Introduction
Renal cell carcinoma (RCC) is the most common type
of renal cancer, and more than half of RCC patients are identified
at the advanced stages and present with a local or systematic
metastasis, resulting in a poor prognosis (1). Patients presenting with advanced RCC
have a poor prognosis due to the chemo- and radioresistance of this
disease (2,3). Specifically, tumor cells respond to
radiation via multiple growth arrests and a form of protective
autophagy (4–8). Therefore, radiotherapy is rarely used to
treat RCC in the clinic (9), and
novel anti-tumor agents to reverse the radiosensitivity of RCC are
urgently required.
In cell biology, autophagy or autophagocytosis,
which is defined as ‘self-eating’, is a catabolic process that
involves the degradation of the components of a cell via the
lysosomal machinery (10,11). The important role of autophagy in
cancer therapy has become increasingly apparent. Under normal
conditions, autophagy is a mechanism that ensures the turnover of
proteins and elimination of damaged organelles to maintain cell
homeostasis (12). By contrast, it
functions as an adaptive cell response under pathological
conditions, allowing the cell to survive bio-energetic stress
(13). However, extensive or
persistent autophagy also results in cell death (14). Therefore, autophagy is a decisive
factor between cell death and survival, and can be either
protective or lead to autophagic cell death. Notably, the induction
of autophagy enhances the effects of radiation (15–22).
However, the contribution of autophagy to radiation efficacy
remains unclear, particularly in RCC.
Recently, the role of autophagy as an alternative
cell death mechanism has been a topic of debate. Ongoing studies
are attempting to define optimal strategies to modulate autophagy
for cancer prevention and therapy, as well as to exploit autophagy
as a target for anticancer drug discovery (23). The activation of the phosphoinositide
3-kinase (PI3K)/Akt signaling pathway, a well-known method of
inhibiting apoptosis, also inhibits autophagy (24). Additionally, increasing evidence
indicates that DNA damage induces autophagy (20–22), and
that inhibiting Akt enhances the cytotoxicity of DNA-damaging
agents in several cancer cells (25).
In addition, an Akt inhibitor could cause a switch from protective
autophagy to autophagic cell death. This switch enhanced sorafenib
resistance and cell proliferation in RCC cells (8,26,27). However, similar studies on RCC
radiotherapy are rare.
Ubenimex is widely used as an adjunct therapy after
surgery to enhance the function of immunocompetent cells and confer
antitumor effects. Ubenimex has been widely used as a therapy for
leukemia, non-small cell lung cancer, gastric cancer and cervical
cancer (28–30). Our previous study demonstrated that
ubenimex induces the autophagic cell death of RCC cells (31). We propose that autophagic cell death
induced by ubenimex may be associated with an autophagy-related
signaling pathway such as the Akt pathway. Therefore, the objective
of the present study was to determine the ability of ubenimex to
enhance RCC cell sensitivity to radiation by inducing autophagic
cell death and changing the role of autophagy from protective to
harmful. We also sought to determine the association of this effect
with Akt.
Materials and methods
Cell culture
The 786-O and OS-RC-2 RCC cell lines were purchased
from the cell bank of the Chinese Academy of Sciences (Beijing,
China). The cells were maintained in RPMI-1640 medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with
penicillin, streptomycin and 10% fetal bovine serum (Biological
Industries, Cromwell, CT, USA). The cells were incubated at 37°C in
a humidified atmosphere containing 5% CO2.
Irradiation (IR)
The cells were irradiated with γ-rays from a 137Cs
irradiator (GSR D1; Gamma-Service Medical GmbH, Leipzig, Germany)
at a dose rate of 1.7 Gy/min. For IR under hypoxic conditions, the
cells were sealed inside a hypoxia chamber in purpose-built
airtight boxes and then transported to the irradiator. Dosimetry
was performed using EBT2 film (Ashland Inc., Covington, KY, USA)
irradiated in the position of cells. The exposed EBT2 film strips
were scanned, and the optical density values were corrected as
recommended by the manufacturer and converted to a dose using a
calibration curve obtained from previously scanned film strips
irradiated with a range of known doses using 60Co
γ-rays.
Cell viability and colony-formation
assay
The cells were seeded in 96-well plates, treated at
different IR doses (0, 2,4 and 6 Gy) or for different times (0, 12,
24 and 36 h), and then allowed to grow for 72 h. Dimethyl sulfoxide
was used as the vehicle. The cell viability was observed using the
trypan blue dye-exclusion assay, and the viable cells were counted
using a hemocytometer. To determine the long-term effects of
treatment on cell colony formation, the cells were seeded in 6-well
plates at a density of 2,000 cells/well and treated with different
doses (0, 2, 4 and 6 Gy) of IR. After rinsing with fresh medium,
the cells were allowed to grow for 14 days to form colonies, which
were stained with crystal violet (0.5% w/v), photographed with a
scanner and counted.
Western blot analysis
To determine the expression of
microtubule-associated protein 1A/1B-light chain 3B (LC3B), Akt and
phosphorylated (p)-Akt, the proteins were extracted from cells or
tissues by resuspension in radioimmunoprecipitation assay buffer.
The samples were centrifuged at 12,000 × g at 4°C for 30 min, and
the supernatants were recovered for analysis. The protein
concentrations were determined using the Bradford protein method
and a bicinchoninic acid protein assay kit (Sigma-Aldrich, St.
Louis, MO, USA). The proteins (40 µg) were electrophoresed on a
pre-cast Bis-Tris polyacrylamide gel (8%) and then transferred to a
polyvinylidene difluoride membrane. The membranes were blotted with
rabbit anti-p-Akt (1:1,000; catalog no. 9271S; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit anti-LC3B (1:1,000;
catalog no. L7543; Sigma-Aldrich) rabbit anti-Akt (1:1,000; catalog
no. 9272; Cell Signaling Technology, Inc.) or mouse
anti-glyceraldehyde 3-phosphate dehydrogenase (1:3,000; catalog no.
TA08; ZsBio, Beijing, China), followed by incubation with the
corresponding horseradish peroxidase-conjugated secondary
antibodies (1:5,000; catalog nos. ZB2306 and ZB2301; ZsBio),
overnight at 4°C. The immunoblots were visualized using enhanced
chemiluminescence and ImageQuant LAS 4000 (GE Healthcare Life
Sciences).
Lactate dehydrogenase (LDH)
cytotoxicity assay
The levels of LDH release were assessed by
determining the extent of cell death irrespective of the type of
death. A 200-µl volume of cell suspension in complete medium
(5×103 cells/well) was dispensed into each well of a
96-well plate. The cells were treated with ubenimex and/or IR at
different times. The 96-well plates were centrifuged for 5 min at
400 × g, and 120 µl supernatant from each well was then transferred
to a new plate. The plates were incubated at room temperature for
30 min in the dark, and the absorbance was then
spectrophotometrically measured at a wavelength of 560 nm.
Electron microscopy (EM)
The RCC cells were treated with 0.5 mg/ml ubenimex
for 12 h and/or irradiated (4 Gy) for 24 h. The cells were then
fixed with 3% glutaraldehyde and 2% paraformaldehyde in 0.1 M
phosphate-buffered saline (PBS) for 30 min, post-fixed with 1%
osmium tetroxide (Absin Bioscience Inc., Shanghai, China) for 1.5
h, washed, stained in 3% aqueous uranyl acetate (Haoranbio,
Nanchang, China) for 1 h, dehydrated in an ascending series of
ethanol and acetone, and embedded in Araldite® (Huntsman
Advanced Materials LLC, The Woodlands, TX, USA). Ultrathin sections
were cut on a Reichert-Jung ultramicrotome (Leica Microsystems,
Inc., Buffalo Grove, IL, USA), double stained with 0.3% lead
citrate (Alfa Chemistyry, Stony Brook, NY, USA) and examined on a
1200EX electron microscope (JEOL, Ltd., Tokyo, Japan).
Acridine orange (AO)-ethidium bromide
(EB) double staining
The DNA-binding dyes AO and EB are used for the
morphological detection of autophagic cell death (32). A cocktail of EB and AO (100 µg/ml) was
prepared in PBS. AO is uptaken by both viable and non-viable cells
and emits green fluorescence, whereas EB is uptaken only by
non-viable cells and emits red fluorescence via intercalation into
DNA (33). Following IR, the cells
were washed twice with PBS, and fresh medium was added. After a
30-min incubation, the cells were washed again with PBS, stained
with AO/EB and incubated for 30 min in the dark. Next, the cells
were washed with PBS and analyzed by fluorescence microscopy
(34). The percentage of positively
stained cells was calculated to determine the death rate (%), which
is the number of cells undergoing programmed cell death per 100
cells. The experiments were repeated thrice.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) staining
The apoptotic cells were quantified (%) using an
annexin V-FITC)/PI kit (Nanjing KeyGen Biotech, Co. Ltd., Nanjing,
China) and detected by flow cytometry. The RCC cells were harvested
after 12 h of treatment with ubenimex and/or after 24 h of IR.
Next, the cells were resuspended in binding buffer (10 mmol/l
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 140 mmol/l NaCl
and 2.5 mmol/l CaCl2, pH 7.4) and incubated with annexin
V-FITC/PI in the dark for 15 min. A total of 5,000 cells/sample
were analyzed using a FACSCalibur or an EPICS XL flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). The cells in the early
stages of apoptosis stained positive for annexin V-FITC, whereas
those in the late stages of apoptosis stained positive for both
annexin V-FITC and PI.
Statistical analysis
The data were statistically analyzed with Student's
t, χ2 or Fisher's exact tests using SPSS version 19.0
(IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a
statistical significant difference.
Results
Pretreatment with ubenimex
significantly enhances the toxicity of IR and reduces the survival
fraction in a dose-dependent manner compared with IR alone
The viability of cells was determined at different
doses of IR (0, 2, 4 and 6 Gy) for 24 h (Fig. 1A) or different times of IR (0, 12, 24,
36 and 48 h) at a dose of 4 Gy (Fig.
1B). The viability of 786-O and OS-RC-2 cells varied with the
different treatments. The LDH cytotoxicity assay of OS-RC-2 cells
revealed that the number of dead cells positively correlated with
the time and dose of IR (Fig. 1C).
Ubenimex and IR alone or in combination were used to treat 786-O
cells. The combined treatment significantly increased toxicity in
786-O cells compared with ubenimex or IR treatment alone (Fig. 1E). Furthermore, the radiation
dose-response survival curves for 786-O cells with or without
ubenimex treatment were significantly shifted downward for
ubenimex-treated cells (Fig. 1D),
indicating that ubenimex increased IR-induced clonogenic cell death
in 786-O cells.
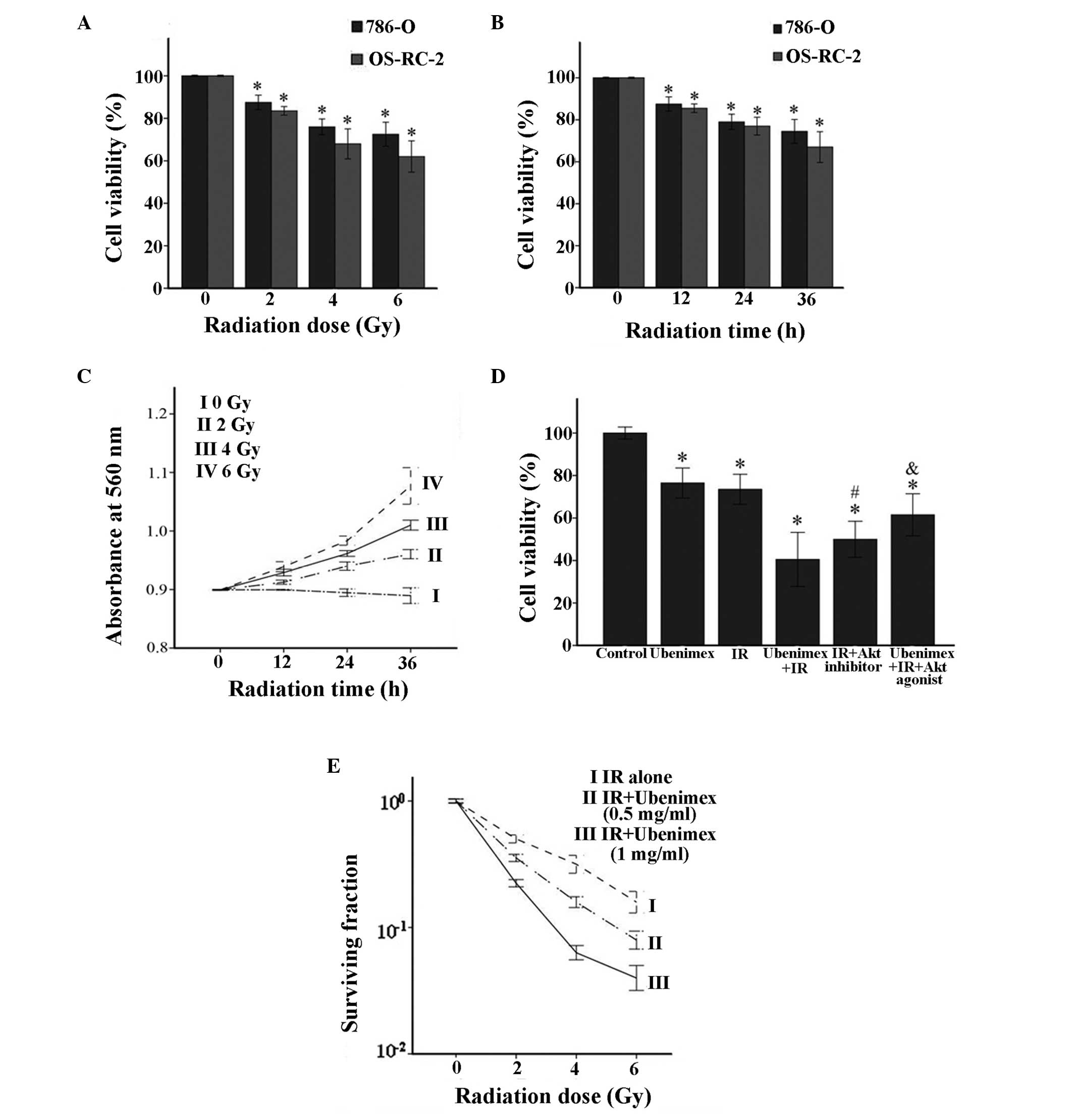 | Figure 1.(A) Dose-dependent effects of IR on
the viability of RCC cells. Cells were treated with 0, 2, 4 or 6 Gy
IR for 24 h. *P<0.05, IR vs. control. (B) Time-dependent effects
of IR on the viability of RCC cells. Cells were treated for 0, 12,
24 or 36 h with 4 Gy IR. *P<0.05, IR vs. control. (C) Lactate
dehydrogenase cytotoxicity assay was performed at different times
and different doses of IR treatment in OS-RC-2 cells. *P<0.05
vs. untreated cells. (D) Cytotoxic effects in 786-O cells treated
with IR (4 Gy) and/or ubenimex (0.5 mg/ml). The IR+Akt inhibitor
and IR+ubenimex+Akt agonist were also tested. *P<0.05 vs.
control. #P<0.05, IR+ubenimex or IR+Akt inhibitor vs.
IR treatment. &P<0.05, IR+ubenimex+Akt agonist vs.
IR+ubenimex treatment. (E) Radiation dose-response survival curves
of 786-O cells with or without ubenimex. All results are presented
as the mean ± standard deviation from three independent
experiments. IR, irradiation; RCC, renal cell carcinoma. |
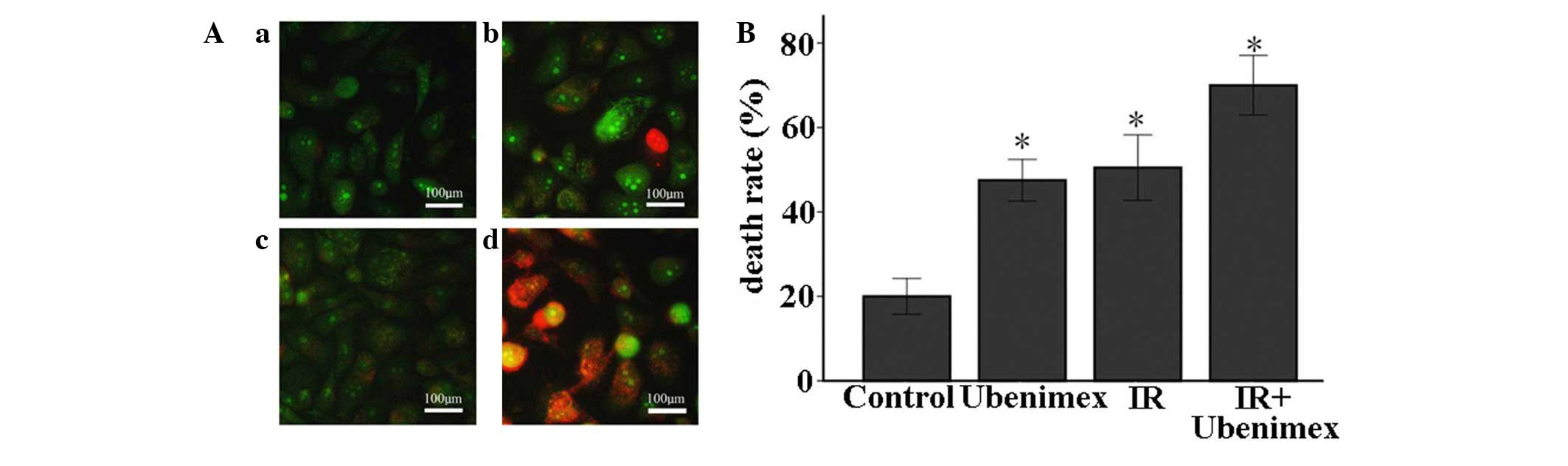
Compared with IR alone, pretreatment
with ubenimex induced cell death, including autophagic cell death
and apoptosis
IR plays a key role in cancer therapy because it
directly induces cell death (35).
AO-EB staining is a sensitive tool for the estimation of autophagic
cell death (32), requiring only a
small number of cells. The AO-EB staining of OS-RC-2 cells
indicated that the combined treatment of ubenimex and IR
significantly enhanced cell death compared with ubenimex or IR
treatment alone. Additionally, this combined treatment
significantly increased the number of EB-positive cells compared
with ubenimex treatment alone. These results suggest that
autophagic cell death may be involved in the anti-proliferative
effects of the above combined treatment on OS-RC-2 cells (Fig. 2).
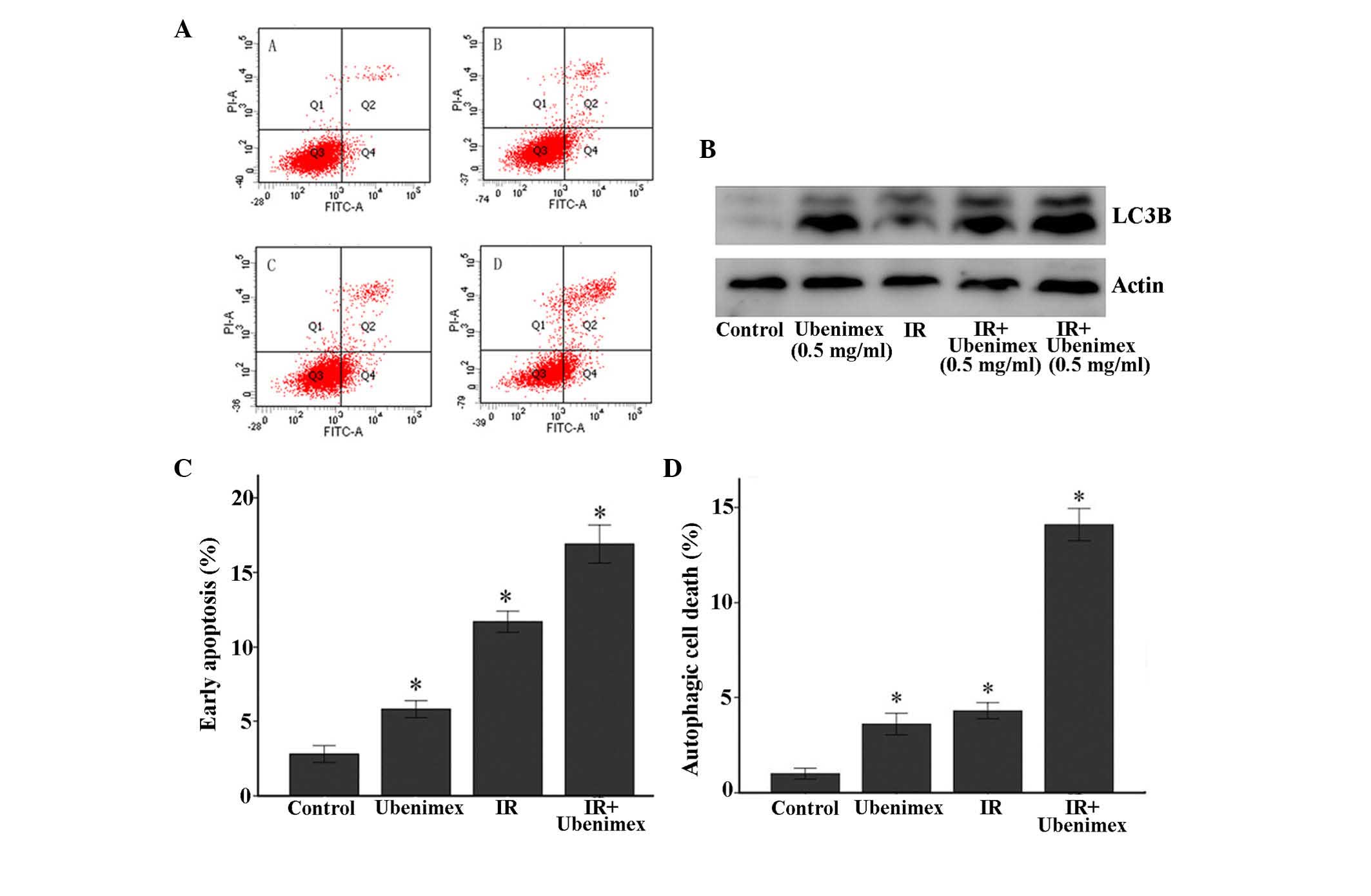
Compared with IR alone, pretreatment
with ubenimex enhances early apoptosis and autophagic cell death in
OS-RC-2 cells
Flow cytometry was used to further detect cell
death. As represented in Fig. 3,
early apoptosis and autophagic cell death in OS-RC-2 cells was
measured by flow cytometry with an annexin V apoptosis detection
kit (Fig. 3). The quantitative
results revealed that the combined treatment of ubenimex and IR
induced significant early apoptosis and autophagic cell death in
OS-RC-2 cells compared with treatment with ubenimex or IR
alone.
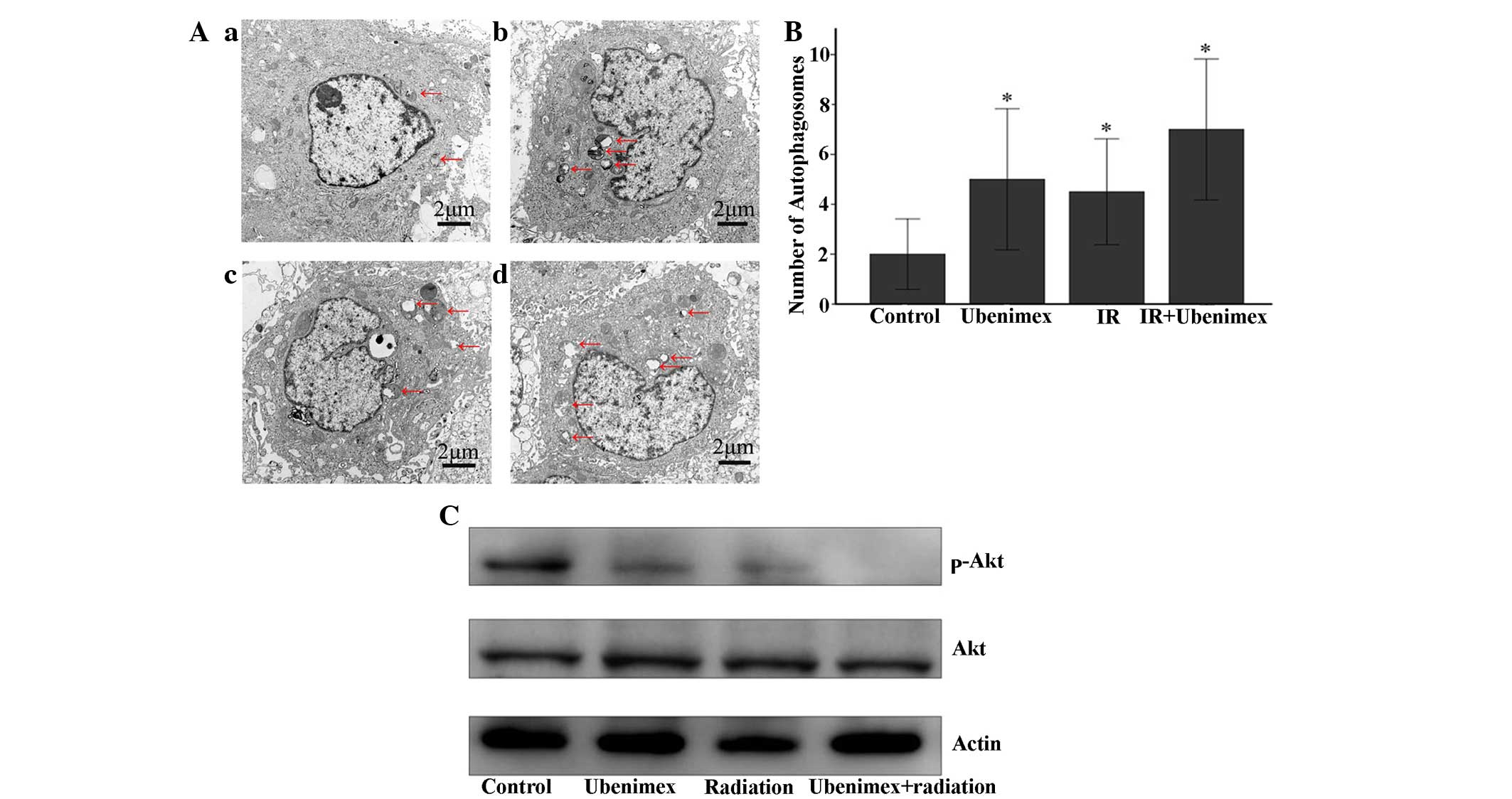
Combined treatment induces type II
programmed cell death (autophagic cell death)
The ultrastructure of the OS-RC-2 cells for each
treatment group was observed by EM photomicrography (Fig. 4A). The combined treatment resulted in
a large number of autophagic vacuoles and autolysosomes in the
cytoplasm (Fig. 4B). In addition,
chromatin condensation or nuclear pyknosis, which are
characteristic of apoptosis (36),
were not observed in any of the treated cells. To detect the
expression of autophagy-related proteins, the lysates from OS-RC-2
cells subjected to different treatments were examined by western
blotting (Fig. 3B). The combined
treatment of ubenimex and IR increased the expression levels of the
LC3B protein, suggesting that the combined treatment induced
autophagic cell death in OS-RC-2 cells (Fig. 3B).
Akt signaling pathway is involved in
the effect of the combined treatment of ubenimex and IR, changing
autophagy from protective to harmful in OS-RC-2 cells
Previous studies have demonstrated that the Akt
signaling pathway is involved in the regulation of autophagy
(26). Specifically, stress appears
to activate the Akt signal transduction pathway in tumor cells,
which results in protective autophagy (8,27).
Therefore, to determine the involvement of the Akt signaling
pathway in the effect of the above combined treatment, the levels
of protein phosphorylation were detected by western blotting
(Fig. 4C). The phosphorylated
proteins involved in the Akt signaling pathways were also examined.
The combined treatment decreased the phosphorylation of Akt
compared with ubenimex treatment or IR alone. As indicated in
Figs. 1E and 4, the autophagic cell death induced by
radiation, but IR combined with an Akt inhibitor or ubenimex
strongly induced autophagic cell death, whereas the addition of an
Akt agonist significantly decreased these effects (Fig. 1E).
Discussion
Radioresistance may be associated with protective
autophagy. As previously reported (37), IR treatment induced autophagy in RCC
cells. However, it did cause significant cell death compared with
ubenimex (32). Akt may be the main
mechanism of autophagic cell death, since the ectopic
overexpression of Akt partly rescues cancer cells from death when
they are stressed (8). Additionally,
previous studies have demonstrated that stress activated the Akt
signal transduction pathway in tumor cells and induced protective
autophagy, whereas the inhibition of the Akt signaling pathway
synergistically killed cancer cells (27). In the present study, the addition of
ubenimex or an Akt inhibitor significantly induced autophagic cell
death, whereas an Akt agonist reduced this effect. Therefore, it
was concluded that IR induces protective autophagy, but the
addition of ubenimex or an Akt inhibitor inhibits the Akt signaling
pathway, thus switching the role of autophagy from protective to
lethal.
Autophagic cell death is a death mechanism that is
distinguishable from apoptosis (23).
Autophagy could lead to cell death, but in certain cases, such as
when faced with chemotherapy, it could be a way for tumor cells to
survive. In multiple examples, autophagy is unequivocally the mode
of tumor cell death (23). Although
the multiple roles of autophagy in cancer require further
clarification, autophagy is directly involved in numerous important
physiological processes, including metabolism, response to stress
and cell death pathways in cancer cells (38). Both tumor-suppressor genes and
oncogenes are implicated in autophagy regulation (39). Accordingly, the role of autophagy in
cancer raises a number of questions. Our previous study revealed
that ubenimex induces autophagy in RCC cells (31). In this context, the present data
indicates that the induction of pro-death autophagy increases the
radiosensitivity of RCC cells. The possibility that RCC cells may
undergo autophagic cell death in response to radiation argues for
the potential utility of autophagy as an alternative pathway based
on the efficacy of drugs and radiation in promoting tumor cell
death.
The Akt signaling pathway plays a crucial role in
the regulation of both apoptosis and autophagy (40). The disruption of PI3K/Akt signaling is
associated with the induction of autophagy by a variety of
antineoplastic agents in cancer cells (40). Viola et al (41) indicated that MG-2477, a tubulin
inhibitor, induces autophagy via the inhibition of the Akt
signaling pathway in A549 cells. Triptolide induces autophagy in
pancreatic cancer cells and also inhibits the Akt pathway (42). The present study demonstrated that the
combined treatment of ubenimex and IR significantly decreased the
expression of p-Akt in cells compared with ubenimex treatment or IR
alone. These results suggest that anticancer agents may commonly
induce autophagy by inhibiting Akt. Additionally, previous studies
revealed that stress activates the Akt signal transduction pathway
in tumor cells, which results in protective autophagy (28). Furthermore, treatment with an Akt
inhibitor changed the role of autophagy from protective to lethal
(27). These findings suggest that
Akt signaling and autophagy are important in the resistance of
tumors to treatment. In the present study, treatment with an Akt
agonist significantly decreased the autophagic cell death induced
by ubenimex as well as radioresistance. This decrease suggests that
ubenimex induces Akt-related autophagic cell death. Furthermore,
this effect switches the role of radiotherapy-induced autophagy
from protective to lethal.
In the present study, ubenimex enhanced the
radiosensitivity of RCC cells, and it was demonstrated that the
combination of ubenimex and IR modulated the radioresistance of RCC
cells. Pretreatment with ubenimex induced pro-death autophagy in
the OS-RC-2 and 786-O cell lines in response to radiation. Since
ubenimex is well tolerated in clinical adjuvant therapy, it has the
potential to be used as a radiosensitizer (28–30).
Radiotherapy is not generally considered for the treatment of RCC
for a number of reasons, including the relative radioresistance of
RCC, the radiosensitivity of the surrounding tissue and the
toleration of nephrectomy (31).
Importantly, the present results show that ubenimex radiosensitizes
RCC, which is essential for the utility of radiotherapy in the
treatment of this disease. However, as a novel therapy, ubenimex is
unlikely to be tested clinically with radiation without supporting
preclinical studies. The present data demonstrate that adding
ubenimex increases the effects of clinically relevant doses of
radiation in RCC cells.
In summary, the results of the present study
demonstrate that the induction of autophagy enhances the
radiosensitivity of RCC cells, and that ubenimex switches the role
of radiation-induced autophagy from protective to lethal, a switch
that is associated with the Akt signaling pathway. In addition, the
present findings demonstrate that combining radiotherapy with
molecularly targeted therapies is a valid approach for the
treatment of RCC that should be further evaluated in preclinical
models. Based on these results, ubenimex appears to be an excellent
adjunct therapy for the treatment of RCC. Combined with rapid
advances in both imaging and radiotherapy technologies, adjunct
therapy with ubenimex and radiotherapy is an obvious treatment
option for RCC in the future.
Acknowledgements
The present study was funded by grants from the
Shandong Provincial Natural Science Foundation (Jinan, China; grant
numbers ZR2014HM111 and ZR2014HP015) and the Science and Technology
Development Plan Project of Shandong Province, China (grant numbers
2014GGH218036, 2015GSF118055 and 2015GGB14008).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hollingsworth JM, Miller DC, Dunn RL,
Montgomery JS, Roberts WW, Hafez KS and Wolf JS Jr: Surgical
management of low-stage renal cell carcinoma: Technology does not
supersede biology. Urology. 67:1175–1180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hollingsworth JM, Miller DC, Daignault S
and Hollenbeck BK: Rising incidence of small renal masses: A need
to reassess treatment effect. J Natl Cancer Inst. 98:1331–1334.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmitt CA, Fridman JS, Yang M, Lee S,
Baranov E, Hoffman RM and Lowe SW: A senescence program controlled
by p53 and p16INK4a contributes to the outcome of cancer therapy.
Cell. 109:335–1146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chu K, Teele N, Dewey MW, Albright N and
Dewey WC: Computerized video time lapse study of cell cycle delay
and arrest, mitotic catastrophe, apoptosis and clonogenic survival
in irradiated 14-3-3sigma and CDKN1A (p21) knockout cell lines.
Radiat Res. 162:270–286. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ichinose Y, Genka K, Koike T, Kato H,
Watanabe Y, Mori T, Iioka S, Sakuma A and Ohta M: NK421 Lung Cancer
Surgery Group: Randomized double-blind placebo-controlled trial of
bestatin in patients with resected stage I squamous-cell lung
carcinoma. J Natl Cancer Inst. 95:605–610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wan J, Liu T, Mei L, Li J, Gong K, Yu C
and Li W: Synergistic antitumour activity of sorafenib in
combination with tetrandrine is mediated by reactive oxygen species
(ROS)/Akt signaling. Br J Cancer. 109:342–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nomiya T, Tsuji H, Hirasawa N, Kato H,
Kamada T, Mizoe J, Kishi H, Kamura K, Wada H, Nemoto K and Tsujii
H: Carbon ion radiation therapy for primary renal cell carcinoma:
Initial clinical experience. Int J Radiat Oncol Biol Phys.
72:828–833. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bursch W: The autophagosomal-lysosomal
compartment in programmed cell death. Cell Death Differ. 8:569–581.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Antalek M, Nguyen L, Li X, Tian X,
Le A and Zi X: The effect of gartanin, a naturally occurring
xanthone in mangosteen juice, on the mTOR pathway, autophagy,
apoptosis, and the growth of human urinary bladder cancer cell
lines. Nutr Cancer. 65(Suppl 1): 68–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mizushima N, Tsukamoto S and Kuma A:
Autophagy in embryogenesis and cell differentiation. Tanpakushitsu
Kakusan Koso. 53:(16 Suppl). S2170–S2174. 2008.(In Japanese).
|
|
14
|
Horita H, Frankel AE and Thorburn A: Acute
myeloid leukemia-targeted toxin activates both apoptotic and
necroptotic death mechanisms. PLoS One. 3:e39092008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ling YH, Aracil M, Zou Y, Yuan Z, Lu B,
Jimeno J, Cuervo AM and Perez-Soler R: PM02734 (elisidepsin)
induces caspase-independent cell death associated with features of
autophagy, inhibition of the AKT/mTOR signaling pathway, and
activation of death-associated protein kinase. Clin Cancer Res.
17:5353–5366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim KW, Moretti L, Mitchell LR, Jung DK
and Lu B: Combined Bcl-2/mammalian target of rapamycin inhibition
leads to enhanced radiosensitization via induction of apoptosis and
autophagy in non-small cell lung tumor xenograft model. Clin Cancer
Res. 15:6096–6105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin CI, Whang EE, Donner DB, Du J, Lorch
J, He F, Jiang X, Price BD, Moore FD Jr and Ruan DT: Autophagy
induction with RAD001 enhances chemosensitivity and
radiosensitivity through Met inhibition in papillary thyroid
cancer. Mol Cancer Res. 8:1217–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiu HW, Lin JH, Chen YA, Ho SY and Wang
YJ: Combination treatment with arsenic trioxide and irradiation
enhances cell-killing effects in human fibrosarcoma cells in vitro
and in vivo through induction of both autophagy and apoptosis.
Autophagy. 6:353–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rajecki M, af Hällström T, Hakkarainen T,
Nokisalmi P, Hautaniemi S, Nieminen AI, Tenhunen M, Rantanen V,
Desmond RA, Chen DT, et al: Mre11 inhibition by oncolytic
adenovirus associates with autophagy and underlies synergy with
ionizing radiation. Int J Cancer. 125:2441–2449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhuang W, Li B, Long L, Chen L, Huang Q
and Liang Z: Induction of autophagy promotes differentiation of
glioma-initiating cells and their radiosensitivity. Int J Cancer.
129:2720–2731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Altmeyer A, Jung AC, Ignat M, Benzina S,
Denis JM, Gueulette J, Noël G, Mutter D and Bischoff P:
Pharmacological enhancement of autophagy induced in ahepatocellular
carcinoma cell line by high-LET radiation. Anticancer Res.
30:303–310. 2010.PubMed/NCBI
|
|
22
|
Gewirtz DA, Hilliker ML and Wilson EN:
Promotion of autophagy as a mechanism for radiation sensitization
of breast tumor cells. Radiother Oncol. 92:323–328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang ZJ, Chee CE, Huang S and Sinicrope F:
Autophagy modulation for cancer therapy. Cancer Biol Ther.
11:169–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodriguez-Rocha H, Garcia-Garcia A,
Panayiotidis MI and Franco R: DNA damage and autophagy. Mutat Res.
711:158–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen W, Wu J, Shi H, Wang Z, Zhang G, Cao
Y, Jiang C and Ding Y: Hepatic stellate cell coculture enables
sorafenib resistance in Huh7 cells through HGF/c-Met/Akt and
Jak2/Stat3 pathways. Biomed Res Int. 2014:7649812014.PubMed/NCBI
|
|
27
|
Zhai B, Hu F, Jiang X, Xu J, Zhao D, Liu
B, Pan S, Dong X, Tan G, Wei Z, et al: Inhibition of Akt reverses
the acquired resistance to sorafenib by switching protective
autophagy to autophagic cell death in hepatocellular carcinoma. Mol
Cancer Ther. 13:1589–1598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishii K, Usui S, Sugimura Y, Yoshida S,
Hioki T, Tatematsu M, Yamamoto H and Hirano K: Aminopeptidase N
regulated by zinc in human prostate participates in tumor cell
invasion. Int J Cancer. 92:49–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fontijn D, Duyndam MC, van Berkel MP,
Yuana Y, Shapiro LH, Pinedo HM, Broxterman HJ and Boven E:
CD13/Aminopeptidase N overexpression by basic fibroblast growth
factor mediates enhanced invasiveness of 1F6 human melanoma cells.
Br J Cancer. 94:1627–1636. 2006.PubMed/NCBI
|
|
30
|
Mina-Osorio P: The moonlighting enzyme
CD13: Old and new functions to target. Trends Mol Med. 14:361–371.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu S, Xie F, Wang H, Liu Z, Liu X, Sun L
and Niu Z: Ubenimex inhibits cell proliferation, migration and
invasion in renal cell carcinoma: The effect is
autophagy-associated. Oncol Rep. 33:1372–1380. 2015.PubMed/NCBI
|
|
32
|
Mujtaba SF, Dwivedi A, Yadav N, Ch R,
Kushwaha HN, Mudiam MK, Singh G and Ray RS: Superoxide mediated
photomodification and DNA damage induced apoptosis by
Benz(a)anthracene via mitochondrial mediated pathway. J Photochem
Photobiol B. 142:92–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu K, Liu PC, Liu R and Wu X: Dual AO/EB
staining to detect apoptosis in osteosarcoma cells compared with
flow cytometry. Med Sci Monit Basic Res. 21:15–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Qi J, Sun L, Cheng B, Pan S, Zhou
M and Sun X: Matrine induces programmed cell death and regulates
expression of relevant genes based on PCR array analysis in C6
glioma cells. Mol Biol Rep. 36:791–799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Burdak-Rothkamm S and Prise KM: New
molecular targets in radiotherapy: DNA damage signalling and repair
in targeted and non-targeted cells. Eur J Pharmacol. 625:151–155.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anbalagan S, Pires IM, Blick C, Hill MA,
Ferguson DJ, Chan DA and Hammond EM: Radiosensitization of renal
cell carcinoma in vitro through the induction of autophagy.
Radiother Oncol. 103:388–393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rosenfeldt MT and Ryan KM: The multiple
roles of autophagy in cancer. Carcinogenesis. 32:955–963. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morani F, Titone R, Pagano L, Galetto A,
Alabiso O, Aimaretti G and Isidoro C: Autophagy and thyroid
carcinogenesis: Genetic and epigenetic links. Endocr Relat Cancer.
21:R13–R29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takeuchi H, Kondo Y, Fujiwara K, Kanzawa
T, Aoki H, Mills GB and Kondo S: Synergistic augmentation of
rapamycin-induced autophagy in malignant glioma cells by
phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer
Res. 65:3336–3346. 2005.PubMed/NCBI
|
|
41
|
Viola G, Bortolozzi R, Hamel E, Moro S,
Brun P, Castagliuolo I, Ferlin MG and Basso G: MG-2477, a new
tubulin inhibitor, induces autophagy through inhibition of the
Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem
Pharmacol. 83:16–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mujumdar N, Mackenzie TN, Dudeja V, Chugh
R, Antonoff MB, Borja-Cacho D, Sangwan V, Dawra R, Vickers SM and
Saluja AK: Triptolide induces cell death in pancreatic cancer cells
by apoptotic and autophagic pathways. Gastroenterology.
139:598–608. 2010. View Article : Google Scholar : PubMed/NCBI
|