aflibercept | 12a | 28 | 11a | 17 | 3 |
| Radiological
response | CR | SD | SD | PR | SD |
| PFS, second line,
months | 8 | 19 | 27 | 11 | 5 |
| OS, second line,
months | >41 | 36 | >36 | 17 | 6 |
| OS, first line,
months | >56 | 47 | >67 | 41 | 12 |
Case 1
A 39 year-old male presented to San Cecilio Clinical
Hospital in October 2011 with rectal bleeding and epigastric pain,
which had been ongoing for 4 months, with no other notable history.
A colonoscopy was performed in October 2011, which revealed the
presence of a stenosing mass 10–12 cm from the anal margin. A
biopsy confirmed adenocarcinoma. Analysis revealed that the patient
had a significant elevation in carcinoembryonic antigen (CEA; 140
ng/ml; normal range, 0–5 ng/ml) and carbohydrate antigen 19–9 (CA
19-9; 544 U/ml; normal range, 0–37 U/ml). Other parameters were
within the normal ranges. A thoracic/abdominal computerised
tomography (CT) scan revealed the presence of three hepatic lesions
(segments VII–VIII, 38 mm in size; segment II, 30 mm; segments
I–VI, 15 mm), all of which were consistent with metastases. In the
primary tumour biopsy, a KRAS mutation was identified at
codon 13 of exon 2 (G13D or 13ASP). The patient received first-line
chemotherapy with FOLFOX6m and bevacizumab, with markers
normalising from the second cycle onward. Subsequent to 9 cycles, a
partial radiological response was achieved, allowing intervention
in May 2012 by lower anterior resection, bisegmentectomy II–III and
metastasectomy of atypical segment VIII; no lesion was identified
in segment I. The pathological stage of the tumour was pT3N0M1,
with moderate differentiation and Mandard tumour regression grade
(TRG) of 3, which indicates predominant fibrosis over residual
cancerous tissue, with two adenocarcinoma liver metastases of 15 mm
and 25 mm. The patient completed 12 cycles of FOLFOX6m-bevacizumab,
without oxaliplatin due to neurotoxicity, and received pelvic
radiotherapy (45 Gy; 1.8 Gy/fraction). The patient underwent
periodic review (tumour markers and CT evaluation every 3 months)
with no evidence of disease progression in October 2012.
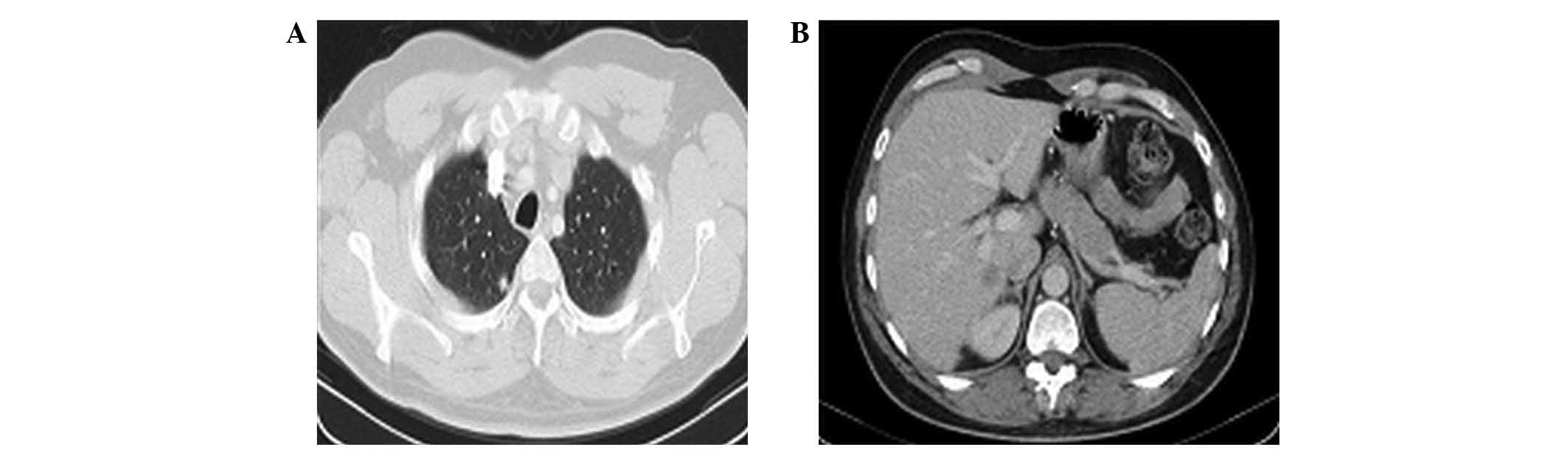
In February 2013, a CT scan revealed bilateral
sub-centimetre pulmonary metastases and a 15 mm liver metastasis on
segment I (Fig. 1). The
Multidisciplinary Committee considered the patient a candidate for
palliative chemotherapy. The patient had an ECOG PS score of 0, and
received second line treatment with FOLFIRI-aflibercept, within the
aflibercept Compassionate Use Program. A follow-up CT scan was
performed in May 2013, following 7 cycles, which revealed a small
remaining pulmonary nodule in the right upper lobe, another part of
a nodule in the right lower lobe next to the sulcus, and another
small nodule in the left lower lobe without hepatic involvement.
The response assessment was partial response (PR) for the lungs and
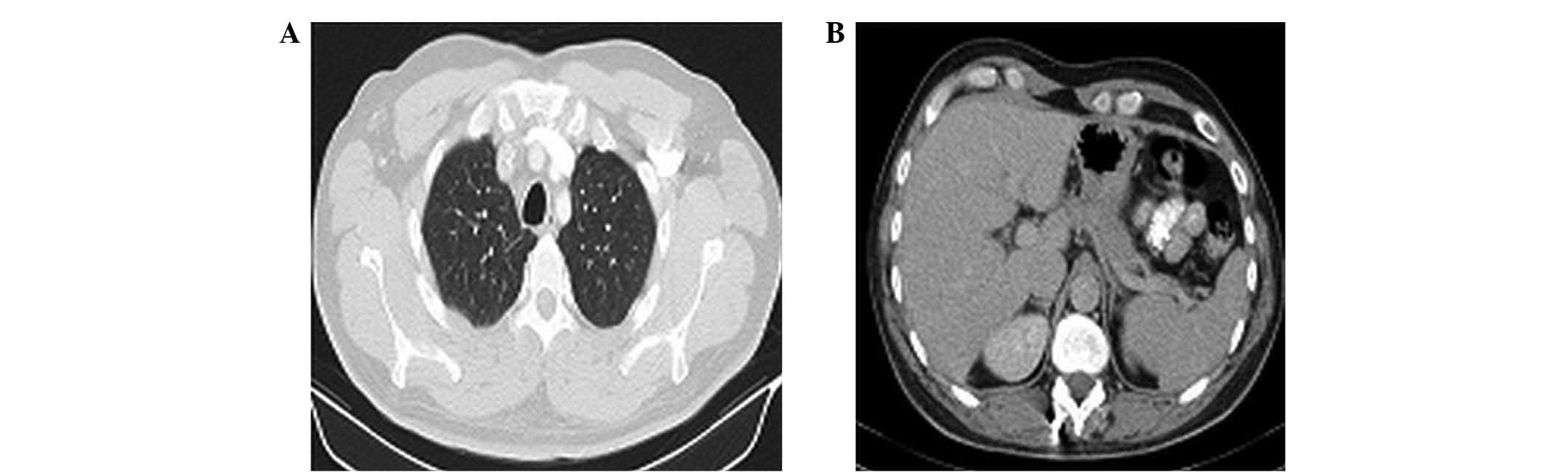
complete response (CR) for liver metastases. In August 2013, the
patient completed 12 cycles of FOLFIRI-aflibercept with a complete
radiological response (Fig. 2).
Subsequently, the patient underwent periodic review. Tumour markers
remained normal throughout the follow-up period.
In October 2013, the patient presented with a
pulmonary relapse of three nodules in the same location. Thoracic
surgery was ruled out due to multiple bilateral and subcentimetric
metastases, as well as a short progression-free interval, and
treatment was resumed with FOLFIRI-aflibercept. The patient
received 14 additional cycles to date, with good tolerance and
radiologically stable disease. Toxicity during treatment was low,
with grade 1–2 diarrhoea, and grade 1 alopecia and hypertension,
which was controlled with enalapril and amlodipine. In cycles 5, 13
and 14, grade 2 proteinuria was detected, which prompted the
administration of FOLFIRI without aflibercept. The patient is
currently alive with pulmonary and hepatic disease, under
regorafenib treatment.
Case 2
A 53 year-old woman presented to San Cecilio
Clinical Hospital in February 2012 with increased intestinal
transit and hematochezia. Following a positive faecal occult blood
test, a colonoscopy revealed the presence of a mass 5–8 cm from the
anal margin, which occupied the circumference of the lumen, but
allowed endoscope entry. A biopsy confirmed the presence of
invasive adenocarcinoma. Analysis revealed that the patient had
increased levels of CEA (269.5 ng/ml) and CA 19–9 (1,096.0 U/ml).
There was also evidence of multiple lung and liver metastases
following a CT scan. Subsequent to detection of mutated KRAS
in codon 12 of exon 2 (12Asp/G12D), the patient was administered
with chemotherapy of FOLFOX6m-bevacizumab. Following 12 cycles, the
patient continued chemotherapy with 5 additional cycles of
5FU/leucovorin-bevacizumab, without oxaliplatin, due to a
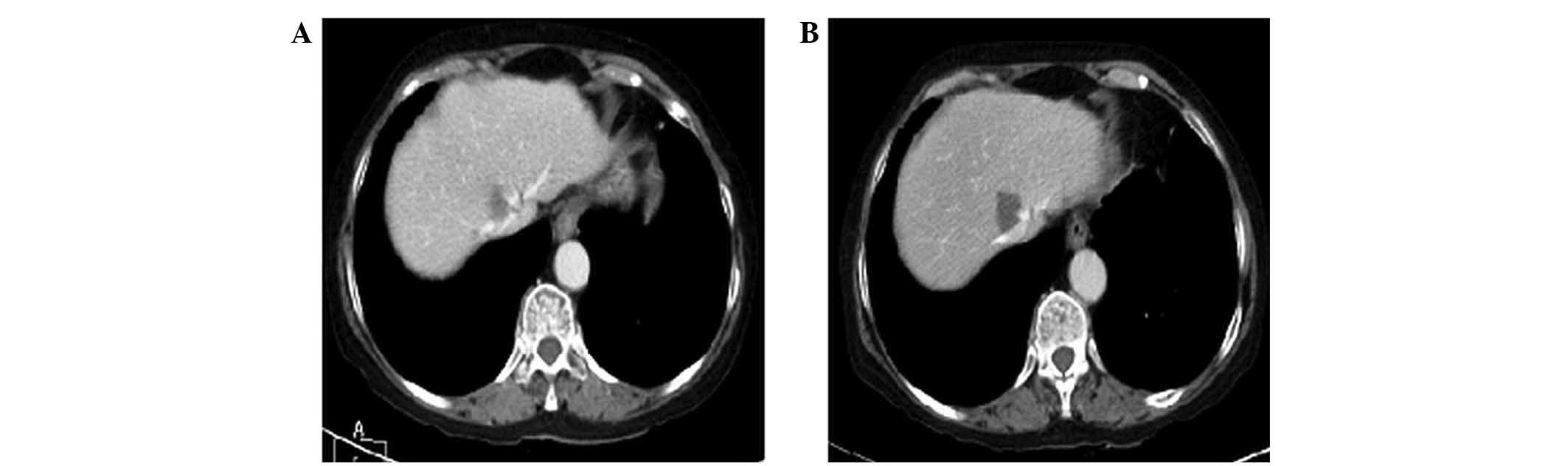
hypersensitivity reaction. At follow-up in February 2013, the
patient presented with tumour progression (Fig. 3), and second-line treatment with
FOLFIRI-aflibercept was recommended. The patient had a PS score of
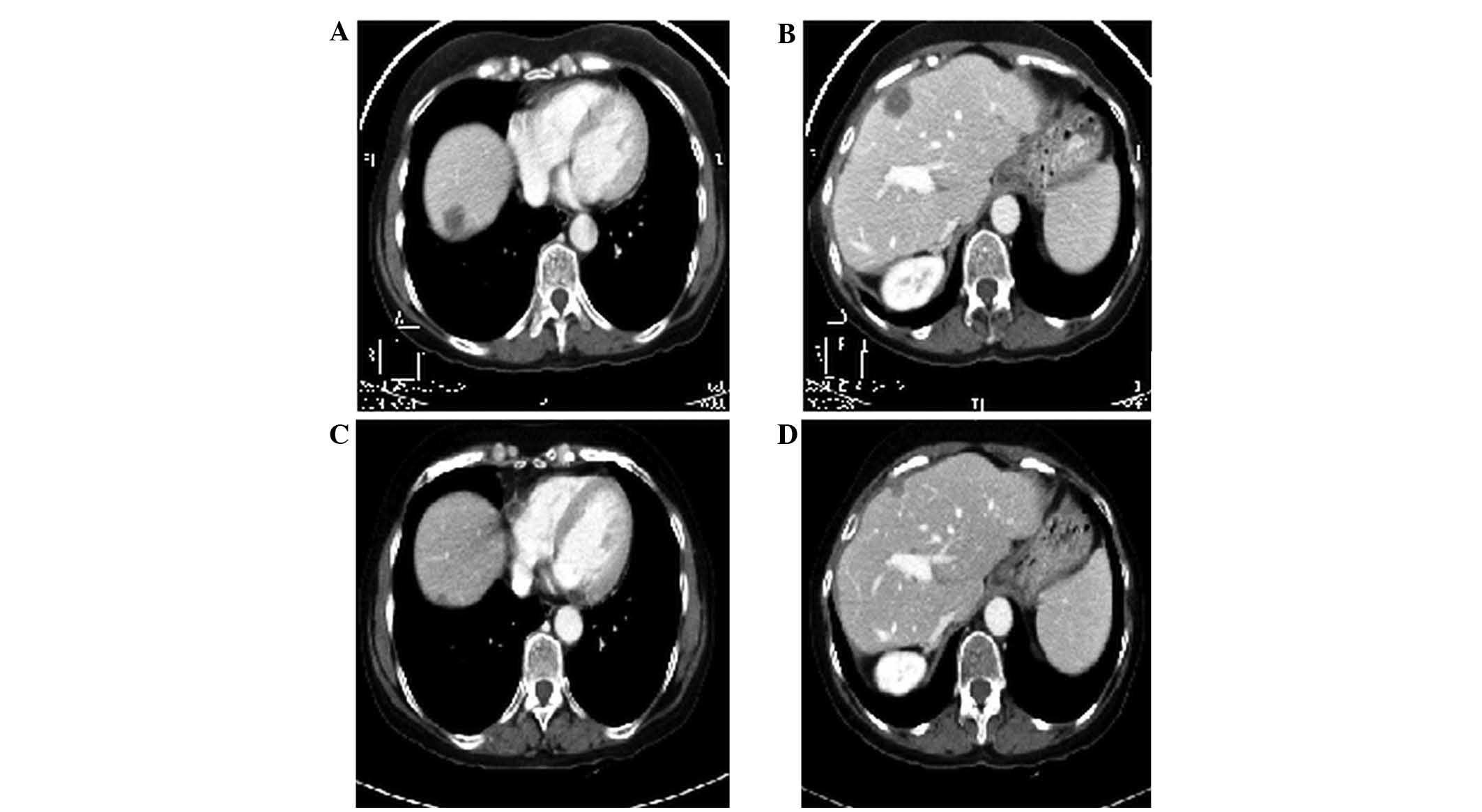
0 when second-line treatment was commenced. The patient received 29
cycles of FOLFIRI-aflibercept, with radiological disease stability
(Fig. 4). Regarding toxicity, the
patient presented with grade 2 palmar-plantar erythrodysesthesia,
and recovered following a week of rest and a 15% reduction in the
5FU dose. Re-evaluation visits were made every 3 months to assess
tumour markers and CT scans, until September 2014, when tumour
progression was observed. The patient succumbed to the disease in
February 2016.
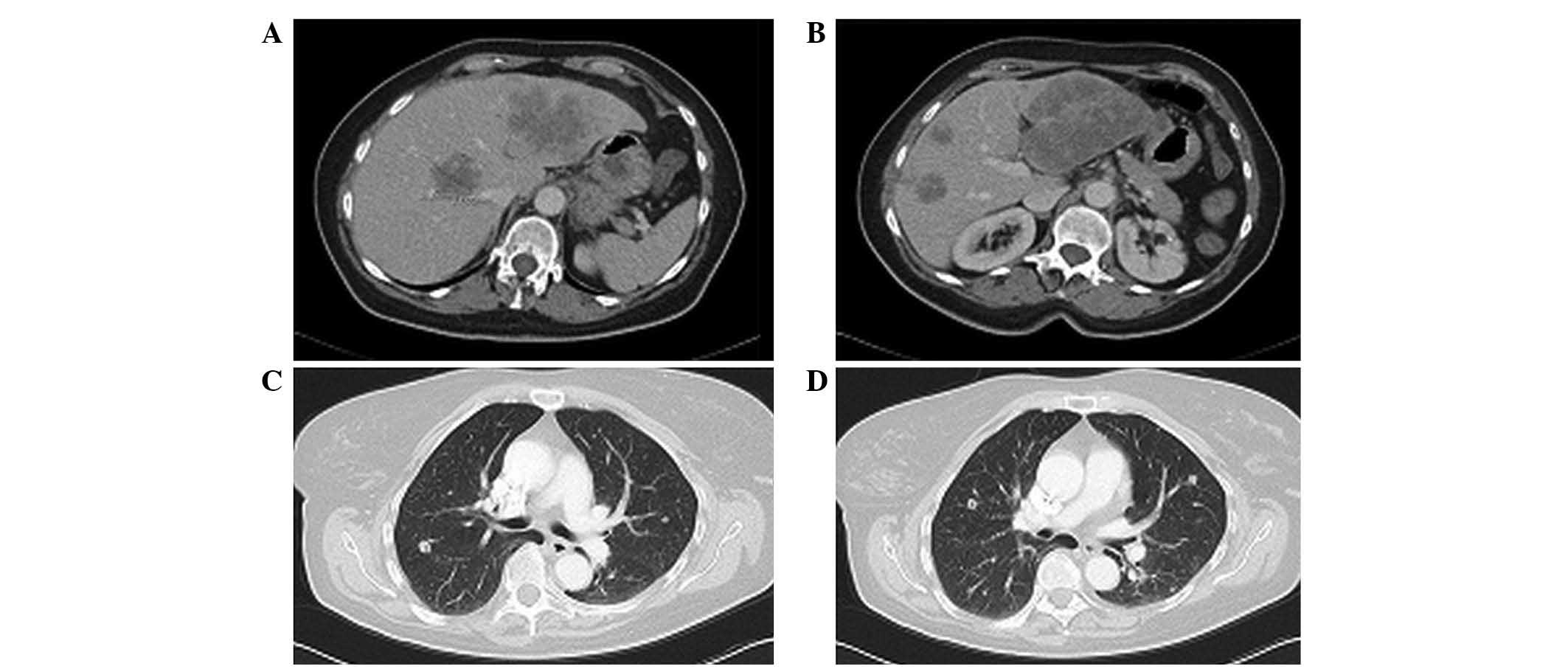 | Figure 3.Case 2. Thoracic-abdominal computed
tomography scan following FOLFOX6m (oxaliplatin, leucovorin and
5-fluorouracil)-bevacizumab treatment revealing tumour progression.
(A) In September 2012, scans revealed hepatic lesions at segments
IV (4.5 cm in size), LI (10 cm), VI (2 cm), VIII (2.2 cm) and VIII
(3.3 cm). (B) In February 2013, hepatic lesions were observed at
segments IV (4.5 cm in size), LI (10 cm), VI (2 cm), VIII (2.2 cm)
and VIII (3.3 cm). (C) In September 2012, right lung metastasis was
detected. (D) In February 2013, bilateral pulmonary metastasis was
detected. |
Case 3
A 66 year-old woman presented to Costa del Sol
Hospital in October 2010 with a history of hypertension and
hypercholesterolemia, and was diagnosed with stage IV colon
adenocarcinoma, due to liver metastases. A mutation at codon 12 of
the KRAS gene (Arg12/G12R) was identified. Tumour markers
(CEA and CA 19-9) were normal at baseline. In December 2010, the
patient began treatment with FOLFOX6m in combination with
bevacizumab for a total of 5 cycles. A follow-up CT scan (February
2011) revealed a slight reduction in liver metastases, which were
more hypodense than before, hence a radiological stabilisation was
noted based on Response Evaluation Criteria In Solid Tumours
(15). The patient underwent surgical
intervention in April 2011, with transverse colectomy,
segmentectomy of VII and liver metastasectomy. The pathological
stage of the tumour was pT3N1M1, and four liver metastases 1.7,
1.4, 0.8 and 5 cm in size were resected. The tumour had a Mandard
TRG of 3. Three of the metastases extended focally to the margin of
resection. Subsequent to surgery, the patient resumed treatment
with FOLFOX-bevacizumab, and completed a total of 12 cycles of
treatment in September 2011.
The patient was followed up with CEA and CT scans
every 3–6 months until June 2013, when a single 15-mm liver
recurrence was detected on a CT scan (Fig. 5A). The patient's tumour markers were
normal and the patient had an ECOG PS of 1. Based on the
localisation of the hepatic recurrence, it was not considered
resectable or a candidate for ablation therapy. Treatment was
initiated with aflibercept in combination with FOLFIRI within the
Compassionate Use Program from July 2013, with an initial 75%
reduction in FOLFIRI dose, due to toxicity from a prior regimen. A
CT scan subsequent to 6 cycles revealed the presence of a lesion 19
mm in diameter, with more defined contours, increased hypodensity,
and without contrast enhancement, which was characterised as
complete necrosis (Fig. 5B). The
patient received a total of 14 cycles of treatment, 11 of these
with aflibercept.
A CT scan in February 2014 did not reveal remission
of the tumour. Therefore, following discussion of the case with the
Multidisciplinary Committee, the patient underwent exploratory
surgery and assessment of resection of liver metastasis in segment
VIII in March 2014. Due to its anatomical location, intraoperative
ablation of the lesion was assessed prior to performing a resection
to establish the surgical margins. However, on further inspection a
second suspicious recurrent lesion was detected on the scar from
the prior liver metastasectomy, which was attached to the
diaphragm, making it unresectable. A biopsy of this lesion
confirmed it as metastatic. Therefore, the surgical plan could not
be completed. As predicted by the optimal morphological response
obtained at imaging, the pathology report of the sample confirmed a
significant Mandard TRG of 2, which indicates rare residual cancer
cells. She experienced a new progression of the disease in October
2015, and was rechallenged with FOLFIRI-aflibercept. To date, the
patient has achieved a survival of 67 months from the beginning of
first-line treatment, and 36 months following second-line
FOLFIRI-aflibercept-based treatment. Toxicity of the FOLFIRI
treatment was moderate, with grade 1 diarrhoea, dysphonia,
epistaxis and conjunctival toxicity, grade 2 alopecia, fatigue and
hyporexia, and grade 3 neutropenia, which required a delay of cycle
12. Blood pressure was controlled throughout treatment with the
usual medication, and proteinuria was detected during the last
cycle via urine test strip, with a urine protein-creatinine ratio
of <1.
Case 4
A 57 year-old woman was diagnosed at Costa del Sol
Hospital in May 2010 with stage IV adenocarcinoma of the colon,
based on the identification of multiple liver metastases on a CT
scan. In addition to a lesion in segment II, 8 lesions on the right
hepatic lobe and a 12 mm adenopathy in the gastrocolic ligament
area were observed. Upon diagnosis, tumour markers CEA (144 ng/ml),
CA 19-9 (558 U/ml), and lactate dehydrogenase (975 U/l) were
elevated, and a slight iron-deficiency anaemia was detected. The
patient had a KRAS mutation in codon 12 (VAL12 or G12V).
Following evaluation of the case by the Multidisciplinary
Committee, chemotherapy treatment was administered with
XELOX-bevacizumab, and the patient received 6 cycles between May
and August 2010. The response of the patient was verified
biochemically and radiologically, with a PR at the hepatic level.
Subsequent intervention was performed in October 2010, which
included a left colectomy and right hepatectomy and resection of
five left liver metastases. The pathological stage of the tumour
was pT1N1M1, with total resection of the liver disease R0, and
strong evidence of tumoural regression (Mandard TRG 3).
In January 2011, the patient had completed 6 months
of systemic therapy, with 2 additional cycles of capecitabine and
bevacizumab administered following surgery, but without
oxaliplatin, due to neurotoxicity. The patient underwent periodic
review of CEA and CT scans every 3–6 months, until April 2012, when
two new liver metastases were observed in the follow-up CT scan.
Positron emission tomography-CT was performed, which revealed
questionable retroperitoneal adenopathy. Therefore, in May 2012, an
exploratory laparotomy was performed, which confirmed the tumoural
adenopathy with extension to the inferior vena cava vascular wall
and aorta. Consequently, due to the assessment of unresectable
disease and a PS of 0, FOLFIRI-based palliative chemotherapy was
recommended, which was administered in June 2012. Aflibercept was
administered from the third cycle following authorisation for use
from the Compassionate Use Program. A CT scan performed following 7
cycles in October 2012, revealed a PR with a reduction in hepatic
and ganglionic metastases. Subsequent to completion of 12 cycles, a
CT scan in December 2012 revealed that the liver metastases were
greatly reduced compared with the baseline CT scan (Fig. 6). Therefore, it was decided to
continue with aflibercept monotherapy until progression.
With regard to toxicity, the patient had symptoms
consistent with grade 1 acute neurotoxicity from irinotecan
following completion of the first infusion, and grade 2 infusion
reaction to aflibercept with lumbago in the fourth and fifth
cycles, for which dexchlorpheniramine premedication was
administered in later cycles. Cycles 8 and 12 were delayed, due to
grade 2 neutropenia and respiratory infection. With the latter, the
patient also had grade 2 mucositis and hypertension. During the
maintenance period with aflibercept, the patient presented with
hypertension, which reached grade 3, but improved with adjustments
to medication, as did grade 2 pharyngotonsillitis without
neutropenia.
In April 2013, radiological progression was detected
in the liver. The patient had persistent residual neurotoxicity
from oxaliplatin. Subsequently, the patient was included in the
MUTEX clinical trial at Virgen del Rocio Hospital (Seville, Spain),
where the patient received cetuximab treatment until August 2013.
During this time, the patient reported jaundice secondary to novel
hepatic disease progression, which improved following placement of
biliary endoprosthesis. In September 2013, the patient began 75%
FOLFOX. Concurrently, multiple vertebral fractures were discovered
with magnetic resonance imaging at the dorsolumbar level, due to
osteoporosis, causing significant pain and requiring the use of a
clamshell body jacket. Chemotherapy was suspended following 1
cycle, due to toxicity and deterioration of the patient's ECOG PS.
The patient continued with supportive treatment until she succumbed
to the disease in October 2013. The patient had reached an overall
survival time (OS) of 41 months since diagnosis, and survival from
the beginning of second-line treatment with aflibercept was 17
months. Progression free survival (PFS) following second-line
treatment was 11 months.
Case 5
A 60 year-old male with no notable medical history,
but with a family history of colon, gastric, pulmonary and
testicular cancer, presented with fatigue in September 2012, and
subsequently postprandial discomfort, slight diarrhoea and
hyporexia. Following the detection of anaemia during routine
testing in November 2012, the patient was diagnosed at Costa del
Sol Hospital with well-differentiated stage IV adenocarcinoma at
the hepatic angle of the colon with hepatic tumour involvement,
along with lymph node metastases, which was assessed as
unresectable. The KRAS gene of the patient was mutated in
codon 13 (13ASP or G13D). The patient began palliative chemotherapy
with FOLFOX6m-bevacizumab in December 2012, receiving 12 cycles of
chemotherapy until June 2013, when tumour progression was detected.
A CT scan revealed a suspicious rib lesion, which was confirmed to
be metastatic, while growth of a pulmonary nodule from the primary
tumour and the intra-abdominal ganglionic metastases was also
observed. As the patient had a PS of 1 and elevated tumour markers
(CEA, 8.4 ng/ml; CA 19-9, 2351.6 U/ml), the patient began
second-line chemotherapy with FOLFIRI-aflibercept, with a 80%
reduction in 5FU dose, due to prior toxicity. Unfortunately, the
patient presented with a complication involving the 5FU infuser,
which was considered unrelated to aflibercept treatment, and
removal of the portacath was required. For this reason, after
receiving 5 cycles and presenting with disease stabilisation at
biochemical and radiological levels, irinotecan monotherapy was
administered. To date, the patient presented with grade 2
hypertension, hyporexia, nausea, and diarrhoea, as well as grade 3
fatigue, requiring a reduction of the FOLFIRI dosage to improve
tolerance. In November 2013, following a total of 8 cycles of
treatment, chemotherapy was suspended following suspicion of tumour
progression, due to progressively elevated tumour markers (CEA, 26
ng/ml; CA 19-9, 2,200 U/ml) and clinical deterioration, with
asthenia, abdominal pain and worsening PS. The patient succumbed to
the disease in December 2013. The patient had a survival time of 6
months following the administration of second-line treatment with
aflibercept.
Discussion
In the present case series, patients 1 and 5
presented with mutated KRAS in codon 13, specifically
G13D/13ASP mutation, with the poorest evolution in survival
occurring in patient 5. The other three patients presented with
mutations in codon 12. Several studies have investigated the
prognostic and predictive significance of different KRAS
mutations, the G13D/13Asp mutation in particular exhibits an
especially poor prognosis for patients (16–19). De
Roock et al (16) suggested
that in patients treated with supportive care with a KRAS
G13D mutation have a poorer OS time (median, 3.6 months) compared
with patients with wild-type KRAS or other KRAS
mutations (median OS time, 5.0 and 4.7 months, respectively).
Another study demonstrated that patients with KRAS G13D
mutation, who were treated with chemotherapy alone, without
anti-EGFR treatment, presented with poorer response rates compared
with patients with other mutations (response rate, 22.0 vs. 43.2%,
respectively; odds ratio, 0.40; P=0.032) (17). These findings support the OS time of
12 months reported in case 5 in the present case series, which was
lower than the OS time reported for the other present cases, and
lower than the median OS time for patients with mCRC treated with
similar therapy regimens (9).
The choice of first-line treatment in all cases in
the present study was FOLFOX or XELOX in combination with
bevacizumab (9). Until progression of
disease is observed, possible treatment plans are FOLFIRI (20), FOLFIRI-bevacizumab (21) or FOLFIRI-aflibercept (13). The present study chose the latter
based on the VELOUR study results (13). VELOUR was a multi-centre, randomised,
placebo-controlled phase III trial, which aimed to compare the
efficacy of aflibercept vs. placebo in combination with FOLFIRI as
a second-line treatment for patients with mCRC, who were previously
treated with oxaliplatin. In the VELOUR study, patients with mCRC
were randomised to receive FOLFIRI and aflibercept at 4 mg/kg
intravenously every 2 weeks (n=614) vs. FOLFIRI and placebo
(n=612), with stratification by PS and prior bevacizumab received.
The aflibercept group demonstrated a superior median OS time [13.50
vs. 12.06 months; hazards ratio (HR) 0.817; P=0.0032], PFS (6.90
vs. 4.67 months; HR 0.758; P<0.0001) and overall response rate
(19.8 vs. 11.1%; P=0.0001) compared with the placebo group
(13). In the subgroup analysis
subsequently published, aflibercept was confirmed to be effective
in all specified subgroups, even for patients that had been treated
with first-line bevacizumab (~30%), with a median OS time of 12.5
vs. 11.7 months and PFS of 6.7 vs. 3.9 months for the experimental
and control arm, respectively (20).
This is consistent with results from other studies, which support
the use of a continued antiangiogenic strategy for the treatment of
mCRC (22–24).
The question remains whether aflibercept may offer
an advantage over bevacizumab maintenance therapy, if the patient's
disease has progressed following first-line treatment that already
included bevacizumab. To date, no randomised study has answered
that question. However, there is available data from pre-clinical
studies that have made a comparative assessment of the activity of
the two drugs. A recent study with mCRC tumour tissue models
extracted from patients and xenografted mice (PDX cancer models)
revealed differences in tumour growth control between the
antiangiogenic treatments bevacizumab and aflibercept; the latter
was revealed to be more effective in preventing tumour growth
(25). Chiron et al (26) verified this data, also using PDX
models, and revealed that administrating aflibercept during cancer
progression following bevacizumab treatment facilitates maintenance
of tumour growth inhibition, thus achieving a greater response
compared with bevacizumab maintenance following tumour progression
(26). This superior activity may be
explained by the effect aflibercept has on VEGF and PIGF, since it
better neutralises the resistance mechanisms that are activated by
the tumour compared with bevacizumab (27,28). In
addition, it has been suggested that PIGF presence in patient serum
may aid in the prediction of the optimal time for switching to
antiangiogenic therapy prior to tumour progression taking place
(25).
In the present case series, the combination of
FOLFIRI-aflibercept was revealed to be effective in patients with
mutated KRAS, who were treated previously with a combination
of oxaliplatin and bevacizumab. The majority of patients received a
greater number of aflibercept cycles than the median reported in
the VELOUR study (present study, 12 cycles of FOLFIRI-aflibercept;
VELOUR study, 7 cycles of aflibercept) (13), with manageable and reversible
toxicity. In the present study, a partial radiological response was
recorded in one patient and one CR was obtained in another patient.
In addition, in the other present cases, the optimal morphological
response observed in the CT scan image was able to predict an
improved pathological response, which is known to be associated
with prolonged survival (29).
Furthermore, the majority of the present patients obtained a PFS
greater than the median reported in the VELOUR study and in a
subgroup of patients previously treated with bevacizumab (21). In addition, a median survival time of
~47 months from the initial treatment of the disease was reached,
which is remarkable for a series entirely consisting of
KRAS-mutated cases.
To the best of our knowledge, the present study is
the first study to publish data from mCRC patients treated with
aflibercept in routine clinical practice, outside of the clinical
trial environment. The present study serves to contrast efficacy
and safety results obtained from the pivotal VELOUR trial, and
confirms that aflibercept is active and well-tolerated following
bevacizumab treatment.
Acknowledgements
Medical writing assistance, supported financially by
Sanofi Spain (Barcelona, Spain), was provided by Mrs. A. Del Campo
(Pivotal S.L., Madrid, Spain).
Glossary
Abbreviations
Abbreviations:
|
mCRC
|
metastatic colorectal cancer
|
|
VEGF
|
anti-vascular endothelial growth
factor
|
|
ECOG
|
eastern cooperative oncology Group
|
|
PS
|
performance status
|
|
CT
|
computed tomography
|
|
TRG
|
tumour regression grade
|
|
EGFR
|
epidermal growth factor receptor
|
|
PFS
|
progression-free survival
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schmoll HJ, Van Cutsem E, Stein A,
Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde
CJ, Balmana J, Regula J, et al: ESMO Consensus Guidelines for
management of patients with colon and rectal cancer. A personalized
approach to clinical decision making. Ann Oncol. 23:2479–2516.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heinemann V, Douillard JY, Ducreux M and
Peeters M: Targeted therapy in metastatic colorectal cancer - An
example of personalised medicine in action. Cancer Treat Rev.
39:592–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Casado-Saenz E, Feliu J, Gomez-España MA,
Sanchez-Gastaldo A and Garcia-Carbonero R; SEOM: SEOM clinical
guidelines for the treatment of advanced colorectal cancer 2013.
Clin Transl Oncol. 15:996–1003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sorich MJ, Wiese MD, Rowland A,
Kichenadasse G, McKinnon RA and Karapetis CS: Extended RAS
mutations and anti-EGFR monoclonal antibody survival benefit in
metastatic colorectal cancer: A meta-analysis of randomized
controlled trials. Ann Oncol. 26:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hassabo HM, Sahin IH and Kazmi SHA:
Associations between patient (pt) colorectal cancer (CRC) tumour
KRAS and BRAF mutation (mut) status and overall survival (OS). J
Clin Oncol. 32:4732014.
|
|
8
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomised phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kubicka S, Greil R, André T, Bennouna J,
Sastre J, Van Cutsem E, von Moos R, Osterlund P, Reyes-Rivera I,
Müller T, et al: Bevacizumab plus chemotherapy continued beyond
first progression in patients with metastatic colorectal cancer
previously treated with bevacizumab plus chemotherapy: ML18147
study KRAS subgroup findings. Ann Oncol. 24:2342–2349. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holash J, Davis S, Papadopoulos N, Croll
SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, et
al: VEGF-Trap: A VEGF blocker with potent antitumour effects. Proc
Natl Acad Sci USA. 99:11393–11398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Temraz S, Mukherji D, Alameddine R and
Shamseddine A: Methods of overcoming treatment resistance in
colorectal cancer. Crit Rev Oncol Hematol. 89:217–230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Cutsem E, Tabernero J, Lakomy R,
Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko
V, Ferry D, et al: Addition of aflibercept to fluorouracil,
leucovorin, and irinotecan improves survival in a phase III
randomised trial in patients with metastatic colorectal cancer
previously treated with an oxaliplatin-based regimen. J Clin Oncol.
30:3499–3506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
European Medicines Agency, . Zaltrap
Technical datasheet. http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/002532/WC500139484.pdflast
accessed. 21st–July. 2016
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Roock W, Jonker DJ, Di Nicolantonio F,
Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M,
Piessevaux H, et al: Association of KRAS p. G13D mutation with
outcome in patients with chemotherapy-refractory metastatic
colorectal cancer treated with cetuximab. JAMA. 304:1812–1820.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tejpar S, Celik I, Schlichting M,
Sartorius U, Bokemeyer C and Van Cutsem E: Association of KRAS G13D
tumour mutations with outcome in patients with metastatic
colorectal cancer treated with first-line chemotherapy with or
without cetuximab. J Clin Oncol. 30:3570–3577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andreyev HJ, Norman AR, Cunningham D,
Oates JR and Clarke PA: Kirsten ras mutations in patients with
colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer
Inst. 90:675–684. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pérez-Ruiz E, Rueda A, Pereda T, Alcaide
J, Bautista D, Rivas-Ruiz F, Villatoro R, Pérez D and Redondo M:
Involvement of K-RAS mutations and amino acid substitutions in the
survival of metastatic colorectal cancer patients. Tumour Biol.
33:1829–1835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomised GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tabernero J, Van Cutsem E, Lakomý R,
Prausová J, Ruff P, van Hazel GA, Moiseyenko VM, Ferry DR,
McKendrick JJ, Soussan-Lazard K, et al: Aflibercept versus placebo
in combination with fluorouracil, leucovorin and irinotecan in the
treatment of previously treated metastatic colorectal cancer:
Prespecified subgroup analyses from the VELOUR trial. Eur J Cancer.
50:320–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bennouna J, Sastre J, Arnold D, Österlund
P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C,
et al: Continuation of bevacizumab after first progression in
metastatic colorectal cancer (ML18147): A randomised phase 3 trial.
Lancet Oncol. 14:29–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grothey A, Sugrue MM, Purdie DM, Dong W,
Sargent D, Hedrick E and Kozloff M: Bevacizumab beyond first
progression is associated with prolonged overall survival in
metastatic colorectal cancer: Results from a large observational
cohort study (BRiTE). J Clin Oncol. 26:5326–5334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simkens LH, van Tinteren H, May A, ten
Tije AJ, Creemers GJ, Loosveld OJ, de Jongh FE, Erdkamp FL, Erjavec
Z, van der Torren AM, et al: Maintenance treatment with
capecitabine and bevacizumab in metastatic colorectal cancer
(CAIRO3): A phase 3 randomised controlled trial of the Dutch
Colorectal Cancer Group. Lancet. 385:1843–1852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiron M, Bagley RG, Pollard J, Mankoo PK,
Henry C, Vincent L, Geslin C, Baltes N and Bergstrom DA:
Differential antitumour activity of aflibercept and bevacizumab in
patient-derived xenograft models of colorectal cancer. Mol Cancer
Ther. 13:1636–1644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiron M, Bagley RG, Pollard J, Henry C,
Mankoo P, Vincent L, Geslin C, Kloss T and Bergstrom DA: Switching
to aflibercept treatment resulted in greater tumour responses than
continuous bevacizumab treatment in patient-derived xenograft
models of colorectal cancer (abstract). Proceedings of the
AACR-NCI-EORTC International Conference. Boston, MA. Mol Cancer
Ther. 1211 Suppl. (B2)2013.
|
|
27
|
Cao Y: Positive and negative modulation of
angiogenesis by VEGFR1 ligands. Sci Signal. 2:re12009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kopetz S, Hoff PM, Morris JS, Wolff RA,
Eng C, Glover KY, Adinin R, Overman MJ, Valero V, Wen S, et al:
Phase II trial of infusional fluorouracil, irinotecan, and
bevacizumab for metastatic colorectal cancer: Efficacy and
circulating angiogenic biomarkers associated with therapeutic
resistance. J Clin Oncol. 28:453–459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shindoh J, Loyer EM, Kopetz S,
Boonsirikamchai P, Maru DM, Chun YS, Zimmitti G, Curley SA,
Charnsangavej C, Aloia TA and Vauthey JN: Optimal morphologic
response to preoperative chemotherapy: An alternate outcome end
point before resection of hepatic colorectal metastases. J Clin
Oncol. 30:4566–4572. 2012. View Article : Google Scholar : PubMed/NCBI
|