Introduction
Osteosarcoma is the most common primary malignant
bone neoplasm in adolescents and young adults, and usually occurs
in growing long bones such as the humerus, femur and tibia
(1,2).
It is a highly aggressive tumor that metastasizes primarily to the
lung (3,4). The main cause of mortality in
osteosarcoma is lung metastasis. In general, lung metastasis is a
sign of deterioration (5). The
prognosis is extremely poor owing to the lack of effective
treatment methods. Therefore, innovative approaches that target
invasion and metastasis, particularly to the lung, from the primary
osteosarcoma site are urgently required. Until now, the molecular
mechanisms of invasion and metastasis in osteosarcoma have remained
unclear. Therefore, clarification of the molecular mechanisms of
the pathogenesis and biology of metastatic osteosarcoma is a
critical factor for improving the curative effect and identifying
potential therapeutic targets.
Tumor invasion and metastasis are complicated
processes, involving the activities of tumor cells and host cells,
which are regulated by multiple tumor-related genes (6,7). One of
the most significant steps in the invasion and metastasis cascade
involves the destruction of the extracellular matrix (ECM) and
basement membranes, allowing tumor cells to invade into and grow at
sites distant from the original tumor site (8–10). The
interaction between ECM proteins (including collagen and
fibronectin) and tumor cell surface receptors is a critical initial
step in the invasion and metastasis process (11,12). Tumor
cells are able to express ECM-degrading enzymes, including matrix
metalloproteinases (MMPs). MMPs have been demonstrated to play a
key role in permitting cancer cells to invade through the ECM and
form metastatic lesions. The protein expression levels of MMPs in
certain tumors are considered as an index of tumor invasion and
metastatic potential (13–16). Therefore, the control of MMP activity
and adhesion to ECM components may prevent invasion and metastasis
development.
MMPs are a family of highly homologous
protein-degrading zinc-dependent enzymes endopeptidases, among
which MMP-2 and −9 are notably correlated with tumor invasion and
metastasis (17,18). A number of studies have demonstrated
that MMP-2 and −9 are overexpressed in osteosarcoma, and promote
osteosarcoma cell migration and invasion by degrading components of
the ECM and basement membranes (19–21). A
large number of studies reveal that the nuclear factor-κB (NF-κB)
gene is an upstream regulator of MMPs, and is closely associated
with tumor invasion and migration (22,23). In
addition, the phosphatidylinositol 3-kinase (PI3K)/Akt pathway is
considered to be a significant regulatory factor in NF-κB
activation. Notably, activation of Akt has been revealed to be
critical for degradation of the inhibitor of NF-κB and κB (IκB)
(24,25). Therefore, the PI3K/Akt/NF-κB signaling
pathway may be a treatment target to suppress osteosarcoma cell
invasion and migration.
Traditional Chinese medicine, a significant
component of complementary and alternative medicine, may serve as a
useful model for scientific inquiry since it has a standardized
system of diagnostics and therapies, and is used worldwide.
Celastrol is a triterpene extracted from the Chinese ‘Thunder God
Vine’, which inhibits cancer cell growth and induces apoptosis in a
number of cancer cell lines (26–29). In
our previous studies, we demonstrated that Celastrol could induce
apoptosis of human osteosarcoma cells via the
mitochondrial-dependent pathway. In the present study, we
identified that Celastrol could suppress osteosarcoma U-2OS cell
metastasis via downregulation of the PI3K/Akt/NF-κB signaling
pathway in vitro.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), dimethyl sulphoxide (DMSO) and trypsin were
purchased from Transgen (Beijing, China). Phosphate-buffered saline
(PBS) was obtained from Solarbio (Beijing, China). Phosphatase
inhibitor cocktail was purchased from Roche (Penzberg, Germany).
Antibodies against phosphorylated PI3K, Akt, inhibitor of κB kinase
(IKK)α/β, inhibitor of κB α (IκBα), nuclear factor-κB (NF-κB
subunit p65) and β-actin were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA), and antibodies against MMP-2
and −9 were purchased from Abcam (Cambridge, UK). Horseradish
peroxidase (HRP)-conjugated secondary antibodies were purchased
from Cell Signaling Technology, Inc. and ZSGB-BIO (Beijing, China).
Matrigel was purchased from Becton-Dickinson (San Jose, CA, USA).
The Transwell invasion chamber was purchased from Costar
(Cambridge, MA, USA). Celastrol was obtained from Nanjing ZeLang
Medical Technology Co., Ltd. (Nanjing, China). Stock solutions of
Celastrol were prepared by dissolving the Celastrol powder in DMSO
to a concentration of 1 M, and stored at −20°C. The working
concentrations of Celastrol were made by diluting the stock
solution with the culture medium. The final concentration of DMSO
in the medium was <0.5%.
Cell culture
Human osteosarcoma U-2OS cell lines were obtained
from the American Type Culture Collection (Manassas, VA, USA).
Cells were grown in culture medium consisting of DMEM supplemented
with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. They
were all placed in a humidified atmosphere containing 5%
CO2 at 37°C. The cells used in this study were subjected
to less than 20 cell passages, and all cells used in this study
were in the logarithmic phase. The present study was approved by
the Ethical Review Committee of The First Affiliated Hospital of
Nanchang University Medical School (Nanchang, China).
Boyden chamber Transwell assays
Invasion of U-2OS cells was determined using
Matrigel-coated Transwell cell culture chambers (8 µm pore size).
Briefly, cells were cultured for 24 h in DMEM, then collected and
resuspended in serum-free medium. Isolated cells (1×104
cells/well) were then added to the upper chamber of the Transwell
insert and treated with Celastrol (0, 2.5 and 4 µM), and the lower
wells were filled with complete growth medium. All samples were
incubated for 48 h at 37°C in a humidified atmosphere with 95% air
and 5% CO2. Non-invading cells on the upper surface of
the membranes were removed using a cotton swab, and invading cells
on the lower surface of the membranes were fixed and counted under
a phase-contrast microscope in three random fields (magnification,
×200).
Wound healing assays
Migration of U-2OS cells was measured using wound
healing assays. U-2OS cells (1×105 cells/well) were
seeded in a six-well culture plate to form a confluent monolayer,
and then cells were wounded with a sterile 200-µl pipette tip. All
cells in the plates were treated with Celastrol at final
concentrations of 0, 2.5 and 4 µM, and then incubated in fresh DMEM
with 1% FBS for 48 h. Scratch wounds were then inspected using a
phase-contrast microscope and images of each wound were
captured.
Western blot analysis
U-2OS cells were seeded in six-well plates at a
concentration of 2×105 cells/well. Following treatment
with 0, 2.5 and 4 µM Celastrol for 48 h, cells were collected and
lysed in RIPA buffer containing phenylmethane sulfonyl fluoride and
phosphatase inhibitor cocktail. Each sample was centrifuged at
12,000 rpm for 10 min at 4°C to remove cell debris and to collect
the supernatant for immunoblotting. Protein concentrations were
calculated using bovine serum albumin as the standard. The same
amounts of proteins were loaded and separated by electrophoresis on
12% sodium dodecyl sulphate-polyacrylamide gels under a reducing
condition using 100 V for 2 h. Following electrophoresis, the
proteins were transferred to polyvinylidene fluoride (PVDF)
membranes in a tris-glycine transfer buffer using a semi-dry
blotting system, and incubated with antibodies against
phosphorylated PI3K, Akt, IKKα/β, IκBα, NF-κB subunit p65, MMP-2,
MMP-9 and β-actin (1:1,000) overnight at 4°C. After washing the
membranes in TBST three times, secondary HRP-conjugated antibodies
were added at a 1:2000 dilution for 1 h at room temperature and the
membranes were washed again in Tris-buffered saline and Tween-20
three times. Immunoreactive proteins were detected by enhanced
chemiluminescence (ECL kit, Transgen, China) and exposed to X-ray
film.
Statistical analysis
Each experiment was performed at least three times
independently, and the quantitative data were expressed as the
means ± standard deviation. Data were analyzed using the SPSS
package for Windows (version 17.0; SPSS, Inc., Chicago, IL, USA).
Statistical analysis of the data was performed using Student's
t-test and analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
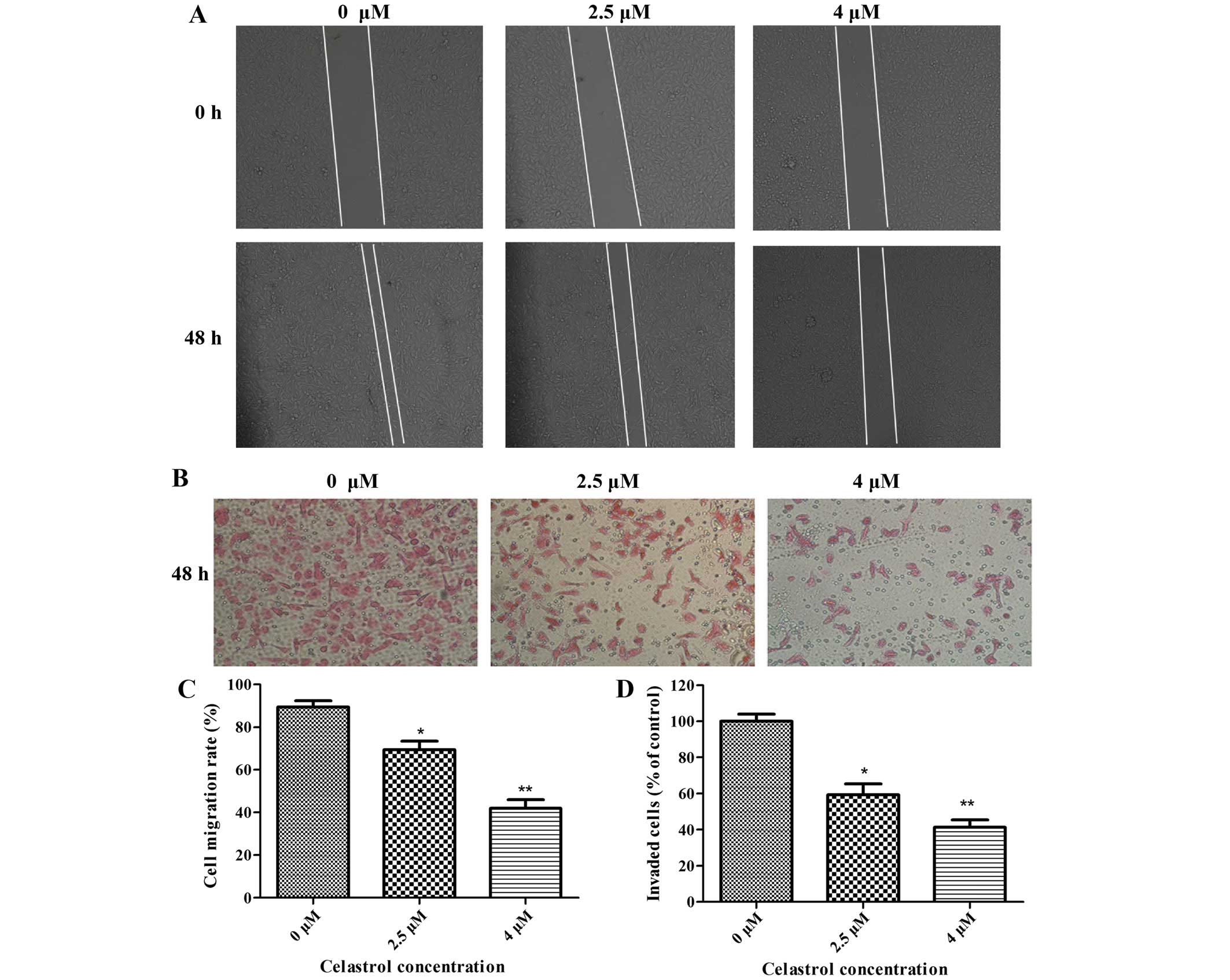
Celastrol inhibits cell migration and
invasion in U-2OS cells
The effect of Celastrol on the migration and
invasion of osteosarcoma U-2OS cells was measured by wound healing
assays and Boyden chamber Transwell assays, respectively. U-2OS
osteosarcoma cell lines were treated with Celastrol (0, 2.5 and 4
µM) for 48 h. As shown in Fig. 1A and
C, Celastrol inhibited the migration of U-2OS cells in a
dose-dependent manner. In the Boyden chamber Transwell assays,
Celastrol significantly reduced the invasion ability of U-2OS cells
in a dose-dependent manner (Fig. 1B and
D).
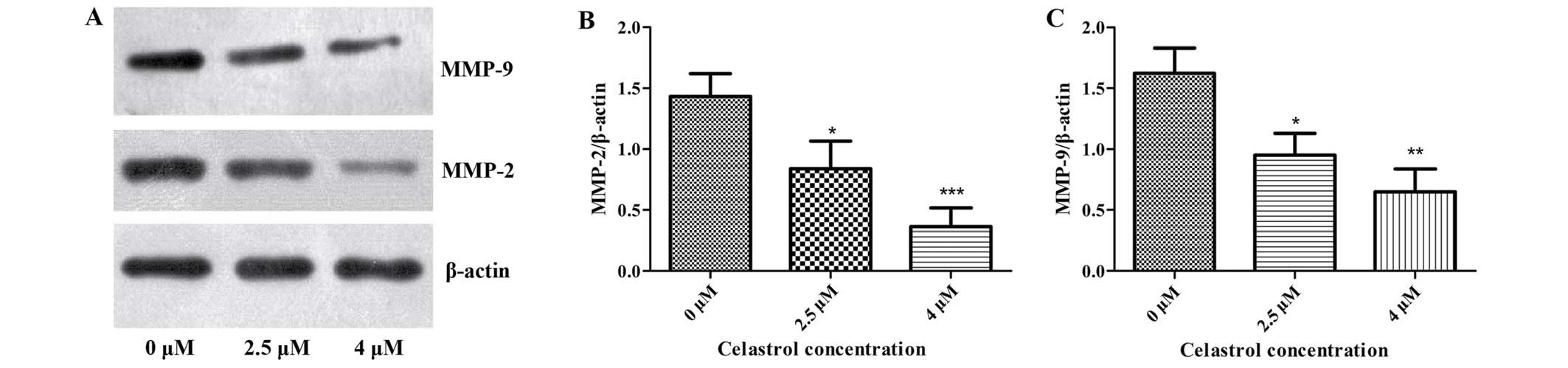
Celastrol decreases the expression of
MMP-2 and MMP-9
It is well known that osteosarcoma cells produce
MMPs to facilitate cell invasion and migration, among which MMP-2
and −9 play the most significant roles. We determined whether
Celastrol could inhibit the expression of MMP-2 and −9 in U-2OS
cells. As shown in Fig. 2, the
results of western blot analysis revealed that Celastrol treatment
caused a marked increase in MMP-2 and MMP-9 when compared with
these levels in the control. This indicates that Celastrol
inhibited cell migration and invasion in U-2OS cells by
downregulating the expression of MMP-2 and −9.
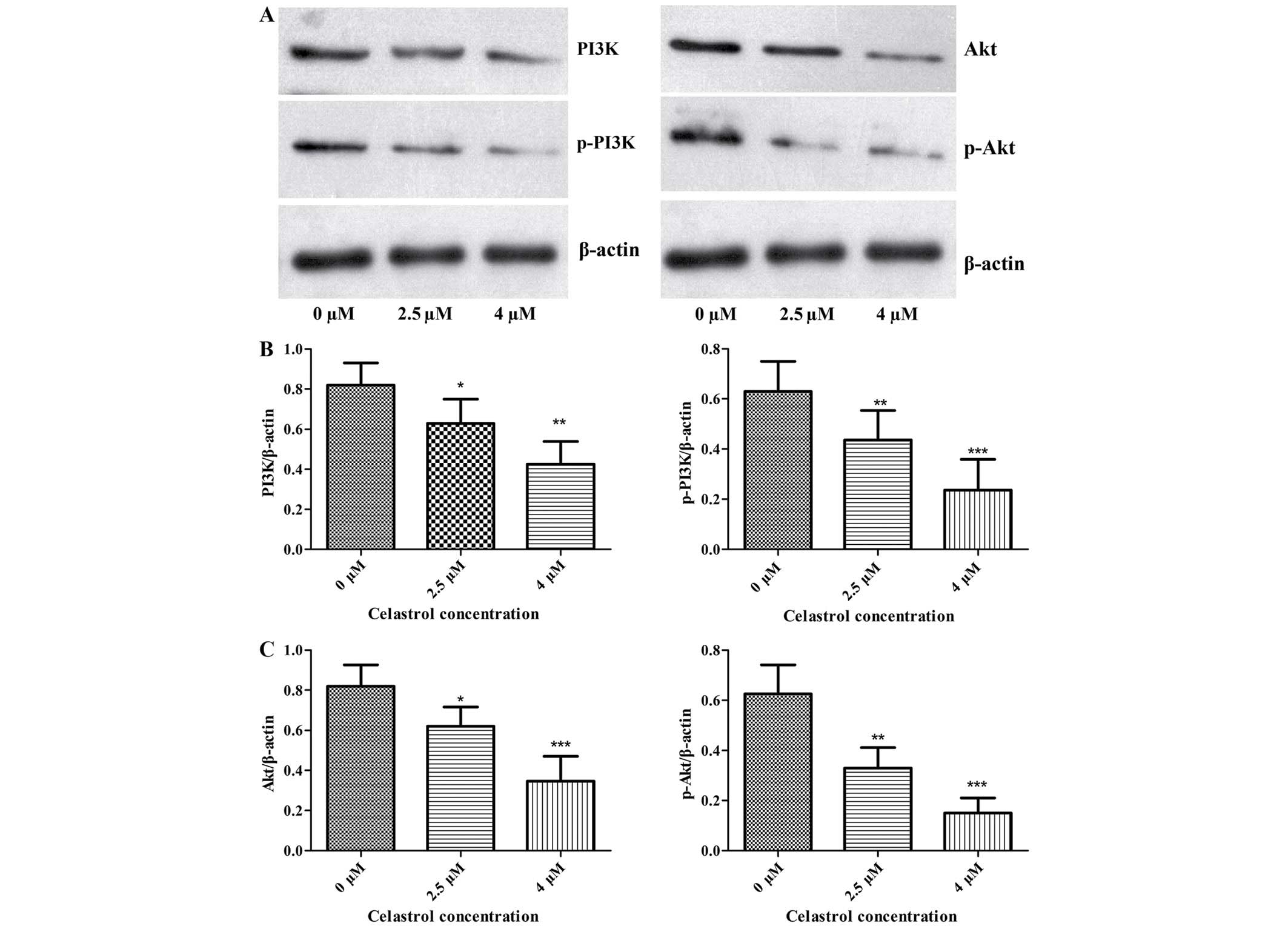
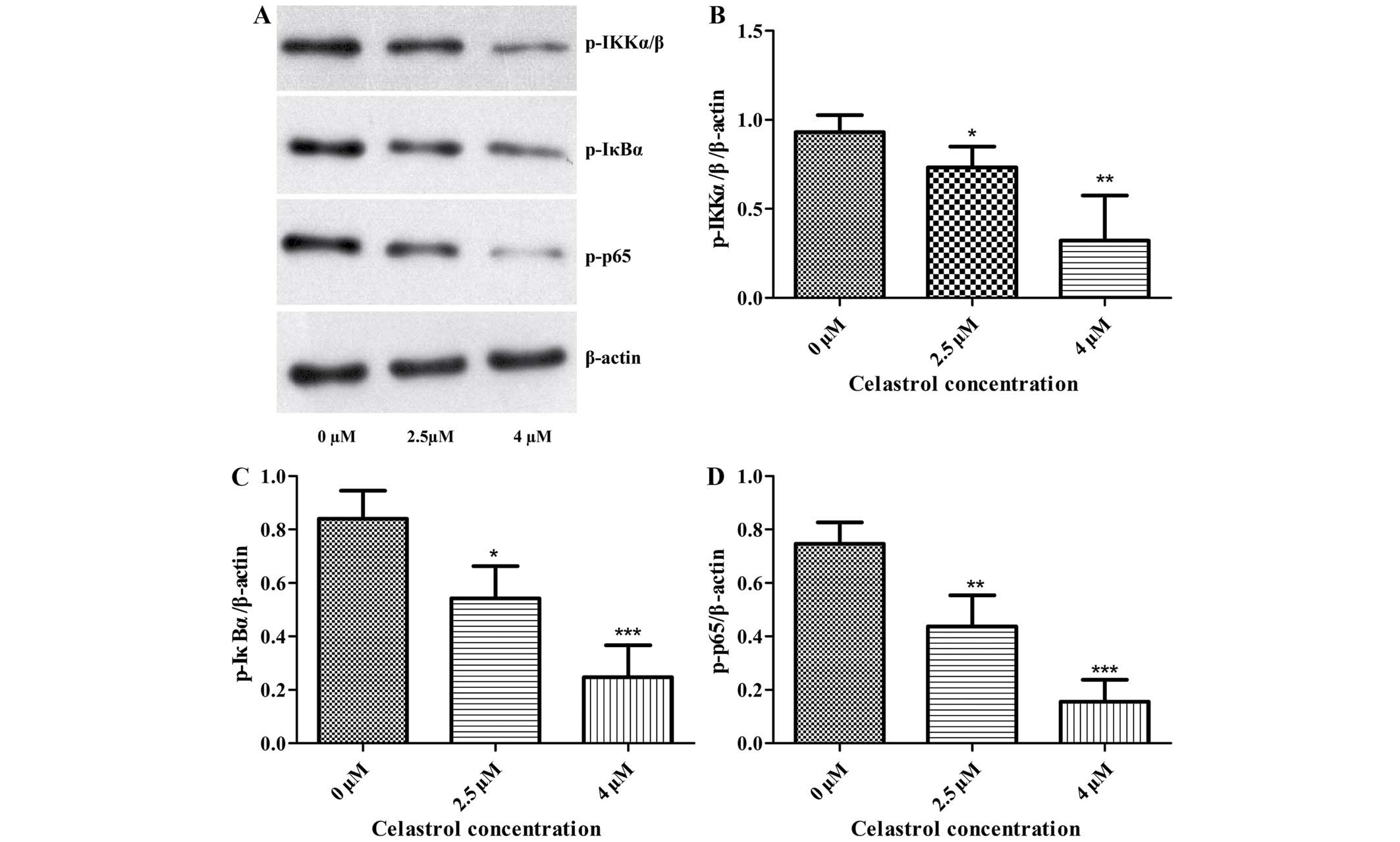
Effect of celastrol inhibition on
PI3K/Akt/NF-κB signaling pathway
The effects of Celastrol on the levels of proteins
associated with migration and invasion in U-2OS cells were examined
using western blot analysis. The expression of phosphorylated PI3K,
Akt, IKKα/β, IκBα and NF-κB subunit p65 was significantly decreased
following Celastrol treatment when compared with these levels in
the control (Figs. 3 and 4). This indicates that Celastrol
downregulates the expression of MMP-2 and −9 and inhibits cell
migration and invasion by inhibiting the PI3K/Akt/NF-κB signaling
pathway in U-2OS cells.
Discussion
Osteosarcoma is the most common primary bone
malignancy, particularly among children and adolescents, with an
incidence of four to five cases per million (30,31). The
symptoms of osteosarcoma are chronic bone pain and swelling in the
leg or arm. The current therapeutic strategies for osteosarcoma
include wide tumor excision, radiotherapy and neoadjuvant
chemotherapy, all of which have notably improved the prognosis of
patients with osteosarcoma (32–34).
However, osteosarcoma has a high tendency for local aggression and
to metastasize to the lung and distant bones, which is a common
cause of mortality (35,36). Therefore, it is an urgent requirement
to identify molecular mechanisms of invasion and metastasis in
osteosarcoma, and to develop an effective adjuvant therapy to
prevent osteosarcoma metastasis.
The interaction of cancer cells with the ECM is
essential for metastasis, and this is performed through a series of
steps including cell attachment, invasion and migration. These
steps are regulated by an extremely complex molecular mechanism
(37). The PI3K/Akt pathway is
considered to be one of the most significant oncogenic pathways in
human cancer. An increasing body of evidence has suggested that
this pathway is frequently activated in osteosarcoma and
contributes to disease development, including proliferation,
invasion and migration (38,39). A number of studies indicate that the
inhibition of this pathway could downregulate the expression of
NF-κB, which is an upstream regulator of MMPs. Therefore,
inhibition of this pathway could decrease the expressions of MMPs
(22–25). It is well known that MMPs, which
destroy the ECM and basement membranes, play a vital role in
osteosarcoma invasion and metastasis. Therefore, we may infer that
the PI3K/Akt/NF-κB signaling pathway may be a treatment target to
suppress osteosarcoma cell invasion and migration.
Celastrol, a triterpene, is an active component
extracted from the traditional Chinese medicine ‘Thunder God Vine’,
and has been used in the treatment of autoimmune and
neurodegenerative diseases (40–42).
Celastrol has previously attracted great attention due to its
significant anticancer activity in vitro and in vivo,
including the induction of apoptosis in a number of cancer cell
lines (26–29). In our previous studies, we
demonstrated that Celastrol could induce apoptosis of human
osteosarcoma cells via the mitochondrial-dependent pathway
(43). However, the effects of
Celastrol on the migration and invasion of human osteosarcoma are
still to be elucidated. In previous studies, the IC50 value for
U-2OS cells treated with Celastrol was 2.5 µM at 48 h in the MTT
assay (43). Therefore, U-2OS cells
were treated with Celastrol at concentrations of 0, 2.5 and 4 µM
for 48 h in the present study.
In the present study, cell migration and invasion
were assessed by wound healing and Boyden chamber Transwell assays.
The results revealed that the migratory and invasive capabilities
were inhibited by Celastrol. These results indicate that Celastrol
may be an effective agent for chemotherapy in the treatment of
osteosarcoma. Furthermore, protein expression levels of
phosphorylated PI3K, Akt, IKKα/β, IκBα, NF-κB subunit p65 and MMP-2
and −9 were assessed by western blot analysis. The results revealed
that the PI3K/Akt/NF-κB signaling pathway was inhibited following
Celastrol treatment. In addition, the expression levels of MMP-2
and −9 proteins were also markedly reduced following Celastrol
treatment.
Taken together, our findings suggest that Celastrol
could suppress osteosarcoma cell migration and invasion via
downregulation of the PI3K/Akt/NF-κB signaling pathway in
vitro, and that Celastrol may be an effective chemotherapeutic
agent for osteosarcoma. In addition, further experiments on the
in vivo effect of Celastrol on U-2OS xenograft tumors in
nude mice are in progress.
Acknowledgements
This project was supported by the Natural Science
Foundation of Jiangxi Province (20132BAB205081), the Foundation of
Health Department of Jiangxi Province on traditional Chinese
medicine (2012A136) and the Engineering Technology Research Center
Construction Project of Jiangxi Province (20132BCD40026).
References
|
1
|
Damron TA, Ward WG and Stewart A:
Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: National Cancer
Data Base Report. Clin Orthop Relat Res. 459:40–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poletajew S, Fus L and Wasiutyński A:
Current concepts on pathogenesis and biology of metastatic
osteosarcoma tumors. Ortop Traumatol Rehabil. 13:537–545. 2011.(In
English and Polish). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gill J, Ahluwalia MK, Geller D and Gorlick
R: New targets and approaches in osteosarcoma. Pharmacol Ther.
137:89–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guise TA, O'Keefe R, Randall RL and Terek
RM: Molecular biology and therapeutics in musculoskeletal oncology.
J Bone Joint Surg Am. 91:724–732. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salah S and Toubasi S: Factors predicting
survival following complete surgical remission of pulmonary
metastasis in osteosarcoma. Mol Clin Oncol. 3:157–162.
2015.PubMed/NCBI
|
|
6
|
Stefanatos RK and Vidal M: Tumor invasion
and metastasis in Drosophila: a bold past, a bright future. J Genet
Genomics. 38:431–438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mareel M, Oliveira MJ and Madani I: Cancer
invasion and metastasis: interacting ecosystems. Virchows Arch.
454:599–622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen A, Zhang Y, Yang H, Xu R and Huang G:
Overexpression of ZEB1 relates to metastasis and invasion in
osteosarcoma. J Surg Oncol. 105:830–834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe H: Extracellular
matrix-regulation of cancer invasion and metastasis. Gan To Kagaku
Ryoho. 37:2058–2061. 2010.(In Japanese). PubMed/NCBI
|
|
10
|
Lin YM, Chang ZL, Liao YY, Chou MC and
Tang CH: IL-6 promotes ICAM-1 expression and cell motility in human
osteosarcoma. Cancer Lett. 328:135–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Zijl F, Krupitza G and Mikulits W:
Initial steps of metastasis: cell invasion and endothelial
transmigration. Mutat Res. 728:23–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Willis AL, Sabeh F, Li XY and Weiss SJ:
Extracellular matrix determinants and the regulation of cancer cell
invasion stratagems. J Microsc. 251:250–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Polette M, Nawrocki-Raby B, Gilles C,
Clavel C and Birembaut P: Tumour invasion and matrix
metalloproteinases. Crit Rev Oncol Hematol. 49:179–186. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moss LA Shuman, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lynch CC: Matrix metalloproteinases as
master regulators of the vicious cycle of bone metastasis. Bone.
48:44–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Halbersztadt A, Haloń A, Pajak J,
Robaczyński J, Rabczynski J and St Gabryś M: The role of matrix
metalloproteinases in tumor invasion and metastasis. Ginekol Pol.
77:63–71. 2006.(In Polish). PubMed/NCBI
|
|
17
|
Khasigov PZ, Podobed OV, Gracheva TS,
Salbiev KD, Grachev SV and Berezov TT: Role of matrix
metalloproteinases and their inhibitors in tumor invasion and
metastasis. Biochemistry (Mosc). 68:711–717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancerr Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
19
|
Korpi JT, Hagström J, Lehtonen N,
Parkkinen J, Sorsa T, Salo T and Laitinen M: Expression of matrix
metalloproteinases-2, −8, −13, −26, and tissue inhibitors of
metalloproteinase-1 in human osteosarcoma. Surg Oncol. 20:e18–e22.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bjørnland K, Flatmark K, Pettersen S,
Aaasen AO, Fodstad O and Maelandsmo GM: Matrix metalloproteinases
participate in osteosarcoma invasion. J Surg Res. 127:151–156.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loukopoulos P, O'Brien T, Ghoddusi M,
Mungall BA and Robinson WF: Characterisation of three novel canine
osteosarcoma cell lines producing high levels of matrix
metalloproteinases. Res Vet Sci. 77:131–141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Felx M, Guyot MC, Isler M, Turcotte RE,
Doyon J, Khatib AM, Leclerc S, Moreau A and Moldovan F:
Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving
the transcription factor NF-kappaB in human osteosarcoma. Clin Sci
(Lond). 110:645–654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang XX, Fu Z, Zhang Z, Miao C, Xu P,
Wang T, Yang L and Cheng S: Microcystin-LR promotes melanoma cell
invasion and enhances matrix metalloproteinase-2/-9 expression
mediated by NF-κB activation. Environ Sci Technol. 46:11319–11326.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahmad A, Biersack B, Li Y, Kong D, Bao B,
Schobert R, Padhye SB and Sarkar FH: Targeted regulation of
PI3K/Akt/mTOR/NF-κB signaling by indole compounds and their
derivatives: mechanistic details and biological implications for
cancer therapy. Anticancer Agents Med Chem. 13:1002–1013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuan YH, Huang FM, Li YC and Chang YC:
Proinflammatory activation of macrophages by bisphenol
A-glycidyl-methacrylate involved NF-κB activation via PI3K/Akt
pathway. Food Chem Toxicol. 50:4003–4009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shrivastava S, Jeengar MK, Reddy VS, Reddy
GB and Naidu VG: Anticancer effect of celastrol on human triple
negative breast cancer: possible involvement of oxidative stress,
mitochondrial dysfunction, apoptosis and PI3K/Akt pathways. Exp Mol
Pathol. 98:313–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li PP, He W, Yuan PF, Song SS, Lu JT and
Wei W: Celastrol induces mitochondria-mediated apoptosis in
hepatocellular carcinoma Bel-7402 cells. Am J Chin Med. 43:137–148.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mi C, Shi H, Ma J, Han LZ, Lee JJ and Jin
X: Celastrol induces the apoptosis of breast cancer cells and
inhibits their invasion via downregulation of MMP-9. Oncol Rep.
32:2527–2532. 2014.PubMed/NCBI
|
|
29
|
Zhao X, Gao S, Ren H, Huang H, Ji W and
Hao J: Inhibition of autophagy strengthens celastrol-induced
apoptosis in human pancreatic cancer in vitro and in vivo models.
Curr Mol Med. 14:555–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sampo M, Koivikko M, Taskinen M, Kallio P,
Kivioja A, Tarkkanen M and Böhling T: Incidence, epidemiology and
treatment results of osteosarcoma in Finland - a nationwide
population-based study. Acta Oncol. 50:1206–1214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dai X, Ma W, He X and Jha RK: Review of
therapeutic strategies for osteosarcoma, chondrosarcoma, and
Ewing's sarcoma. Med Sci Monit. 17:RA177–RA190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ando K, Heymann MF, Stresing V, Mori K,
Rédini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers (Basel). 5:591–616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lamoureux F, Trichet V, Chipoy C,
Blanchard F, Gouin F and Redini F: Recent advances in the
management of osteosarcoma and forthcoming therapeutic strategies.
Expert Rev Anticancer Ther. 7:169–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Liao Q, Li K, Zhong D, Weng X and Mi
M: Knockdown of endothelin A receptor expression inhibits
osteosarcoma pulmonary metastasis in an orthotopic xenograft mouse
model. Mol Med Rep. 5:1391–1395. 2012.PubMed/NCBI
|
|
36
|
Kato H, Wakabayashi H, Naito Y, Kato S,
Nakagawa T, Matsumine A and Sudo A: Anti-tumor necrosis factor
therapy inhibits lung metastasis in an osteosarcoma cell line.
Oncology. 88:139–146. 2015.PubMed/NCBI
|
|
37
|
Daw NC, Chou AJ, Jaffe N, Rao BN, Billups
CA, Rodriguez-Galindo C, Meyers PA and Huh WW: Recurrent
osteosarcoma with a single pulmonary metastasis: a
multi-institutional review. Br J Cancer. 112:278–282. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hou CH, Lin FL, Tong KB, Hou SM and Liu
JF: Transforming growth factor alpha promotes osteosarcoma
metastasis by ICAM-1 and PI3K/Akt signaling pathway. Biochem
Pharmacol. 89:453–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Allison AC, Cacabelos R, Lombardi VR,
Alvarez XA and Vigo C: Celastrol, a potent antioxidant and
anti-inflammatory drug, as a possible treatment for Alzheimer's
disease. Prog Neuropsychopharmacol Biol Psychiatry. 25:1341–1357.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cleren C, Calingasan NY, Chen J and Beal
MF: Celastrol protects against MPTP- and 3-nitropropionic acid
induced neurotoxicity. J Neurochem. 94:995–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jung HW, Chung YS, Kim YS and Park YK:
Celastrol inhibits production of nitric oxide and proinflammatory
cytokines through MAPK signal transduction and NF-kappaB in
LPS-stimulated BV-2 microglial cells. Exp Mol Med. 39:715–721.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu X, Zhou X, Fu C, Wang Q, Nie T, Zou F,
Guo R, Liu H, Zhang B and Dai M: Celastrol induces apoptosis of
human osteosarcoma cells via the mitochondrial apoptotic pathway.
Oncol Rep. 34:1129–1136. 2015.PubMed/NCBI
|