Introduction
Cervical cancer (Ca) is the second most common type
of cancer (after breast cancer) in women worldwide. Globally, the
mortality ratio is 52%, with ~275,000 mortalities from Ca annually
(1). Estimated age-adjusted Ca
mortality rates reach 10/100,000 women in developing countries, as
opposed to 3/100,000 women in the majority of developed countries
(2). However, there have been
dramatic reductions in the incidence and mortality of invasive Ca
due to the widespread availability of cytological screening
(3). Ca is caused by persistent
infection with ≥1 oncogenic human papillomaviruses (HPVs) (4), with the prevalence of HPV infection
reaching 99.7% in cancer patients (5). However, HPV is detected in 3–30% of
asymptomatic controls, indicating the importance of co-factors in
the development of cancer of the uterine cervix (6,7).
Malignancies arising from the uterine cervical
epithelium are preceded by long periods (occasionally decades) of
precancerous dysplastic lesions, usually involving progression from
mild, reversible changes to severe irreversible ones (4). The dysplastic lesions were
conventionally graded as cervical intraepithelial neoplasia I, II
and III, and more recently, as low- or high-grade squamous
intraepithelial lesions (LG-SIL or HG-SIL, respectively). Various
studies have demonstrated that the simultaneous use of colposcopy
and cytology as a screening modality exhibits a high sensitivity
(95.0–99.4%) (8).
Mammalian target of rapamycin (mTOR) is a
serine/threonine protein kinase of the
phosphatidylinositol-3-kinase/AKT signaling pathway, with a
critical role in controlling cancer cellular growth, metabolism and
cell cycle progression (9). When
activated, the mTOR signaling pathway regulates ribosomal
biogenesis and protein synthesis through phosphorylation and
inactivation of the repressor of messenger RNA (mRNA) translation
eukaryotic initiation factor 4E (eIF4E) and 4E binding protein 1
(4EBP1) (9), which in turn releases
its inhibitory effect on eIF4E. The latter functions as the
initiating factor in protein translation machinery. Another
downstream effector of the mTOR kinase is the phosphorylation of
the ribosomal protein S6 kinase and the subsequent phosphorylation
of the ribosomal protein S6 to stimulate protein translation and
ribosome biogenesis (9).
A strong body of evidence suggests that the mTOR
signaling pathway is activated in a number of solid tumors,
including head and neck squamous cell carcinoma (10–13).
Notably, mTOR signaling activation has been reported in both
HPV-negative and HPV-associated head and neck carcinomas, as well
as in cervical carcinoma (13)
tissues and cell lines. Furthermore, mTOR inhibitors effectively
decreased mTOR activity in vivo and caused a remarkable
decrease in tumor burden (13). In
addition, overexpression and/or activation of the eIF4E/4EBP1 axis
has been reported in numerous human tumors, and has been associated
with poor prognosis (14–17).
In the present study, it was hypothesized that the
critical downstream effectors of mTOR signaling, 4EBP1 and eIF4E,
which are important in protein synthesis control, may be involved
in the progression of precancerous lesions and cancer of the
uterine cervix. Therefore, the present study investigated the
expression patterns of both proteins and correlated the findings
with patient characteristics and HPV infection status. The present
data demonstrate that high-grade dysplasia and Cas are
characterized by overexpression of 4EBP1 and eIF4E proteins, which
significantly correlates with oncogenic HPV types, and may provide
novel targets for experimental therapies for these patients.
Patients and methods
Patients
Biopsy specimens were obtained from 73 participants
at the ‘Elena Venizelou’ General Maternity Hospital and ‘Alexandra’
General Hospital (Athens, Greece) between January 2011 and December
2011. The present study was conducted according to the ethical
committee guidelines of the University of Athens (Athens, Greece).
Participants were healthy women who had never undergone any kind of
therapy for cervical pathology. All women provided written informed
consent and answered a behavioral questionnaire (Table I). Women were excluded from the study
if they refused HPV DNA testing or if they stated that they had
never had sexual intercourse. The present prospective study was
restricted to those participants who met the protocol criteria, who
were stratified into four groups: i) Normal with non-dysplastic,
non-cancerous tissue; ii) LG-SIL; iii) HG-SIL; and iv) Ca. All
patients underwent Papanicolaou (Pap) test, HPV DNA test with
polymerase chain reaction (PCR) and colposcopic evaluation.
Cervical liquid-based cytology samples were gathered, and HPV DNA
detection and genotyping plus cytopathological examination using
the Bethesda system were performed (7). Cervical samples and biopsy material were
tested by PCR for DNA from 14 oncogenic HPV types. The primer
sequences are identical to those previously described (18). The cycling conditions were as follows:
Incubation at 94°C for 10 min, followed by 40 cycles of
denaturation for 1 min at 94°C, annealing for 1 min at 55°C and
elongation for 1 min at 72°C. The last cycle was followed by a
final extension step of 7 min at 72°C. Cervical abnormalities were
confirmed by cytological abnormalities on Pap smears or
histologically. Colposcopy is important in diagnosis, since this
method has the best accuracy for the delineation and biopsy of the
truly positive area of the most atypical site (3). From each patient, two biopsies were
submitted, one for histological assessment and one for storage at
−80°C in liquid nitrogen. All primary samples were transferred in a
special collector with liquid nitrogen at the Department of
Pathology of the University of Athens. All stored samples in liquid
nitrogen were used for western blotting. The Institutional Research
Board at the University of Athens approved the study.
 | Table I.Questionnaire-based clinical and
behavioral characteristics of patients included in the present
study (n=73). |
Table I.
Questionnaire-based clinical and
behavioral characteristics of patients included in the present
study (n=73).
| Risk
factor/categories | Frequency
distribution at study entry, n (%) |
|---|
| Age group, years |
|
|---|
|
17–25 | 12 (16.4) |
|
26–45 | 41 (56.1) |
| ≥46 | 20 (27.4) |
| Marital status |
|
|---|
|
Married | 37 (50.7) |
|
Single | 36 (49.3) |
| Cigarette smoking,
packs/day |
|
|---|
| Never
smoked | 39 (53.4) |
|
<1 | 16 (22.0) |
| 1 | 9 (12.3) |
|
>1 | 9 (12.3) |
| Age at first sexual
intercourse, years |
|
|
<15 | 5 (6.8) |
|
16–20 | 47 (64.3) |
|
21–25 | 18 (24.6) |
|
>26 | 3 (4.1) |
| Lifetime number of
sexual partners |
|
| 1 | 19 (26.0) |
|
>1 | 54 (74.0) |
| Condom use |
|
|
Yes | 42 (57.5) |
| No | 31 (42.4) |
| Hormonal
contraception |
|
| Yes
(past) | 20 (27.4) |
| Yes
(current) | 1 (1.4) |
| No | 52 (71.2) |
| History of sexually
transmitted infections |
|
|
Yes | 1 (1.4) |
| No | 72 (98.6) |
| Pregnancy with ≥1
delivery at term |
|
|
Yes | 42 (57.5) |
| No | 31 (42.4) |
Western blot analysis
A total of 40 tissue samples were analyzed by
western blotting following total protein extraction. Total proteins
were extracted using lysis buffer supplemented with phosphatase and
protease inhibitors, as previously described (19). Western blot analysis was also
performed using standard methods described previously (19). The monoclonal antibodies used in the
present study were specific for eIF4E (catalog no. 9742; dilution,
1:1,000) and 4E-BP1 (catalog no. 9644; dilution, 1:1,000) (Cell
Signaling Technology, Inc., Danvers, MA, USA) and β-actin
(Sigma-Aldrich, St. Louis, MO, USA). Detection was performed using
enhanced chemiluminescence (Amersham™ ECL™ Western Blotting
Detection reagent; GE Healthcare Life Sciences, Chalfont, UK). The
KARPAS 299 cell line, previously shown to overexpress eIF4E and
4EBP1 proteins (19), served as a
positive control in all immunoblots.
Immunohistochemistry
Immunohistochemical analysis was performed on 42
specimens, using previously published methods (19). Briefly, formalin-fixed,
paraffin-embedded tissue sections (4-µm thick) were obtained, and
subsequently deparaffinized in xylene and rehydrated in graded
series of alcohol solutions. Monoclonal antibodies specific for
total eIF4E (catalog no. 9742; dilution, 1:100) and 4EBP1 (catalog
no. 9644; dilution, 1:100) (Cell Signaling Technology, Inc.) were
used for immunostaining. Heat-induced antigen retrieval was
conducted using preheated target retrieval solution (Dako,
Glostrup, Denmark). Based on the intensity of immunostaining in
tissues, eIF4E and 4EBP1 expression levels were semi-quantitatively
graded as ‘strongly positive’ (2+), ‘weakly positive’ (1+) or
negative (0). A formalin-fixed, paraffine-embedded cell block with
KARPAS 299 cells (a gift from Dr Marshall Kadin, Boston University
and Roger Williams Medical Center, Providence, RI, USA) exhibiting
overexpression of eIF4E and 4EBP1 proteins (17), served as a positive control.
PCR analysis of HPV types
Detection and subtyping of HPV was based on a nested
PCR method using suitable sets of primers, which were specific for
all 14 high-risk HPV (HR-HPV) types, as described previously
(18). Genomic DNA was extracted from
paraffin sections of all 73 tissue specimens using standard methods
(18).
Statistical analysis
Statistical analysis was performed to compare high
(2+) vs. low/absent expression (1+/0) of eIF4E and 4EBP1 with the
clinicopathological parameters and HPV status of the patients using
the χ2 and Fisher's exact tests. P<0.05 was considered to
indicate a statistically significant difference. Statistical
calculations were performed using StatView (version 5.0; SAS
Institute Inc., Cary, NC, USA).
Results
Colposcopy and histology findings
Colposcopy of the cervix was performed after the
application of dilute 5% acetic acid. The ability of histology to
define the true level of disease is dependent on accurately
biopsied abnormal-appearing lesions (7). However, certain patients had areas of
acetowhite changes and vessel abnormalities in the transformation
zone. Besides, other patients with certain vaginal infections had
also vessels with changes. In all these cases, a biopsy was
necessary to establish the origin of acetowhite or angiogenic
change. The colposcopic findings in association with histologic
diagnosis are summarized in Table
II. In total, 68 cases with available data were statistically
analyzed. The colposcopy and biopsy groups were stratified into
three categories: High, low and negative; 28
specimens were high (HG-SIL or Ca), 29 low (LG-SIL)
and 11 negative. Of the 28 biopsy specimens with HG-SIL or
Ca, 24 were evaluated as HG-SIL or cancer and only 4 as LG-SIL on
colposcopy. Of the 29 biopsy specimens with LG-SIL, 26 were
categorized as LG-SIL and only 3 as negative
colposcopically. Overall, the association between the colposcopic
findings and histologic diagnosis was very strong (P<0.0001, χ2
test). In summary, these data indicate sufficiency in the
diagnostic accuracy of colposcopically-directed cervical biopsies
in the present study group.
 | Table II.Expression of 4EBP1/eIF4E proteins in
association with patient characteristics. |
Table II.
Expression of 4EBP1/eIF4E proteins in
association with patient characteristics.
|
| WB | IHC |
|---|
|
|
|
|
|---|
| Patient | Age
groupa | Colposcopy | Pap test | Biopsy | HR-HPV | HPV16/18 | 4EBP1 | eIF4E | 4EBP1 | eIF4E |
|---|
| 1 | 2 | Neg | Neg | Neg | Yes | No | Low | Low | 0 | 1+ |
| 2 | 1 | Low | LG-SIL | LG-SIL | No | No | Low | Low | 1+ | 0 |
| 3 | 2 | Low | Susp | LG-SIL | Yes | Yes | Low | Low | 1+ | 1+ |
| 4 | 3 | Neg | Neg | Neg | No | No | Low | Low | NP | NP |
| 5 | 2 | High | HG-SIL | HG-SIL | NP | NP | High | High | 1+ | 1+ |
| 6 | 2 | Low | LG-SIL | LG-SIL | Yes | No | Low | Low | 1+ | 1+ |
| 7 | 2 | Low | LG-SIL | LG-SIL | Yes | Yes | Low | Low | 1+ | 2+ |
| 8 | 1 | Low | Neg | LG-SIL | No | No | Low | Low | 2+ | 1+ |
| 9 | 2 | Low | LG-SIL | LG-SIL | Yes | Yes | NP | NP | 1+ | 1+ |
| 10 | 2 | Low | LG-SIL | HG-SIL | NP | NP | High | High | 2+ | 2+ |
| 11 | 2 | Low | LG-SIL | LG-SIL | Yes | Yes | Low | Low | 1+ | 0 |
| 12 | 2 | Low | LG-SIL | HG-SIL | NP | NP | High | Low | NP | NP |
| 13 | 1 | Low | LG-SIL | HG-SIL | Yes | Yes | Low | Low | NP | NP |
| 14 | 2 | High | HG-SIL | HG-SIL | Yes | No | High | High | 2+ | 1+ |
| 15 | 3 | High | NP | SCC | Yes | Yes | High | High | 2+ | 2+ |
| 16 | 3 | Low | LG-SIL | LG-SIL | No | No | Low | Low | 1+ | 1+ |
| 17 | 2 | Low | LG-SIL | LG-SIL | No | No | Low | Low | NP | NP |
| 18 | 3 | Low | Susp | LG-SIL | Yes | Yes | NP | NP | 1+ | 0 |
| 19 | 3 | Neg | Neg | Neg | Yes | Yes | Low | Low | 0 | 0 |
| 20 | 3 | High | Neg | SCC | Yes | No | High | High | 2+ | 2+ |
| 21 | 2 | High | HG-SIL | HG-SIL | Yes | Yes | Low | Low | 2+ | 1+ |
| 22 | 3 | High | NP | SCC | Yes | Yes | High | High | 2+ | 2+ |
| 23 | 3 | High | NP | SCC | Yes | Yes | High | High | 2+ | 2+ |
| 24 | 2 | High | Susp | SCC | NP | NP | High | High | 2+ | 2+ |
| 25 | 2 | High | NP | SCC | NP | NP | NP | NP | 2+ | 2+ |
| 26 | 1 | High | LG-SIL | HG-SIL | Yes | No | Low | Low | 2+ | 2+ |
| 27 | 3 | High | HG-SIL | SCC | Yes | No | High | High | 2+ | 2+ |
| 28 | 2 | High | HG-SIL | SCC | NP | NP | High | High | 2+ | 2+ |
| 29 | 2 | High | HG-SIL | SCC | Yes | Yes | NP | NP | 2+ | 2+ |
| 30 | 2 | High | HG-SIL | HG-SIL | Yes | Yes | Low | Low | 2+ | 2+ |
| 31 | 2 | High | HG-SIL | HG-SIL | NP | NP | NP | NP | 2+ | 2+ |
| 32 | 2 | High | HG-SIL | HG-SIL | Yes | Yes | NP | NP | 2+ | 2+ |
| 33 | 2 | High | HG-SIL | SCC | Yes | Yes | High | High | 2+ | 2+ |
| 34 | 2 | Low | HG-SIL | LG-SIL | No | No | High | Low | 1+ | 1+ |
| 35 | 3 | High | NP | SCC | Yes | Yes | High | High | 2+ | 2+ |
| 36 | 3 | High | NP | SCC | Yes | Yes | High | High | 2+ | 2+ |
| 37 | 2 | Low | HG-SIL | LG-SIL | No | No | Low | Low | 1+ | 1+ |
| 38 | 2 | Low | Neg | Neg | No | No | Low | Low | 1+ | 0 |
| 39 | 3 | Low | Neg | Neg | No | No | Low | Low | 0 | 1+ |
| 40 | 2 | Low | Neg | Neg | Yes | No | Low | Low | 0 | 0 |
| 41 | 2 | Low | Neg | Neg | Yes | No | Low | Low | 0 | 0 |
| 42 | 1 | Low | Neg | Neg | Yes | Yes | Low | Low | 1+ | 0 |
| 43 | 2 | Low | Neg | Neg | No | No | Low | Low | 1+ | 1+ |
| 44 | 2 | Low | Neg | Neg | Yes | Yes | Low | Low | 1+ | 1+ |
| 45 | 3 | High | Neg | HG-SIL | Yes | No | NP | NP | 2+ | 2+ |
| 46 | 2 | High | Susp | HG-SIL | Yes | Yes | NP | NP | 2+ | 2+ |
| 47 | 1 | High | LG-SIL | HG-SIL | Yes | Yes | Low | Low | NP | NP |
| 48 | 1 | High | LG-SIL | HG-SIL | Yes | Yes | Low | Low | NP | NP |
Detection of eIF4E and 4EBP1 protein
levels in dysplastic lesions and cancer of the uterine cervix in
immunoblots
Western blot analysis revealed high expression
levels of eIF4E and 4EBP1 proteins in all cancer specimens tested
(Fig. 1). The eIF4E and 4EBP1
expression level and its association with clinicopathological
parameters are summarized in Table
II. Among 19 evaluable samples with high-grade dysplasia or
carcinomas, overexpression of 4EBP1 and eIF4E proteins was observed
in 14 of 19 (73%) and 13 of 19 (68%) samples, respectively,
compared with 1 of 10 (10%) and 0 of 10 (0%) samples in patients
with low-grade lesions (P=0.0004 and P=0.0017 for 4EBP1 and eIF4E,
respectively, Fisher's exact test). In addition, strong
co-expression of 4EBP1 and eIF4E proteins was also noted in the
entire patient group (P<0.0001, χ2 test).
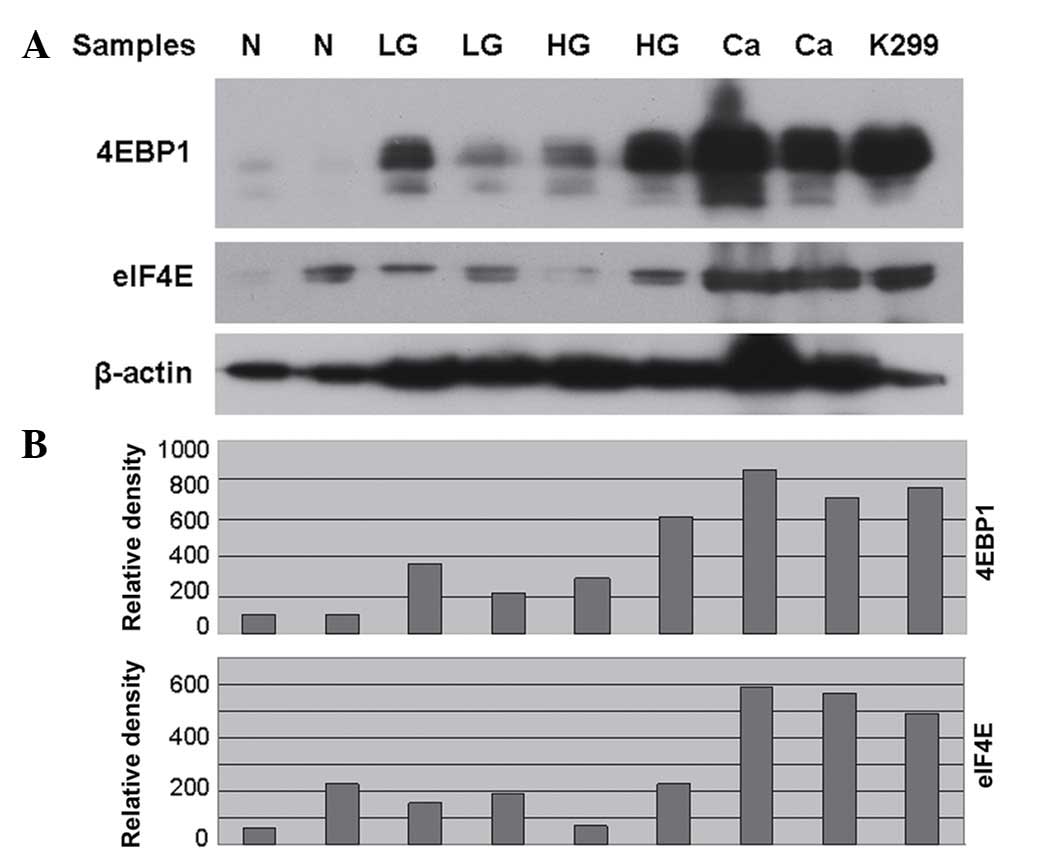 | Figure 1.(A) Representative immunoblot of the
protein expression levels of 4EBP1 and eIF4E in whole protein
extracts from patient samples with N, LG-SIL, HG-SIL and Ca. The
KARPAS 299 cell line served as a positive control for high
expression levels of both proteins. (B) Bar graphs obtained by
densitometric analysis revealed the differences in the expression
levels of 4EBP1 and eIF4E among N, LG-SIL, HG-SIL and Ca samples.
The Y-axis represents density units, as measured by
AlphaImager® software (Proteinsimple, San Jose, CA,
USA). N, normal histology; SIL, squamous intraepithelial lesion;
LG, low grade; HG, high grade; Ca, cervical cancer; K299, KARPAS
299; 4EBP1, 4E binding protein 1; eIF4E, eukaryotic initiation
factor 4E. |
Immunohistochemical expression of
eIF4E and 4EBP1 in cervical precancerous lesions and cancer
To further elucidate the expression patterns and
distribution of eIF4E and 4EBP1 in cervical lesions,
immunohistochemical analysis was performed using specific
anti-eIF4E and anti-4EBP1 antibodies. This study subgroup included
42 biopsy specimens from patients with normal histology,
precancerous lesions (LG-SIL and HG-SIL) and Ca. Histologic slides
from a medullary thyroid carcinoma known to overexpress eIF4E and
4EBP1 proteins (20) were used as
positive controls. Overexpression (2+) of eIF4E and 4EBP1 was
observed in all cancer specimens with faint expression in the
surrounding stromal area (Fig. 2). By
immunohistochemistry, overexpression of 4EBP1 and eIF4E was
observed in 20 of 21 (95%) and 17 of 21 (81%) samples,
respectively, in patients with high-grade dysplasia and carcinomas,
compared with 1 of 20 (5%) and 2 of 20 (10%) specimens in patients
with low-grade lesions or normal histology (P<0.0001, Fisher's
exact test) (Fig. 2A). In a selected
group of 10 4EBP1-positive cases with high-grade dysplasia or
carcinomas and 8 4EBP1-negative cases with low-grade lesions or
normal histology, immunohistochemistry for the phosphorylated form
of 4EBP1 (P-4EBP1) was performed. All (10/10) 4EBP1-positive cases
were also positive for P-4EBP1, compared with none (0/8) of
4EBP1-negative cases with low-grade lesions or normal histology
(P<0.0010, Fisher's exact test) (Fig.
2B). Of note, in both cases with LG-SIL histology and eIF4E
overexpression, squamous metaplasia was present in the lesions
(Fig. 3). Immunohistochemical
co-expression of the proteins reached significance (P<0.0001, χ2
test).
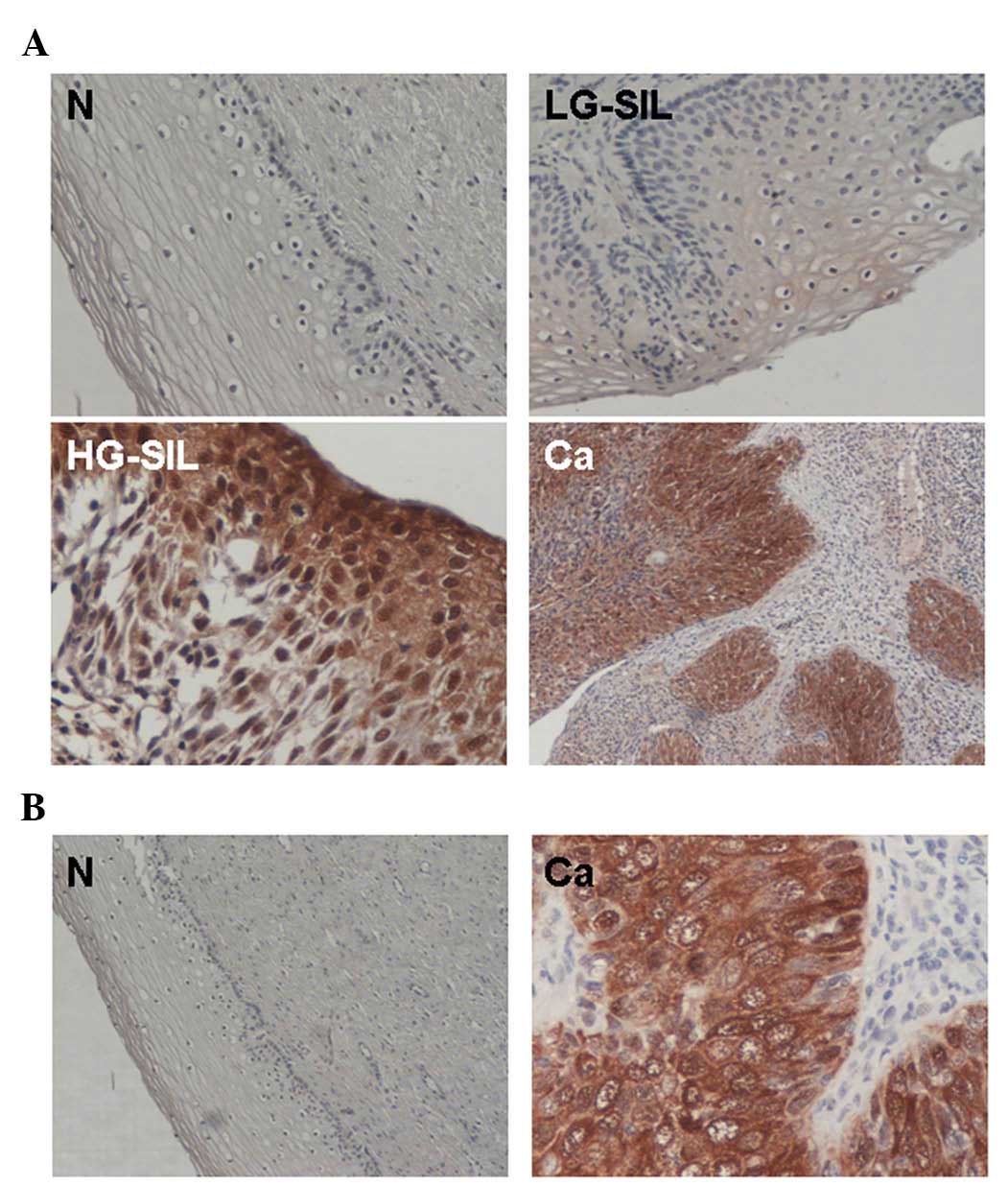 | Figure 2.(A) Immunohistochemical expression of
4EBP1 in cervical tissue specimens from patients with N, LG-SIL,
HG-SIL and Ca. 4EBP1 expression was minimal in the surrounding
stromal tissue, compared with the strong expression observed in
specimens with HG-SIL and cancer of the uterine cervix. Original
magnification, ×100 (N and LG-SIL), ×200 (Ca) and ×400 (HG-SIL).
(B) Representative examples of expression of P-4EBP1. Left,
P-4EBP1-negative specimen of cervical epithelium with normal
histology. Right, P-4EBP1-positive specimen of carcinoma of the
uterine cervix. Original magnification, ×100 (N) and ×400 (Ca). N,
normal squamous epithelium; SIL, squamous intraepithelial lesion;
LG, low grade; HG, high grade; Ca, cervical cancer; 4EBP1, 4E
binding protein 1; P-4EBP1, phosphorylated 4E binding protein
1. |
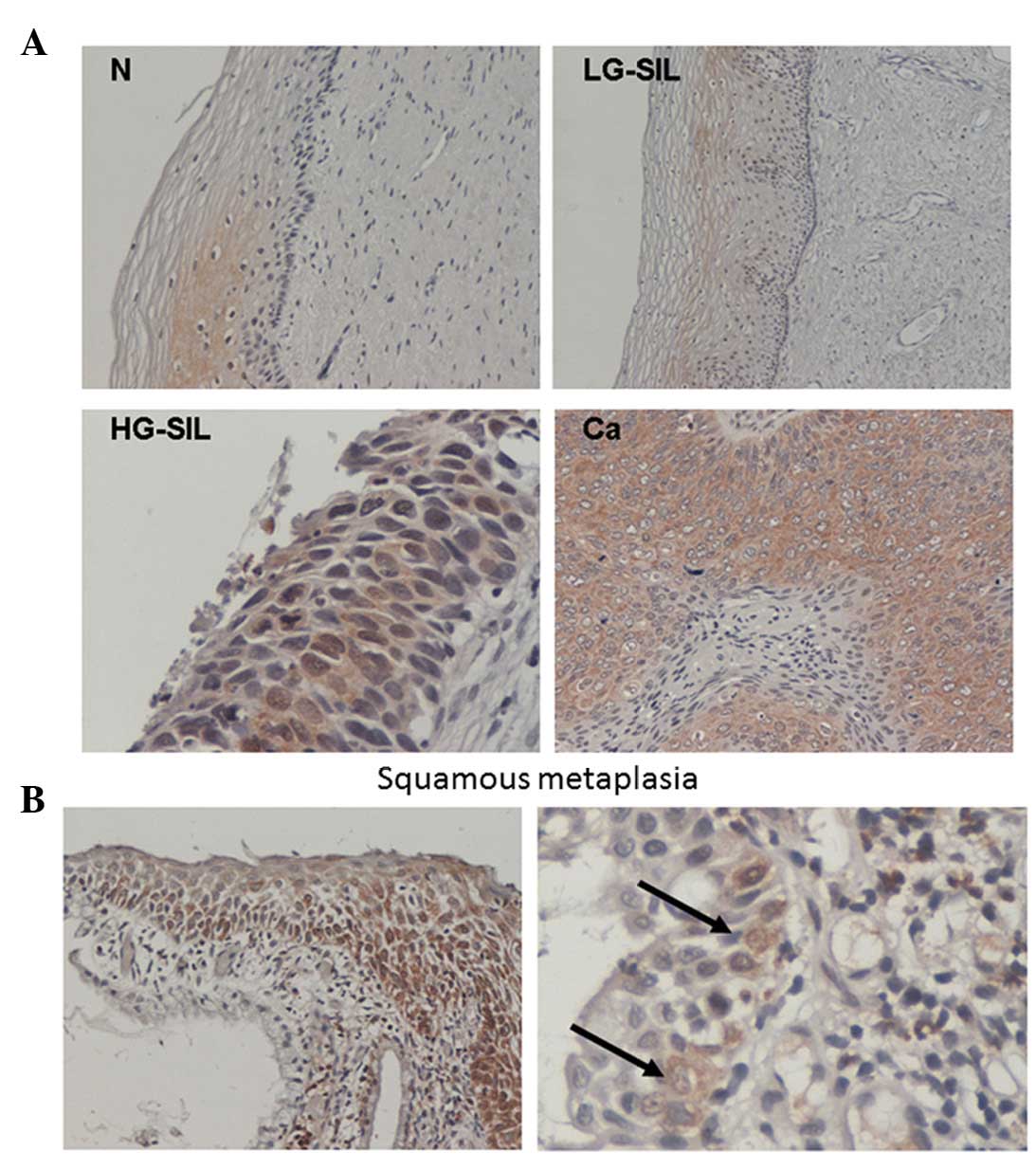 | Figure 3.(A) Immunohistochemical expression of
eIF4E in cervical tissue specimens from patients with N, LG-SIL,
HG-SIL and Ca, being at the highest level in high-grade dysplasia
and carcinoma tissue. eIF4E expression was minimal or undetectable
in the surrounding stromal tissue. Original magnification, ×100 (N
and LG-SIL), ×200 (Ca) and ×400 (HG-SIL). (B) Immunohistochemical
expression of eIF4E in cervical tissue specimens with squamous
metaplasia and presence of high-risk human papilloma virus type 16.
eIF4E was detected at a high level in the majority (left panel) or
a subset (right panel) of metaplastic cells in the basal layer.
Arrows indicate metaplastic squamous epithelial cells
strongly-positive for eIF4E. Original magnification, ×100 (left)
and ×400 (right). N, normal squamous epithelium; SIL, squamous
intraepithelial lesion; LG, low grade; HG, high grade; Ca, cervical
cancer; eIF4E, eukaryotic initiation factor 4E. |
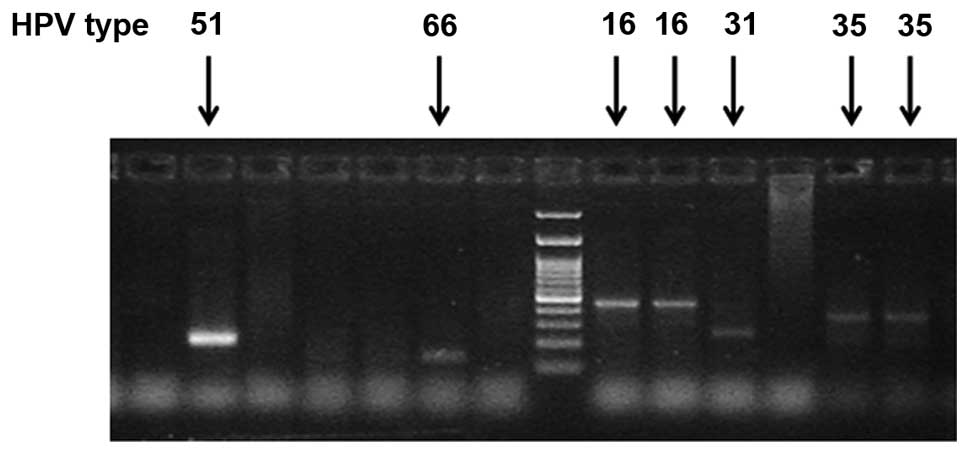
Associations with HPV status
Detection of any of the 14 HR-HPV types as a group
(Fig. 4) was significantly associated
with colposcopy findings and histologic diagnosis (P=0.0040 and
P=0.0030, respectively, Fisher's exact test) in the present study
group. Overexpression of 4EBP1 and eIF4E statistically correlated
with the presence of HR-HPV oncogenic types (P=0.0400 and P=0.0100,
respectively, Fisher's exact test, Table III). In addition, detection of the
most oncogenic HPV16/18 types was statistically associated with
expression of eIF4E (P=0.0480, Fisher's exact test) but not with
4EBP1 overexpression (P=0.2000, Fisher's exact test).
 | Table III.Correlation between expression of
eIF4E and 4EBP1 and HR-HPV. |
Table III.
Correlation between expression of
eIF4E and 4EBP1 and HR-HPV.
| Expression | HR-HPV
positive | HR-HPV
negative | P-value |
|---|
| 4EBP1 |
|
| 0.0400 |
|
High | 16 | 1 |
|
|
Low/neg | 12 | 7 |
|
| eIF4E |
|
| 0.0100 |
|
High | 15 | 0 |
|
|
Low/neg | 13 | 8 |
|
Discussion
Recent evidence suggests a link between the mTOR
signaling pathway and cancer, as the majority of the upstream and
downstream components of the mTOR signaling pathway are directly
implicated in cancer initiation and progression (9). The present study aimed to investigate
whether the expression of the two main downstream effectors of the
mTOR signaling pathway, 4EBP1 and eIF4E, may be involved in the
progression of dysplasia to cancer of the uterine cervix.
Therefore, the expression levels of these proteins and their
correlation with the degree of the intraepithelial lesion of the
uterine cervix and the presence of HR-HPV types were investigated.
The present study demonstrated that 4EBP1 and eIF4E proteins were
strongly expressed in all Ca specimens using western blot analysis
and immunohistochemical methods. These findings are in agreement
with the results of a previous study demonstrating mTOR kinase
activation in HG-SIL and cancer (21). However, in that study, the expression
levels of 4EBP1 and eIF4E proteins were not analyzed. In addition,
Faried et al (22)
demonstrated that activation (phosphorylation) of AKT and mTOR
kinase is detected in ~1/2 of the Cas tested, and represent
potential predictive and prognostic indicators in these
patients.
The present study also revealed that overexpression
of the 4EBP1/eIF4E axis significantly correlates with the degree of
cervical dysplasia (SIL). These results are in accordance with the
data published by Matthews-Greer et al (23), which reported a progressive increase
in immunohistochemical eIF4E expression with increasing degree of
cervical dysplastic lesions irrespective of HPV status. In a
selected group of cases, the present study also revealed that
overexpression of 4EBP1 correlated with its phosphorylation, which
is a strong indicator of the activity of the 4EBP1/eIF4E axis and
mTOR signaling, which is associated with cancer development and
progression (24). More specifically,
mTOR complex 1 (mTORC1) positively regulates cell growth, and its
inhibition causes cell size decrease at least in part through the
4EBP1/eIF4E axis (24). Inhibition of
4EBP1 due to sequential phosphorylation by mTOR kinase results in
the release of eIF4E, which, in its free form, is capable of
initiating the translation of various mRNAs, including cell cycle
regulators such as cyclin D1 or c-Myc (24). Therefore, eIF4E has been widely
recognized to function as an oncogene, whereas 4EBP1 has
anti-oncogenic properties (24). For
instance, experimental overexpression of eIF4E can cause malignant
transformation (24). Upstream of
mTOR, several oncogenic mechanisms may operate. Inactivation of
tumor suppressors, in particular phosphatase and tensin homolog
(PTEN), but also p53 and neurofibromatosis type 1, has been linked
to mTOR-regulatory-associated protein of mTOR (raptor) activation
(9). Notably, numerous cancer cells
without PTEN function and, therefore, with activated AKT signaling,
are highly sensitive to the anti-proliferative effects of rapamycin
(25,26).
Of note, the only two cases in the present study
with LG-SIL and strong expression of eIF4E were associated with
squamous metaplasia. It is known that squamous metaplasia may
precede epithelial dysplasia of the cervix (7); however, the exact mechanisms underlying
this transition are yet unknown. It is tempting to speculate that
the mTOR-raptor pathway may be involved in the process, but this
merits further investigation.
Besides, it is well established that HPV infection
is necessary for cervical carcinogenesis, and therefore, HR-HPV DNA
testing is used for screening and secondary prevention (27,28). In
the present report, HPV-DNA testing by PCR was used for the
detection of HR-HPV types in order to analyze potential
associations with the expression of 4EBP1 and eIF4E. Overexpression
of 4EBP1 and eIF4E was observed to be significantly associated with
the presence of HR-HPV types. This finding suggests that a
potential biologic link between the mTOR signaling pathway and
oncogenic HPV oncoproteins may exist. This hypothesis is supported
by the results of previous studies, which have demonstrated that i)
eIF4E is upregulated in cervical neoplasia (23); ii) E6 oncoprotein activates mTORC1
signaling and increases protein synthesis (29); and iii) rapamycin significantly
decreases the levels of E7 protein in vitro through the
inhibition of mTORC1 (20).
Furthermore, a recent study demonstrated that transcription of the
eIF4E gene is induced by the E6 oncoprotein of HPV (30). However, previous studies have reported
that activation of AKT may be observed in HPV-negative tumors of
the head and neck (31).
Modulation of the mTOR-raptor activity by mTOR
inhibitors as well as other targeted therapies has demonstrated
promising results in HPV-associated squamous cell carcinomas in
vitro (32). Thus, mTOR
inhibitors currently tested in clinical trials may indeed have a
role in the treatment of carcinomas of the uterine cervix, most
likely in combination with chemotherapeutic agents. For instance,
use of the single agent temsirolimus has demonstrated activity in
cervical carcinoma, with ~2/3 of patients exhibiting stable disease
(33). However, despite the
increasing knowledge, the role of the mTOR signaling pathway in
uterine cervical tumorigenesis requires further investigation.
Better understanding of the mTOR pathway may provide the
mechanistic basis for novel investigational therapeutic approaches
aimed to improve the clinical outcome of women with high-grade
dysplasia or cancer of the uterine cervix.
References
|
1
|
Forman D, de Martel C, Lacey CJ,
Soerjomataram I, Lortet-Tieulent J, Bruni L, Vignat J, Ferlay J,
Bray F, Plummer M and Franceschi S: Global burden of human
papillomavirus and related diseases. Vaccine. 30(Suppl 5): F12–F23.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Health Organization, . Cervix Cancer
ScreeningIARC Handbook of Cancer Prevention. 1st. 10. IARC Press;
Lyon: 2005, pp. 168–173
|
|
4
|
Muñoz N, Castellsagué X, de González AB
and Gissmann L: Chapter 1: HPV in the etiology of human cancer.
Vaccine. 24(Suppl 3): 1–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arends MJ, Buckley CH and Wells M:
Aetiology, pathogenesis, and pathology of cervical neoplasia. J
Clin Pathol. 51:96–103. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richart RM: A modified terminology for
cervical intraepithelial neoplasia. Obstet Gynecol. 75:131–133.
1990.PubMed/NCBI
|
|
9
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Surviladze Z, Sterk RT, DeHaro SA and
Ozbun MA: Cellular entry of human papillomavirus type 16 involves
activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway
and inhibition of autophagy. J Virol. 87:2508–2517. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gulhati P, Cai Q, Li J, Liu J, Rychahou
PG, Qiu S, Lee EY, Silva SR, Bowen KA, Gao T and Evers BM: Targeted
inhibition of mammalian target of rapamycin signaling inhibits
tumorigenesis of colorectal cancer. Clin Cancer Res. 15:7207–7216.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clark C, Shah S, Herman-Ferdinandez L,
Ekshyyan O, Abreo F, Rong X, McLarty J, Lurie A, Milligan EJ and
Nathan CO: Teasing out the best molecular marker in the AKT/mTOR
pathway in head and neck squamous cell cancer patients.
Laryngoscope. 120:1159–1165. 2010.PubMed/NCBI
|
|
13
|
Molinolo AA, Marsh C, El Dinali M, Gangane
N, Jennison K, Hewitt S, Patel V, Seiwert TY and Gutkind JS: mTOR
as a molecular target in HPV-associated oral and cervical squamous
carcinomas. Clin Cancer Res. 18:2558–2568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coleman LJ, Peter MB, Teall TJ, Brannan
RA, Hanby AM, Honarpisheh H, Shaaban AM, Smith L, Speirs V,
Verghese ET, et al: Combined analysis of eIF4E and 4E-binding
protein expression predicts breast cancer survival and estimates
eIF4E activity. Br J Cancer. 100:1393–1399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
No JH, Jeon YT, Park IA, Kim YB, Kim JW,
Park NH, Kang SB, Han JY, Lim JM and Song YS: Activation of mTOR
signaling pathway associated with adverse prognostic factors of
epithelial ovarian cancer. Gynecol Oncol. 121:8–12. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inamdar KV, Romaguera JE, Drakos E,
Knoblock RJ, Garcia M, Leventaki V, Medeiros LJ and Rassidakis GZ:
Expression of eukaryotic initiation factor 4E predicts clinical
outcome in patients with mantle cell lymphoma treated with
hyper-CVAD and rituximab, alternating with rituximab, high-dose
methotrexate, and cytarabine. Cancer. 115:4727–4736. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kouvaraki MA, Liakou C, Paraschi A, Dimas
K, Patsouris E, Tseleni-Balafouta S, Rassidakis GZ and Moraitis D:
Activation of mTOR signaling in medullary and aggressive papillary
thyroid carcinomas. Surgery. 150:1258–1265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sotlar K, Diemer D, Dethleffs A, Hack Y,
Stubner A, Vollmer N, Menton S, Menton M, Dietz K, Wallwiener D, et
al: Detection and typing of human papillomavirus by e6 nested
multiplex PCR. J Clin Microbiol. 42:3176–3184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vega F, Medeiros LJ, Leventaki V, Atwell
C, Cho-Vega JH, Tian L, Claret FX and Rassidakis GZ: Activation of
mammalian target of rapamycin signaling pathway contributes to
tumor cell survival in anaplastic lymphoma kinase-positive
anaplastic large cell lymphoma. Cancer Res. 66:6589–6597. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spangle JM and Münger K: The human
papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling
and increases protein synthesis. J Virol. 84:9398–9407. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng W, Duan X, Liu J, Xiao J and Brown
RE: Morphoproteomic evidence of constitutively activated and
overexpressed mTOR pathway in cervical squamous carcinoma and high
grade squamous intraepithelial lesions. Int J Clin Exp Pathol.
2:249–260. 2009.PubMed/NCBI
|
|
22
|
Faried LS, Faried A, Kanuma T, Sano T,
Nakazato T, Tamura T, Kuwano H and Minegishi T: Predictive and
prognostic role of activated mammalian target of rapamycin in
cervical cancer treated with cisplatin-based neoadjuvant
chemotherapy. Oncol Rep. 16:57–63. 2006.PubMed/NCBI
|
|
23
|
Matthews-Greer J, Caldito G, de Benedetti
A, Herrera GA, Dominguez-Malagon H, Chanona-Vilchis J and
Turbat-Herrera EA: eIF4E as a marker for cervical neoplasia. Appl
Immunohistochem Mol Morphol. 13:367–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neshat MS, Mellinghoff IK, Tran C, Stiles
B, Thomas G, Petersen R, Frost P, Gibbons JJ, Wu H and Sawyers CL:
Enhanced sensitivity of PTEN-deficient tumors to inhibition of
FRAP/mTOR. Proc Natl Acad Sci USA. 98:10314–10319. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Podsypanina K, Lee RT, Politis C, Hennessy
I, Crane A, Puc J, Neshat M, Wang H, Yang L, Gibbons J, et al: An
inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase
activity in Pten+/-mice. Proc Natl Acad Sci USA. 98:10320–10325.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ronco G, Biggeri A, Confortini M, Naldoni
C, Segnan N, Sideri M, Zappa M, Zorzi M, Calvia M, Accetta G, et
al: Health technology assessment report: HPV DNA based primary
screening for cervical cancer precursors. Epidemiol Prev. 36(3-4):
Suppl 1. e1–e72. 2012.
|
|
28
|
Arbyn M, Ronco G, Anttila A, Meijer CJ,
Poljak M, Ogilvie G, Koliopoulos G, Naucler P, Sankaranarayanan R
and Peto J: Evidence regarding human papillomavirus testing in
secondary prevention of cervical cancer. Vaccine. 30(Suppl 5):
F88–F99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oh KJ, Kalinina A, Park NH and Bagchi S:
Deregulation of eIF4E: 4E-BP1 in differentiated human
papillomavirus-containing cells leads to high levels of expression
of the E7 oncoprotein. J Virol. 80:7079–7088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang S, Pang T, Gao M, Kang H, Ding W, Sun
X, Zhao Y, Zhu W, Tang X, Yao Y and Hu X: HPV E6 induces eIF4E
transcription to promote the proliferation and migration of
cervical cancer. FEBS Lett. 587:690–697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Won HS, Jung CK, Chun SH, Kang JH, Kim YS,
Sun DI and Kim MS: Difference in expression of EGFR, pAkt, and PTEN
between oropharyngeal and oral cavity squamous cell carcinoma. Oral
Oncol. 48:985–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aderhold C, Faber A, Umbreit C, Birk R,
Weiss C, Sommer JU, Hörmann K and Schultz JD: Targeting mTOR and
AREG with everolimus, sunitinib and sorafenib in HPV-positive
and-negative SCC. Anticancer Res. 35:1951–1959. 2015.PubMed/NCBI
|
|
33
|
Tinker AV, Ellard S, Welch S, Moens F,
Allo G, Tsao MS, Squire J, Tu D, Eisenhauer EA and MacKay H: Phase
II study of temsirolimus (CCI-779) in women with recurrent,
unresectable, locally advanced or metastatic carcinoma of the
cervix. A trial of the NCIC Clinical Trials Group (NCIC CTG IND
199). Gynecol Oncol. 130:269–274. 2013. View Article : Google Scholar : PubMed/NCBI
|