Introduction
Meningiomas arise from meningeal arachnoid cells. A
key histological characteristic associated with these lesions is
the concentric arrangement of meningioma cells around calcified
tissue, known as psammoma bodies, to form a whorl appearance.
Microscopically, the World Health Organization (WHO) classification
scheme recognizes 15 variations of meningioma, which can be
classified into benign (grade I), atypical (grade II) and malignant
(grade III). Approximately 90% of all meningiomas fall into the
grade-I category, which includes nine histological subtypes:
Meningothelial, fibrous (fibroblastic), transitional (mixed),
psammomatous, angiomatous, microcystic, secretory,
lymphoplasmacyte-rich and metaplastic. Based on molecular
pathogenesis, meningiomas are classified as neurofibromin 2 [NF2;
Drosophila Merlin (Mer)] mutant meningiomas (NF2-associated
meningiomas) and non-NF2 meningiomas, with the loss of NF2 observed
in 40–60% of sporadic meningioma cases (1,2). Hedgehog
(Hh) signaling molecules are considered to be involved in the
pathogenesis of meningioma due to the detection of suppressor of
fused (SUFU), a component of the Hh signaling pathway, in familial
multiple meningioma (3). However, the
frequency of mutations in SUFU is extremely low, with no SUFU
mutations detected in a study of 162 individuals with sporadic
meningioma (3). Previously,
next-generation sequencing analysis of non-NF2 meningiomas
identified novel, recurrent mutations in the following genes: TNF
receptor-associated factor 7, Krüppel-like factor 4, v-akt murine
thymoma viral oncogene homolog 1 and smoothened, frizzled class
receptor (1,2). In addition, it was demonstrated that NF2
meningiomas are associated with the Hippo (Hpo) pathway, which
regulates tissue growth, proliferation and ultimately controls
organ size (4–6).
The present study hypothesized that the whorl
appearance of meningioma may occur as a result of a disturbance in
planar cell polarity (PCP), and the molecular pathogenesis of the
whorl appearance may involve genes that control PCP. In
Drosophila, the PCP signaling molecules have been well
characterized, and include Frizzled [human homologs, frizzled class
receptor (FZD)3 and FZD6], Dishevelled [human homologs, dishevelled
segment polarity protein (DVL)1, DVL2 and DVL3], Flamingo [human
homologs, cadherin, EGF LAG seven-pass G-type receptor (CELSR)1,
CELSR2 and CELSR3], Diego (human homolog, ankyrin repeat domain 6),
Van Gogh [human homologs, VANGL planar cell polarity protein
(VANGL)1 and VANGL2] and Prickle [human homologs, prickle planar
cell polarity protein (PRICKLE)1 and PRICKLE2] (7). Fat (Ft)-Hpo signaling is also involved
in PCP (7). The Ft-Hpo signaling
molecules in Drosophila are as follows: Ft [human homologs,
FAT atypical cadherin (FAT)1, FAT2, FAT3 and FAT4], Dachsous [human
homologs, dachsous cadherin-related (DCHS)1 and DCHS2],
Four-jointed (human homolog, four jointed box 1), Dachs, Expanded
[human homologs, FERM domain containing (FRMD)1 and FRMD2], Mer
(human homolog, NF2), Kibra ortholog [human homologs, WW and C2
domain containing (WWC)1 and WWC2], Salvador (human homolog,
salvador family WW domain containing protein 1), Hpo
[serine/threonine kinase (STK)4 and STK3], Warts [human homologs,
large tumor suppressor kinase (LATS)1 and LATS2], Mats [human
homologs, MOB kinase activator (MOB)1A and MOB1B], Yorkie (human
homologs, yes-associated protein 1 and tafazzin) and Scalloped
[human homologs, TEA domain transcription factor (TEAD)1, TEAD2,
TEAD3 and TEAD4] (8,9). The current study performed whole exome
sequencing (WES) analysis to identify mutated genes in spinal
meningioma by screening the human homologs of Drosophila PCP
genes, in addition to Ft-Hpo signaling genes. A nonsynonymous
mutation was identified in the FAT2 gene in a non-NF2
meningioma, thus indicating a possible association between the
FAT2 gene and the molecular pathogenesis of meningioma.
Patient and methods
A 42-year-old female patient was admitted to Showa
University Fujigaoka Hospital (Yokohama, Japan) in November 2013
after experiencing loss of consciousness and falling down twice
within 2 weeks. Approximately 1 month later, marked muscle weakness
was detected in the left leg. Magnetic resonance imaging of the
thorax, which was performed with a Signa HDxt 1.5T (GE Healthcare
Japan Corporation, Tokyo, Japan), revealed a tumor in the
extramedullary spinal cord and intradural space at the C7 to Th1
level. Surgery was performed to fully remove the tumor, which was
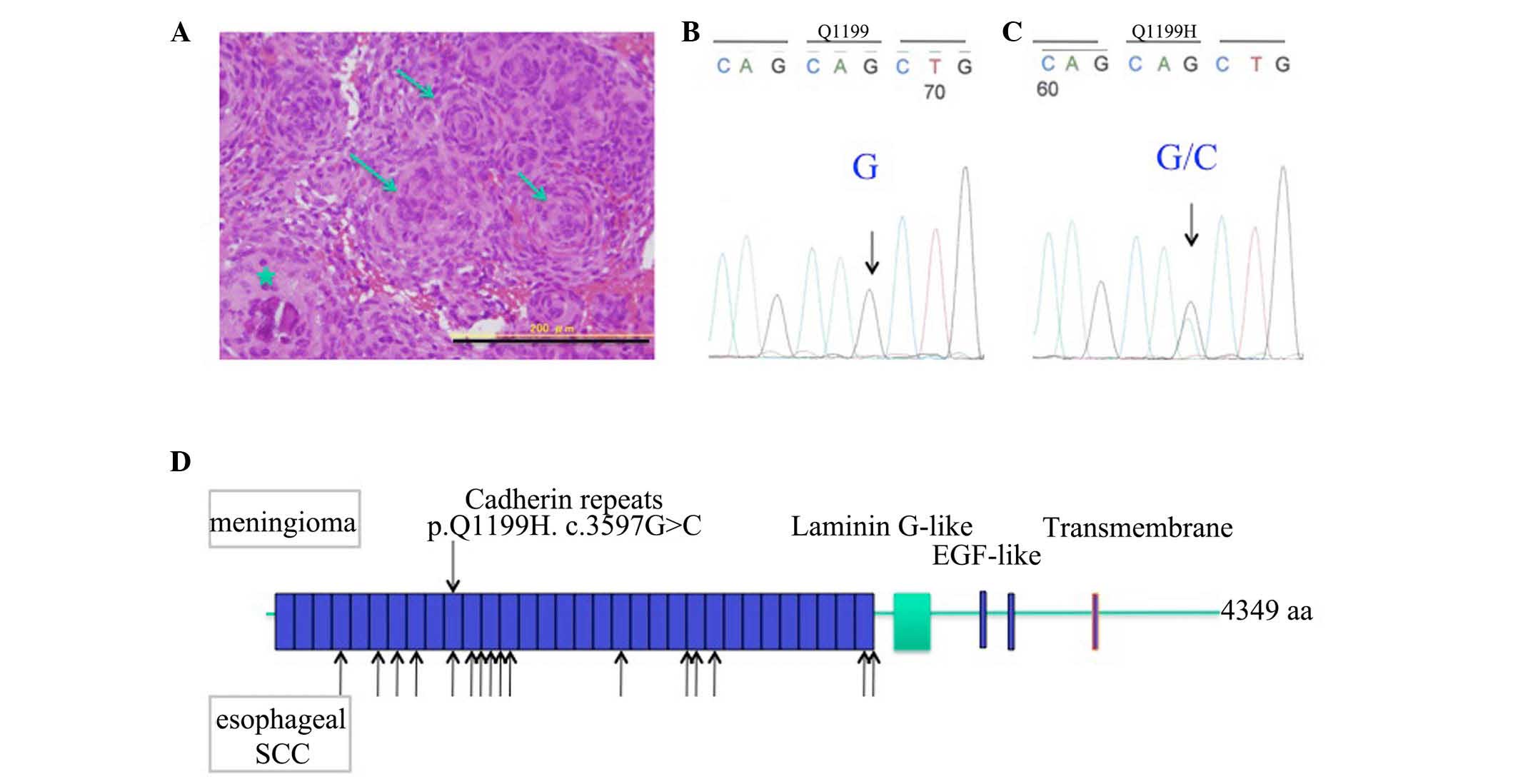
~1 cm in diameter, and histopathological analysis confirmed a
diagnosis of spinal meningioma, which exhibited numerous psammoma
bodies and a whorl formation of the neoplastic arachnoid cells
(Fig. 1A). For the analysis, the
tissue was formalin-fixed, and formalin-fixed paraffin-embedded
sections of 3 µm in thickness were prepared with a CRM-440
microtome (Sakura Seiki Co., Ltd., Tokyo, Japan), and subsequently
stained with hematoxylin and eosin (Merck Japan, Tokyo, Japan) as
previously described (10). Olympus
microscope BX60 and DP73 digital camera (Olympus Corporation,
Tokyo, Japan) with WinROOF2013 software (Mitani Valve Co., Ltd.,
Tokyo, Japan) were used for visual examination. According to the
WHO classification system (11), the
tumor was a grade I meningothelial meningioma.
Genomic DNA was extracted from the meningioma tissue
by phenol-chloroform extraction and ethanol precipitation methods
(12), and was subsequently used for
WES and Sanger sequencing. Peripheral blood mononuclear cells were
used as a DNA source for germline sequence analysis.
Next-generation WES using exome capture via in-solution
hybridization followed by massive parallel sequencing were
conducted as previously described (13). Briefly, ~3 µg of genomic DNA was
sheared to a mean fragment size of 300 bp, and the fragments were
used for Illumina Paired-End DNA library preparation and enrichment
of target sequences (Agilent Technologies, Inc., Santa Clara, CA,
USA). Exon capture was then performed using the SureSelect Human
All Exon v4 kit (Agilent Technologies, Inc.). The mean size of the
sequence library was 450 bp, and the enriched DNA fragments were
sequenced with 100-bp paired-end reads in the HiSeq 2000 sequencing
system (Illumina, Inc., San Diego, CA, USA). The sequencing reads
were aligned to the reference human genome (1000 Genomes;
http://www.1000genomes.org/) using the
Genome Analysis Toolkit (https://software.broadinstitute.org/gatk/) and
Burrows-Wheeler Aligner software (http://bio-bwa.sourceforge.net/). Single-nucleotide
substitutions and small insertions-deletions (indels) were
annotated against the RefSeq database (http://www.ncbi.nlm.nih.gov/refseq/) and The Single
Nucleotide Polymorphism database 137 (http://www.ncbi.nlm.nih.gov/SNP/) using the ANNOVAR
tool (http://annovar.openbioinformatics.org/en/latest/).
In order to validate any mutations, Sanger
sequencing was also performed. The primers used for FAT2
amplification were as follows: Forward,
5′-TCTTGAAGTTGCCTCAGTAAAGT-3′ and reverse,
5′-CTAACATGGCTCCACAAATCACC-3′. Polymerase chain reaction (PCR) was
conducted with 30 cycles of denaturation (98°C for 1 min),
annealing (65°C for 2 min) and extension (72°C for 3 min). PCR was
performed in a Quick Thermo Personal thermal cycler (Nippon
Genetics Co., Ltd., Tokyo, Japan), using deoxynucleotides (Takara
Bio, Inc., Otsu, Japan) and Tris-borate-ethylenediaminetetraacetic
acid (Takara Bio, Inc.) as a buffer. Amplified DNA fragments were
recovered from a low melting temperature agarose gel (2% Agarose L;
Wako Pure Chemical Industries, Ltd,. Osaka, Japan) and subjected to
direct sequencing analysis using an automated ABI PRISM®
377 DNA Sequencer (Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), as previously described (13).
Written informed consent was obtained from the
patient. The present study complied with the Declaration of
Helsinki revised in 2008 (14), and
was conducted according to the ethical guidelines presented
annually by the Committee for Medical Experiment at Showa
University.
Results
WES analysis identified no alterations of the
NF2 gene (22q12.2). Therefore, human genes homologous to
those involved in PCP and Ft-Hpo signaling in Drosophila
were analyzed (9). Subsequent to
screening of the WES data and validation by Sanger sequencing, a
nonsynonymous mutation in exon 4 of the FAT2 gene,
c.3597G>C, that resulted in p.Q1199H, was identified in spinal
meningioma tissue, which was not detected in the germline sequence,
indicating the heterozygous mutation of the FAT2 gene in
spinal meningioma (Fig. 1B and C).
The spinal meningioma mutation of the FAT2 gene was located
in the 10th of 32 cadherin repeats, although the same mutation was
not identified to be common to both esophageal squamous cell
carcinoma (ESCC) and the present case, as indicated by the arrows
in Fig. 1D. No other mutations were
detected among the PCP or Ft-Hpo signaling genes.
Discussion
Psammoma bodies observed in meningioma tissue
exhibit similar histological characteristics to those observed in
well-differentiated ESCC whose tumor cells form a whorl-like
arrangement, known as keratin pearls. These similarities in cell
arrangement in meningioma and esophageal SCC tissue indicate that
the horizontal cell polarity, which is controlled by cell surface
adhesion molecules (including cadherin family members), may be
disturbed in these tumors, whereby the tumor cells proliferate
towards the center of the tumor nest (11). Lin et al (15) identified 34 mutations of human
FAT genes, FAT1, FAT2 and FAT3, in 139 cases
of esophageal SCC. FAT2 gene mutations were reported in 12
of these cases (8 cases of missense and 4 cases of stop-gain,
splicing site, frameshift or indel), although no information
regarding the different histological types of esophageal SCC are
currently available (15).
In vitro and in vivo experiments have
demonstrated the involvement of FAT2 in the molecular
pathogenesis of SCC. Matsui et al (12) reported that human FAT2 is localized at
immature adherens junctions in epidermal keratinocytes, and the
knockdown of human FAT2 by siRNA inhibited the migration of
the cultured HSC-1 human SCC cell line. Furthermore, Lin et
al (15) demonstrated that the
depletion of FAT2 with small hairpin RNA promoted esophageal
SCC growth in vivo.
FAT2 is a member of the cadherin superfamily and is
homologous to Drosophila Ft, which functions as a positive
regulator of PCP in the Drosophila wing (8,9). The human
FAT2 gene (5q33.1) encodes a large, type I transmembrane
protein belonging to the cadherin superfamily, which consists of
4,349 amino acids with an extracellular domain (amino acids
19–4,048), transmembrane region (amino acids 4,049–4,069) and
cytoplasmic domain (amino acids 4,070–4,349). The extracellular
domain consists of 32 cadherin repeats, two epidermal growth
factor-like domains and one laminin G-like domain (9). The FAT2 mutation identified in
the present study was located in the cadherin repeats. How the
signaling of FAT2 with p.1199H differs from that of the wild-type
remains to be elucidated.
The present case of meningioma was classified as WHO
grade I, thus, indicating that the FAT2 mutation may be an
initial or early genetic alteration, as the number of mutated genes
in grade I meningioma is considered to be limited compared with
that in high-grade meningioma.
Immunohistochemistry is regarded as a simple method
to detect FAT2 expression in meningioma. At present, however, no
specific antibodies against human FAT2 are commercially available.
Thus, once an appropriate antibody for use with formalin-fixed,
paraffin-embedded sections has been developed, the performance of
routine medical examination to detect how FAT2 expression differs
among varying grades of meningioma is anticipated to improve. In
addition, in vitro analyses may aid investigation into how
FAT2 with p.1199H differs in its PCP signaling compared with the
wild-type protein in arachnoid cells.
In conclusion, the present study identified the
presence of a novel FAT2 somatic mutation in a non-NF2
spinal meningioma, indicating that the Hpo signaling pathway is
important in NF-2-associated and non-NF2 meningioma. Additionally,
it was hypothesized that a mutation of FAT2 may be involved
in the molecular pathogenesis of non-NF2 meningioma, potentially
providing a molecular target for novel therapeutic drugs for the
treatment of patients with meningioma.
References
|
1
|
Brastianos PK, Horowitz PM, Santagata S,
Jones RT, McKenna A, Getz G, Ligon KL, Palescandolo E, Van Hummelen
P, Ducar MD, et al: Genomic sequencing of meningiomas identifies
oncogenic SMO and AKT1 mutations. Nat Genet. 45:285–289. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clark VE, Erson-Omay EZ, Serin A, Yin J,
Cotney J, Ozduman K, Avşar T, Li J, Murray PB, Henegariu O, et al:
Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7,
KLF4, AKT1 and SMO. Science. 339:1077–1080. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aavikko M, Li SP, Saarinen S, Alhopuro P,
Kaasinen E, Morgunova E, Li Y, Vesanen K, Smith MJ, Evans DG, et
al: Loss of SUFU function in familial multiple meningioma. Am J Hum
Genet. 91:520–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang N, Bai H, David KK, Dong J, Zheng Y,
Cai J, Giovannini M, Liu P, Anders RA and Pan D: The Merlin/NF2
tumor suppressor functions through the YAP oncoprotein to regulate
tissue homeostasis in mammals. Dev Cell. 19:27–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu AM, Wong KF, Jiang X, Qiao Y and Luk
JM: Regulators of mammalian Hippo pathway in cancer. Biochim
Biophys Acta. 1826:357–364. 2012.PubMed/NCBI
|
|
6
|
Dong J, Feldmann G, Huang J, Wu S, Zhang
N, Comerford SA, Gayyed MF, Anders RA, Maitra A and Pan D:
Elucidation of a universal size-control mechanism in Drosophila and
mammals. Cell. 130:1120–1133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Viktorinová I, Pismen LM, Aigouy B and
Dahmann C: Modelling planar polarity of epithelia: The role of
signal relay in collective cell polarization. J R Soc Interface.
8:1059–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Viktorinová I, König T, Schlichting K and
Dahmann C: The cadherin Fat2 is required for planar cell polarity
in the Drosophila ovary. Development. 136:4123–4132. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katoh M: Function and cancer genomics of
FAT family genes (review). Int J Oncol. 41:1913–1918.
2012.PubMed/NCBI
|
|
10
|
Lyle HM: An improved tissue technique with
hematoxylin-eosin stain. Am J Med Technol. 13:178–181.
1947.PubMed/NCBI
|
|
11
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauser BW and Kleihues P: The 2007
WHO classification of tumors of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsui S, Utani A, Takahashi K, Mukoyama
Y, Miyachi Y and Matsuyoshi N: Knockdown of Fat2 by siRNA inhibits
the migration of human squamous carcinoma cells. J Dermatol Sci.
51:207–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tate G, Tajiri T, Kishimoto K and Mitsuya
T: A novel mutation of the axonemal dynein heavy chain gene 5
(DNAH5) in a Japanese neonate with asplenia syndrome. Med Mol
Morphol. 48:116–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams JR: The Declaration of Helsinki
and public health. Bull World Health Organ. 86:650–652. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin DC, Hao JJ, Nagata Y, Xu L, Shang L,
Meng X, Sato Y, Okuno Y, Varela AM, Ding LW, et al: Genomic and
molecular characterization of esophageal squamous cell carcinoma.
Nat Genet. 46:467–473. 2014. View
Article : Google Scholar : PubMed/NCBI
|