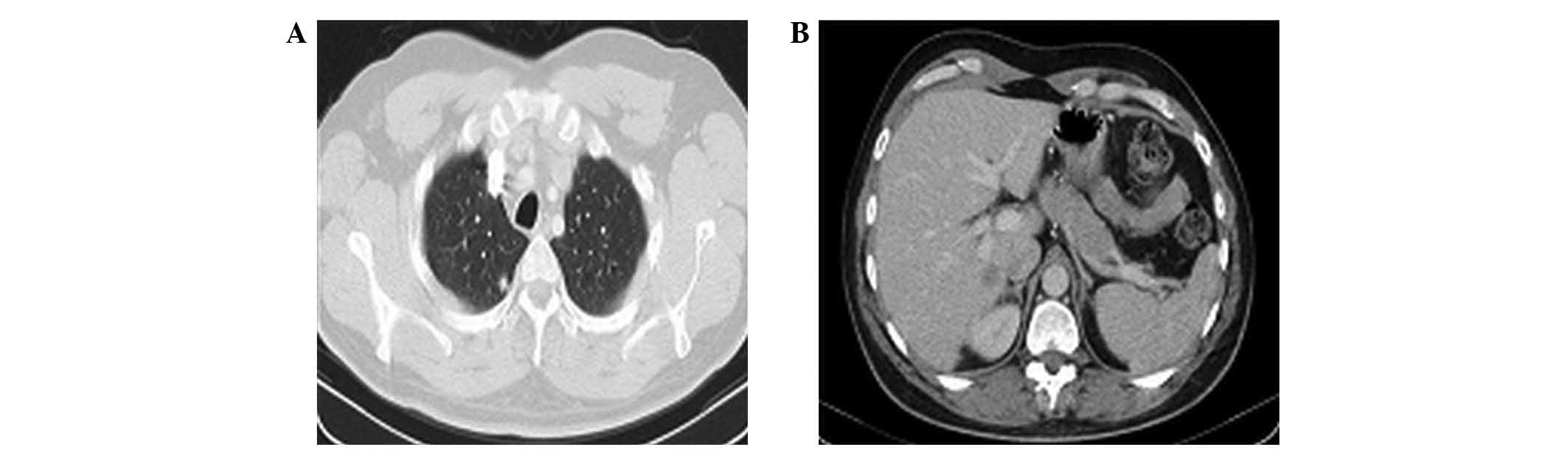
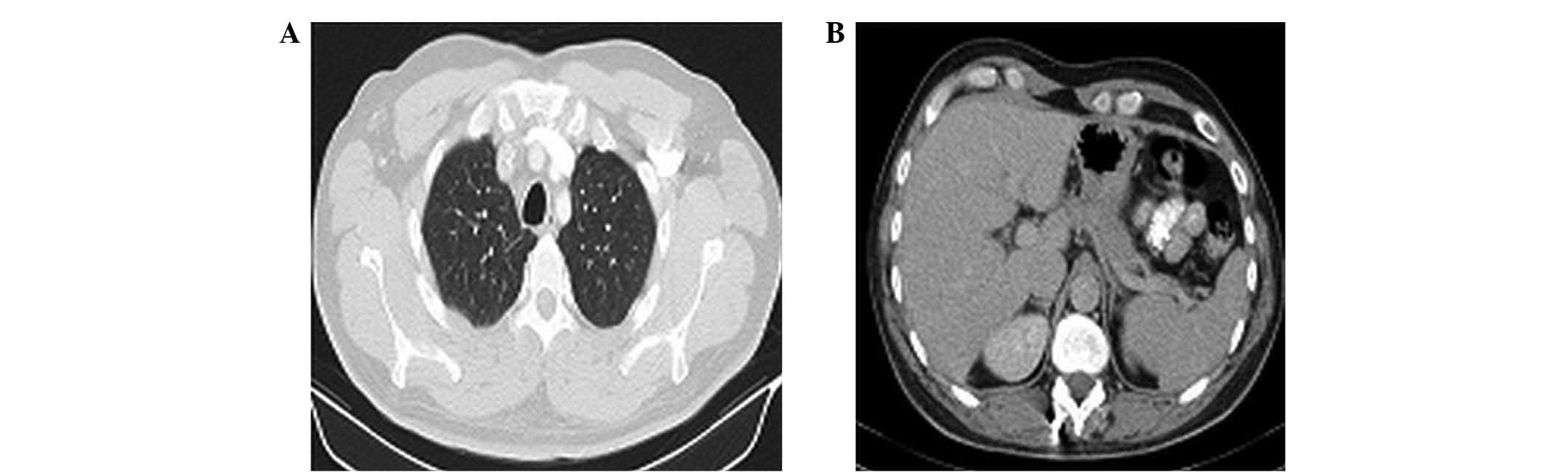
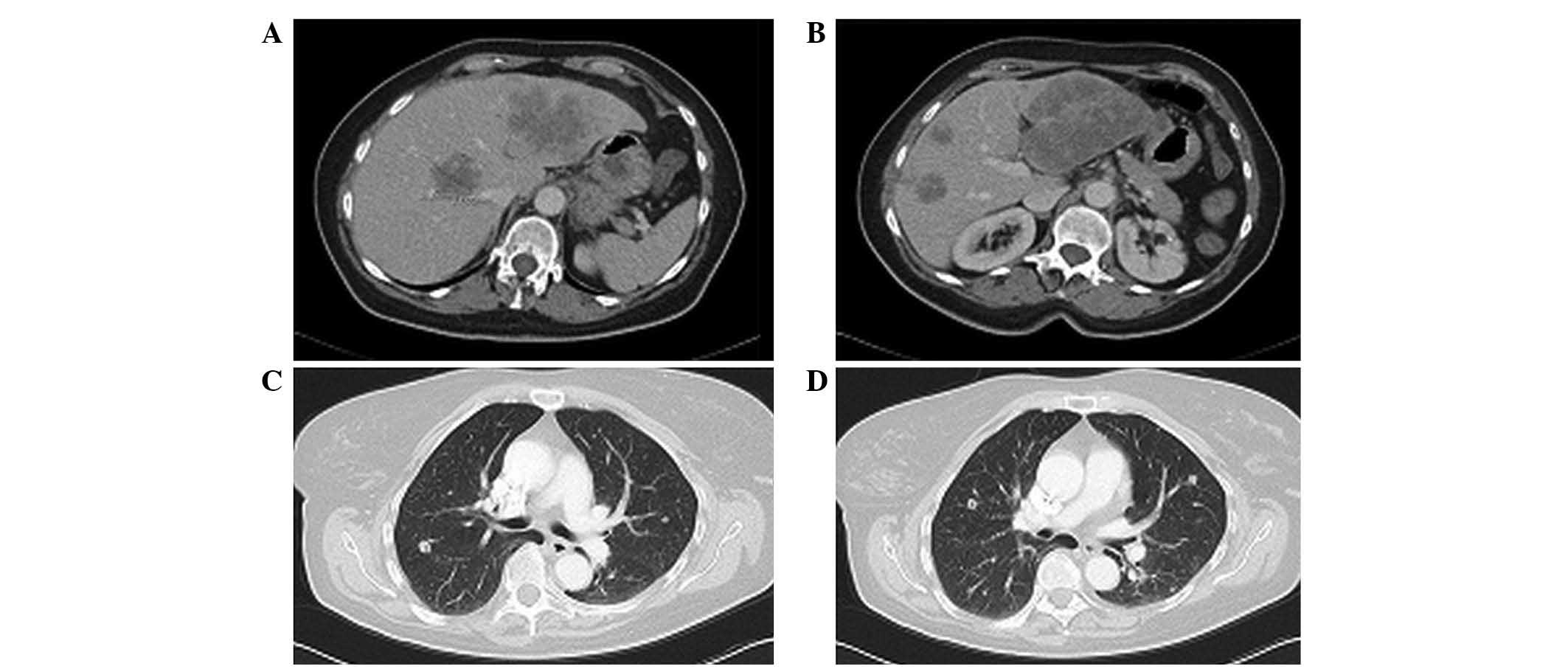
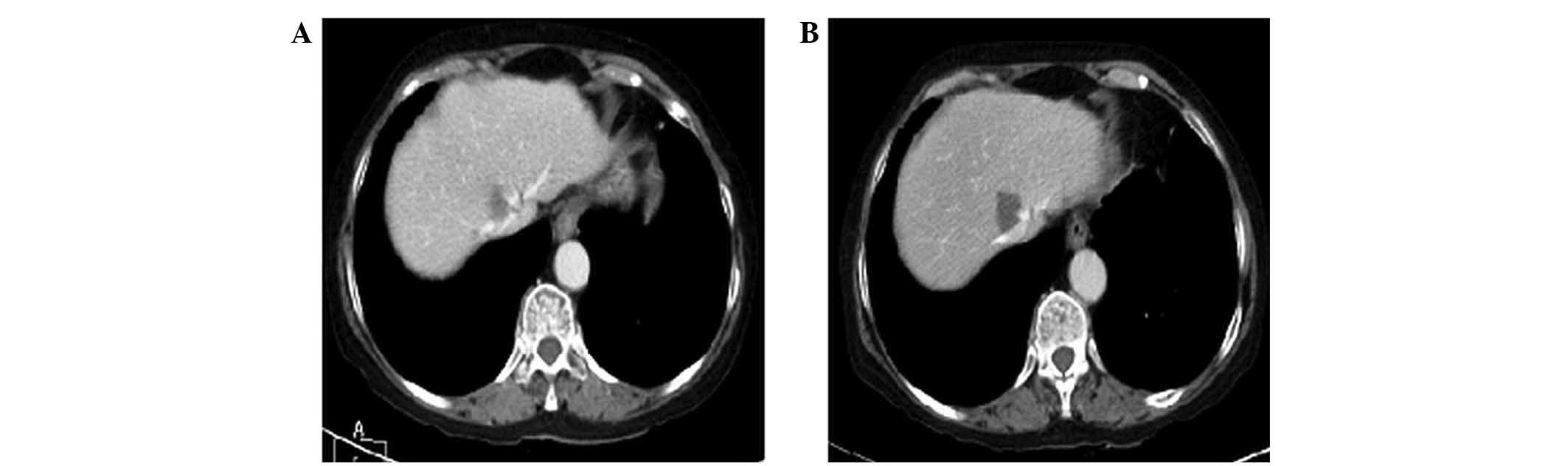
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schmoll HJ, Van Cutsem E, Stein A,
Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde
CJ, Balmana J, Regula J, et al: ESMO Consensus Guidelines for
management of patients with colon and rectal cancer. A personalized
approach to clinical decision making. Ann Oncol. 23:2479–2516.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heinemann V, Douillard JY, Ducreux M and
Peeters M: Targeted therapy in metastatic colorectal cancer - An
example of personalised medicine in action. Cancer Treat Rev.
39:592–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Casado-Saenz E, Feliu J, Gomez-España MA,
Sanchez-Gastaldo A and Garcia-Carbonero R; SEOM: SEOM clinical
guidelines for the treatment of advanced colorectal cancer 2013.
Clin Transl Oncol. 15:996–1003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sorich MJ, Wiese MD, Rowland A,
Kichenadasse G, McKinnon RA and Karapetis CS: Extended RAS
mutations and anti-EGFR monoclonal antibody survival benefit in
metastatic colorectal cancer: A meta-analysis of randomized
controlled trials. Ann Oncol. 26:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hassabo HM, Sahin IH and Kazmi SHA:
Associations between patient (pt) colorectal cancer (CRC) tumour
KRAS and BRAF mutation (mut) status and overall survival (OS). J
Clin Oncol. 32:4732014.
|
|
8
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomised phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kubicka S, Greil R, André T, Bennouna J,
Sastre J, Van Cutsem E, von Moos R, Osterlund P, Reyes-Rivera I,
Müller T, et al: Bevacizumab plus chemotherapy continued beyond
first progression in patients with metastatic colorectal cancer
previously treated with bevacizumab plus chemotherapy: ML18147
study KRAS subgroup findings. Ann Oncol. 24:2342–2349. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holash J, Davis S, Papadopoulos N, Croll
SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, et
al: VEGF-Trap: A VEGF blocker with potent antitumour effects. Proc
Natl Acad Sci USA. 99:11393–11398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Temraz S, Mukherji D, Alameddine R and
Shamseddine A: Methods of overcoming treatment resistance in
colorectal cancer. Crit Rev Oncol Hematol. 89:217–230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van Cutsem E, Tabernero J, Lakomy R,
Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko
V, Ferry D, et al: Addition of aflibercept to fluorouracil,
leucovorin, and irinotecan improves survival in a phase III
randomised trial in patients with metastatic colorectal cancer
previously treated with an oxaliplatin-based regimen. J Clin Oncol.
30:3499–3506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
European Medicines Agency, . Zaltrap
Technical datasheet. http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/002532/WC500139484.pdflast
accessed. 21st–July. 2016
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Roock W, Jonker DJ, Di Nicolantonio F,
Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M,
Piessevaux H, et al: Association of KRAS p. G13D mutation with
outcome in patients with chemotherapy-refractory metastatic
colorectal cancer treated with cetuximab. JAMA. 304:1812–1820.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tejpar S, Celik I, Schlichting M,
Sartorius U, Bokemeyer C and Van Cutsem E: Association of KRAS G13D
tumour mutations with outcome in patients with metastatic
colorectal cancer treated with first-line chemotherapy with or
without cetuximab. J Clin Oncol. 30:3570–3577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andreyev HJ, Norman AR, Cunningham D,
Oates JR and Clarke PA: Kirsten ras mutations in patients with
colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer
Inst. 90:675–684. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pérez-Ruiz E, Rueda A, Pereda T, Alcaide
J, Bautista D, Rivas-Ruiz F, Villatoro R, Pérez D and Redondo M:
Involvement of K-RAS mutations and amino acid substitutions in the
survival of metastatic colorectal cancer patients. Tumour Biol.
33:1829–1835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomised GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tabernero J, Van Cutsem E, Lakomý R,
Prausová J, Ruff P, van Hazel GA, Moiseyenko VM, Ferry DR,
McKendrick JJ, Soussan-Lazard K, et al: Aflibercept versus placebo
in combination with fluorouracil, leucovorin and irinotecan in the
treatment of previously treated metastatic colorectal cancer:
Prespecified subgroup analyses from the VELOUR trial. Eur J Cancer.
50:320–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bennouna J, Sastre J, Arnold D, Österlund
P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C,
et al: Continuation of bevacizumab after first progression in
metastatic colorectal cancer (ML18147): A randomised phase 3 trial.
Lancet Oncol. 14:29–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grothey A, Sugrue MM, Purdie DM, Dong W,
Sargent D, Hedrick E and Kozloff M: Bevacizumab beyond first
progression is associated with prolonged overall survival in
metastatic colorectal cancer: Results from a large observational
cohort study (BRiTE). J Clin Oncol. 26:5326–5334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simkens LH, van Tinteren H, May A, ten
Tije AJ, Creemers GJ, Loosveld OJ, de Jongh FE, Erdkamp FL, Erjavec
Z, van der Torren AM, et al: Maintenance treatment with
capecitabine and bevacizumab in metastatic colorectal cancer
(CAIRO3): A phase 3 randomised controlled trial of the Dutch
Colorectal Cancer Group. Lancet. 385:1843–1852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiron M, Bagley RG, Pollard J, Mankoo PK,
Henry C, Vincent L, Geslin C, Baltes N and Bergstrom DA:
Differential antitumour activity of aflibercept and bevacizumab in
patient-derived xenograft models of colorectal cancer. Mol Cancer
Ther. 13:1636–1644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiron M, Bagley RG, Pollard J, Henry C,
Mankoo P, Vincent L, Geslin C, Kloss T and Bergstrom DA: Switching
to aflibercept treatment resulted in greater tumour responses than
continuous bevacizumab treatment in patient-derived xenograft
models of colorectal cancer (abstract). Proceedings of the
AACR-NCI-EORTC International Conference. Boston, MA. Mol Cancer
Ther. 1211 Suppl. (B2)2013.
|
|
27
|
Cao Y: Positive and negative modulation of
angiogenesis by VEGFR1 ligands. Sci Signal. 2:re12009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kopetz S, Hoff PM, Morris JS, Wolff RA,
Eng C, Glover KY, Adinin R, Overman MJ, Valero V, Wen S, et al:
Phase II trial of infusional fluorouracil, irinotecan, and
bevacizumab for metastatic colorectal cancer: Efficacy and
circulating angiogenic biomarkers associated with therapeutic
resistance. J Clin Oncol. 28:453–459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shindoh J, Loyer EM, Kopetz S,
Boonsirikamchai P, Maru DM, Chun YS, Zimmitti G, Curley SA,
Charnsangavej C, Aloia TA and Vauthey JN: Optimal morphologic
response to preoperative chemotherapy: An alternate outcome end
point before resection of hepatic colorectal metastases. J Clin
Oncol. 30:4566–4572. 2012. View Article : Google Scholar : PubMed/NCBI
|