Introduction
Endometrial carcinoma is the most common
gynecological malignancy, and the incidence is rising (1). Patients are standardly surgically
treated with a hysterectomy and bilateral salpingoophorectomy.
Despite the improved surgical treatment and adjuvant therapy used
in previous studies, the prognosis of endometrial carcinoma has not
improved significantly (2–5). Identifying novel markers that can be
used to predict the risk of endometrial carcinoma progression
remains to be important.
Sineoculis homeobox homolog 1 (SIX1) is a
transcription factor that belongs to the SIX family of
homeoproteins. SIX1 expression level is increased in embryogenesis
and promotes progenitor cell expansion and survival (6–8). SIX1
absence results in the reduction in size or loss of numerous
organs, as a result of inhibited proliferation and increased
apoptosis in mice (9). However, SIX1
expression is low in adult tissues, and aberrant expression of the
SIX1 gene in adult tissue may contribute to carcinogenesis
(10). SIX1 was found to be
overexpressed in human breast, cervical, ovarian and pancreatic
cancers and associated with a poor patient survival (11–15). SIX1
overexpression promotes cancer cell survival and epithelial to
mesenchymal transition (EMT) (16,17).
Overall, these findings suggest that SIX1 is an important
oncoprotein for the development of cancer. However, the clinical
significance and biological role of SIX1 in endometrial carcinoma
remains unexplored. In the present study, endogenous SIX1
expression was examined in endometrial carcinoma specimens. SIX1
expression was overexpressed and downregulated, and the effect on
cell proliferation was investigated. The present study also
investigated the potential molecular signaling pathways underlying
the biological effects of SIX1.
Materials and methods
Patients and specimens
The study protocol was approved by the Institutional
Review Board of Shengjing Hospital of China Medical University
(Shengyang, China). Primary tumor specimens were obtained from 60
female patients (age range, 42–70 years) diagnosed with
endometrioid adenocarcinoma, who underwent resections in the
Shengjing Hospital of China Medical University between January 2010
and December 2012. The tumor sections were evaluated using
histological diagnosis, according to World Health Organization
guidelines (18–20). The International Federation of
Gynecology and Obstetrics (FIGO) staging system was used to
classify patients as stages I, II or III (21). Clinical and histopathological data
were obtained from medical records. The study protocol was approved
by the Institutional Review Board of Shengjing Hospital of China
Medical University (Shenyang, China) and informed consent was
obtained from all patients.
Immunohistochemistry
Tumor specimens were fixed with 10% neutral formalin
and 4-µm thick paraffin sections were cut. Immunostaining was
performed using the Ultrasensitive™ S-P staining kit (Maixin
Biotech Co., Ltd., Fuzhou, China). After antigen retrieval in
citrate buffer (pH 6.0) for 2 min in an autoclave, 0.3% hydrogen
peroxide was applied to the samples for 15 min and then the
sections were incubated with goat serum for 10 min at room
temperature (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The samples were incubated with SIX1 rabbit polyclonal
antibody (dilution, 1:300; SAB2102157; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) at 4°C overnight. Samples were then
incubated with biotinylated goat anti-rabbit serum immunoglobulin G
(IgG) antibodies (dilution, 1:200; A0545; Sigma-Aldrich; Merck
Millipore) for 10 min at room temperature subsequent to washing in
phosphate-buffered saline (PBS). The samples were then incubated
with streptavidin- biotin antibodies conjugated with horseradish
peroxidase (dilution, 1:300; A0185; Sigma-Aldrich; Merck Millipore)
for 10 min at room temperature. The DAB Detection kit (Maixin
Biotech Co., Ltd.) was then used for staining. Counterstaining with
hematoxylin was performed, and the sections were dehydrated in
ethanol prior to mounting.
Two independent pathologists of the Department of
Pathology of Shengjing Hospital of China Medical University
examined the sections. In total, 500 cells were counted for each
slide. The immunostaining of SIX1 was scored on a system that
evaluated the intensity and percentage of tumor cells. Nuclear and
cytoplasmic immunostaining in tumor cells were considered to show
SIX1 staining. The intensity of staining was scored as: 0, no
signal; 1, weak; or 2, strong. Percentage scores were assigned as:
1, 1–25%; 2, 26–50%; 3, 51–75%; or 4, 76–100%. Then the intensity
score and percentage score were multiplied to get a final score
between 0 and 8. Tumor samples receiving a score between 4 and 8
were regarded to show SIX1 overexpression.
Cell culture and transfection
The HEC1B and Ishikawa cell lines were obtained from
American Type Culture Collection (Manassas, VA, USA). Cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc.) containing 10% fetal calf serum
(Invitrogen; Thermo Fisher Scientific, Inc.). Cells were passaged
every 2 days with 0.25% trypsin.
The pCMV6-SIX1 vector was purchased from OriGene
Technologies, Inc. (Rockville, MD, USA). Attractene transfection
reagent (Qiagen, Hilden, Germany) was used for plasmid
transfection. The pCMV6 vector was used as a negative control.
Cells were harvested 48 h subsequent to transfection. Dharmacon
On-TargetPlus SMARTpool small interfering RNA (siRNA) for SIX1 and
ON-TARGETplus Non-targeting siRNA were purchased from Thermo Fisher
Scientific, Inc.. The cells were transfected with siRNA using the
DharmaFECT 1 (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) (SYBR Green method)
Total RNA was extracted using a total RNA extraction
kit (SYBR Select Master Mix; Applied Biosystems; Thermo Fisher
Scientific, Inc.). RT-qPCR was performed using the Applied
Biosystems SYBR Green master mix kit, purchased from Thermo Fisher
Scientific, Inc. PCR was performed using 7900HT Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
β-actin was used as the reference gene. The cycling conditions were
as follows: 50°C for 2 min, 95°C for 2 min, and 45 cycles of 95°C
for 15 sec and 60°C for 40 sec. The relative expression of target
genes were calculated as ΔCq = Cq gene - Cq reference, and the fold
change of target gene expression was calculated by the
2−ΔΔCq method (22).
Experiments were repeated in triplicate. The primer sequences were
as follows: SIX1 forward, 5′-AAGGAGAAGTCGAGGGGTGT-3′ and reverse,
5′-TGCTTGTTGGAGGAGGAGTT-3′; β-actin forward,
5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and reverse,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′.
Western blot analysis
Total proteins from the cells were extracted using
lysis buffer and quantified using the Bradford method (23–25). In
total, 30 µg protein was separated by sodium dodecyl sulfate
polyacrylamide gel electrophoresis (stacking gel, 5%; separating
gel, 10%). Samples were transferred to polyvinylidene fluoride
(PVDF) membranes (EMD Millipore, Billerica, MA, USA) and subjected
to a blocking step with 5% bovine serum albumin (BSA) (5 g BSA +
100 ml Tris-buffered saline with Tween 20; Maixin Biotech Co.,
Ltd.) at room temperature for 45 min. Next, membranes were
incubated overnight at 4°C with antibodies against SIX1 (rabbit
polyclonal antibody; dilution, 1:800; SAB2102157; Sigma-Aldrich;
Merck Millipore), phosphorylated (p)-retinoblastoma protein (Rb)
(rabbit monoclonal antibody; dilution, 1:1,000; 8147; Cell
Signaling Technology, Inc., Danvers, MA, USA), p-ERK (rabbit
polyclonal antibody; dilution, 1:1,000; 9101; Cell Signaling
Technology, Inc.), ERK (rabbit polyclonal antibody; dilution,
1:1,000; 9102; Cell Signaling Technology, Inc.), p-AKT (rabbit
polyclonal antibody; dilution, 1:1,000; 9271; Cell Signaling
Technology, Inc.), AKT (rabbit polyclonal antibody; dilution,
1:1,000; 9272; Cell Signaling Technology, Inc.), cyclin D1 (rabbit
monoclonal antibody; dilution, 1:1,000; 2978; Cell Signaling
Technology, Inc.), cyclin E (rabbit monoclonal antibody; dilution,
1:1,000; 20808; Cell Signaling Technology, Inc.) and glyceraldehyde
3-phosphate dehydrogenase (GAPDH; rabbit polyclonal antibody;
dilution, 1:1,000; G5262; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA). Subsequently, samples were incubated with peroxidase-
coupled anti-mouse (monoclonal antibody; dilution, 1:1,000; 7076,
Cell Signaling Technology, Inc.) or anti-rabbit IgG antibodies
(polyclonal antibody; dilution, 1:1,000; 7074; Cell Signaling
Technology, Inc.) at 37°C for 2 h. Target proteins on the PVDF
membrane were visualized using a Pierce enhanced chemiluminescence
kit (Thermo Fisher Scientific, Inc.) and captured using a DNR
BioImaging system equipped with GelCapture application software
(DNR Bio-Imaging Systems Ltd., Jerusalem, Israel).
Colony formation and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays
For the colony formation assay, cells (~1,000 per
dish) were plated into 6 cm culture dishes, 48 h after
transfection. After two weeks, plates were washed with PBS and
Giemsa staining was performed to visualize the colonies. The
colonies with >50 cells were counted using a microscope.
For the MTT assay, cells (~3,000 per well) were
plated in 96-well plates and cultured for 5 days. In total, 20 µl
of 5 mg/ml MTT solution was added to each well. After incubation
for 4 h, the medium was removed and the remaining MTT formazan was
dissolved in 150 µl of DMSO. The optical density of the solution
was measured at 490 nm using a microplate reader.
Statistical analysis
SPSS version 11.5 for Windows (SPSS, Inc., Chicago,
IL, USA) was used for all statistical analyses. A χ2
test was used to examine the possible associations between SIX1
expression and clinicopathological factors. The Student's t-test
was used to compare differences between control and
SIX1-transfected cells. All P-values were based on a two-sided
statistical analysis, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of SIX1 in human
endometrial carcinoma
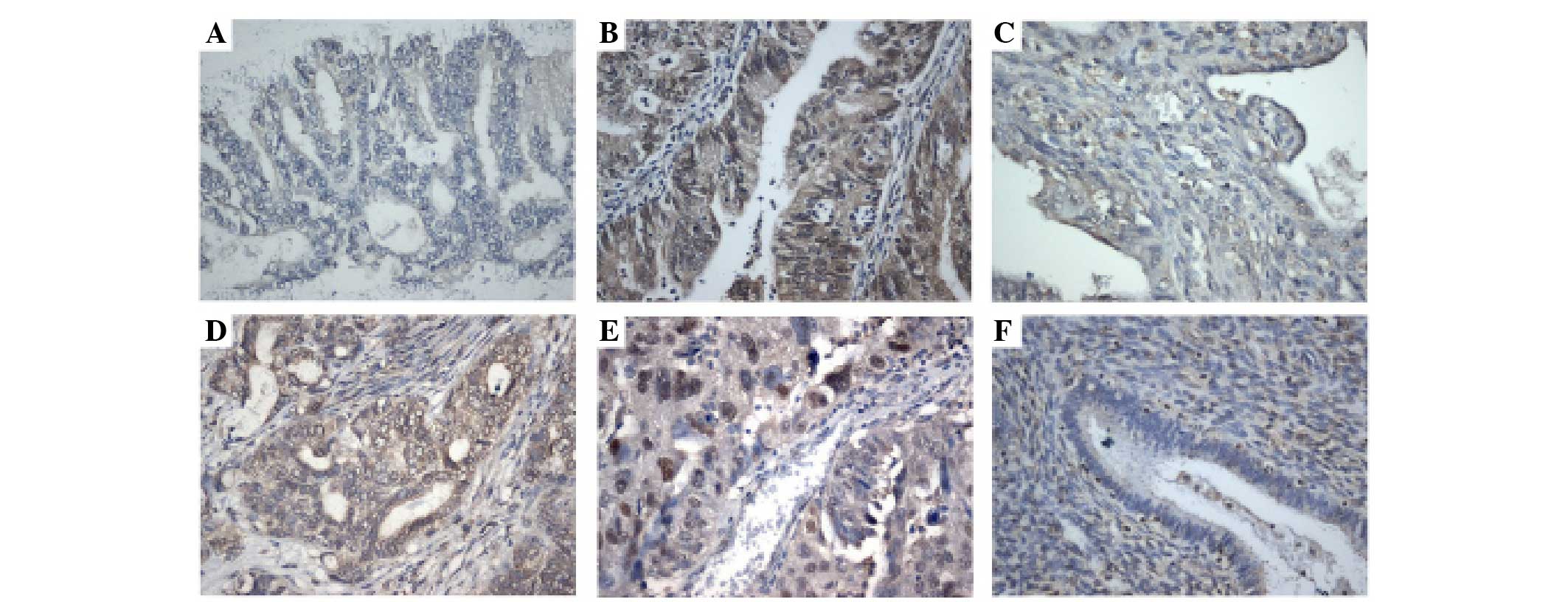
SIX1 immunostaining was observed in 84 cases of
endometrioid adenocarcinoma (Fig. 1).
In normal endometrial tissues, stromal and glandular tissues showed
weak or negative staining. In total, 43/84 cancer tissues showed
positive SIX1 immunoreactivity, and the expression was located in
the nuclear and cytoplasmic compartment of the cancer cells. The
associations between SIX1 expression and the clinicopathological
characteristics of patients is shown in Table I. A high SIX1 immunostaining score in
the primary endometrioid carcinoma was significantly associated
with advanced tumor grade (P=0.0105). However, there was no
association between SIX1 expression levels and other parameters,
including FIGO stage (P=0.7034) or patient age (P=0.6089).
 | Table I.Distribution of SIX1 status in
endometrial carcinomas, according to clinicopathological
characteristics. |
Table I.
Distribution of SIX1 status in
endometrial carcinomas, according to clinicopathological
characteristics.
| Characteristics | No. of patients | SIX1
weak/negative | SIX1 positive | P-value |
|---|
| Age, years |
|
|
| 0.6089 |
|
<60 | 53 | 27 | 26 |
|
| ≥60 | 31 | 14 | 17 |
|
| Tumor grade |
|
|
| 0.0105 |
| 1+2 | 61 | 35 | 26 |
|
| 3 | 23 | 6 | 17 |
|
| FIGO stage |
|
|
| 0.7034 |
| I | 59 | 28 | 31 |
|
|
II+III | 25 | 13 | 12 |
|
SIX1 promotes endometrial cell
proliferation and colony formation
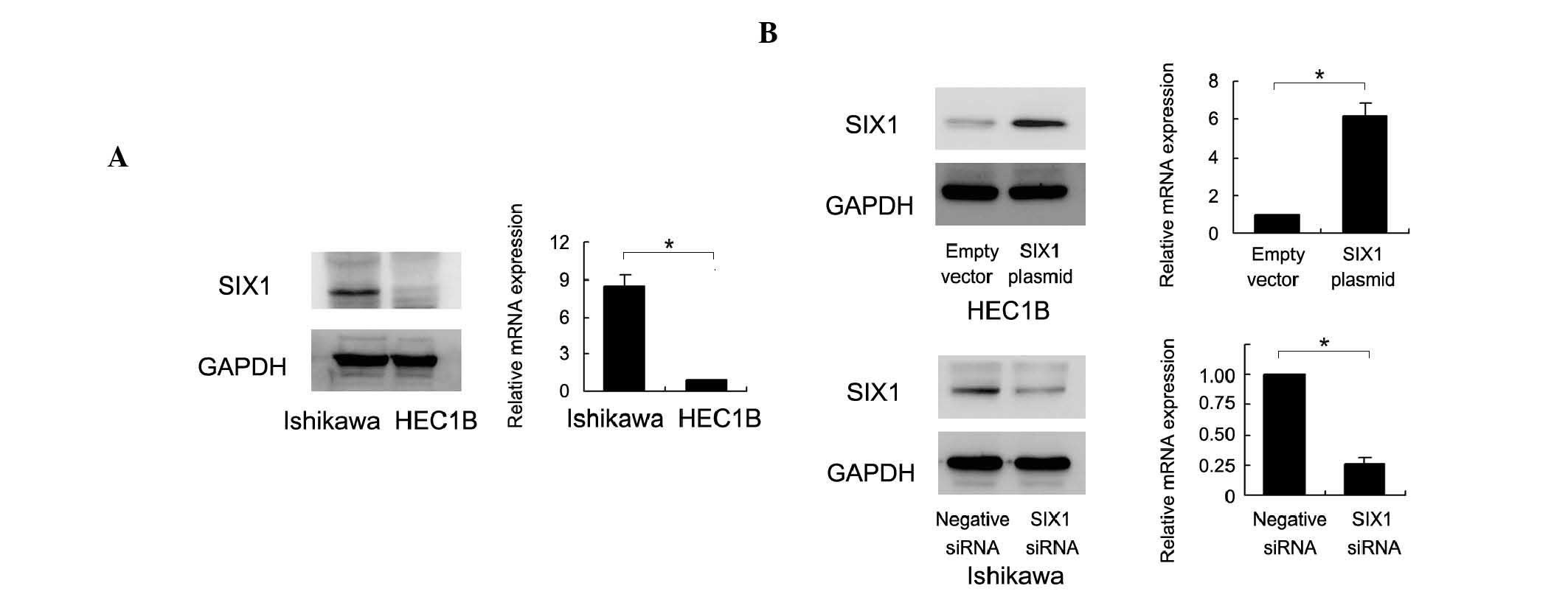
The endogenous expression of SIX1 was examined by
western blot analysis and RT-qPCR in Ishikawa and HEC1B cell lines.
Ishikawa cells possess high endogenous SIX1 expression and HEC1B
cells possess low SIX1 expression (Fig.
2A). To determine the biological role of SIX1 in endometrial
cancer, plasmid transfection and siRNA knockdown were performed. As
shown in Fig. 2B, SIX1 plasmid
transfection significantly upregulated the expression of SIX1
protein and mRNA. SIX1 siRNA transfection significantly
downregulated the expression of SIX1 protein and mRNA. The MTT
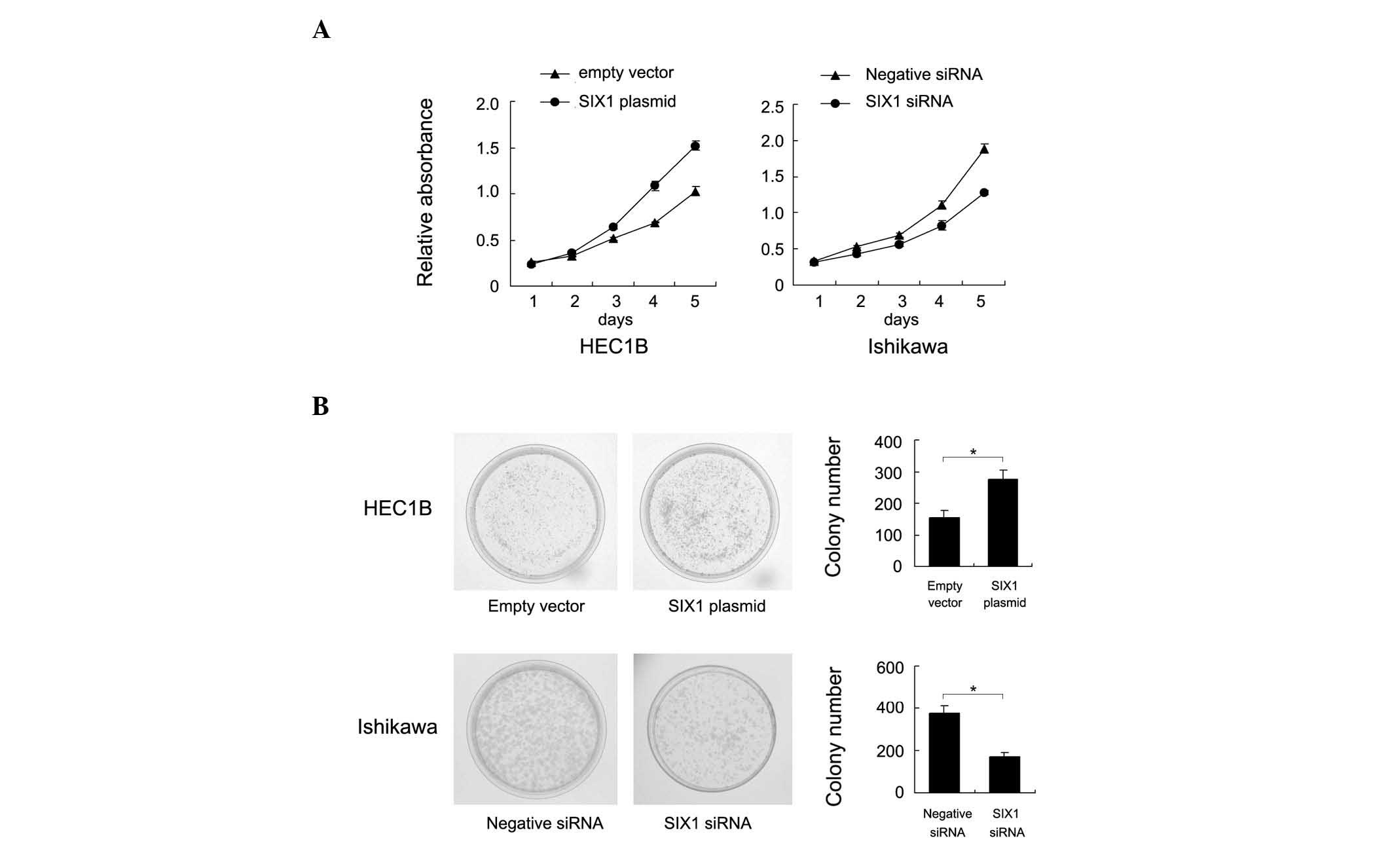
assay revealed that SIX1 upregulation increased the cell
proliferation rate in HEC1B cells and SIX1 depletion decreased the
proliferation rate in Ishikawa cells (Fig. 3A). A colony formation assay was also
performed. As shown in Fig. 3B, SIX1
transfection significant increased the colony number of HEC1B cells
(empty vector vs. SIX1 plasmid, 155±22 vs. 276±28; P=0.041). By
contrast, SIX1 depletion in Ishikawa cells significantly
downregulated colony formation ability (negative siRNA vs. SIX1
siRNA, 378±33 vs. 169±20; P=0.047). Accordingly, SIX1 transfection
was found to upregulate, whereas SIX1 depletion was found to
downregulate, cyclin D1 and cyclin E and p-Rb expression (Fig. 4), which suggests that SIX1 facilitated
cell cycle transition.
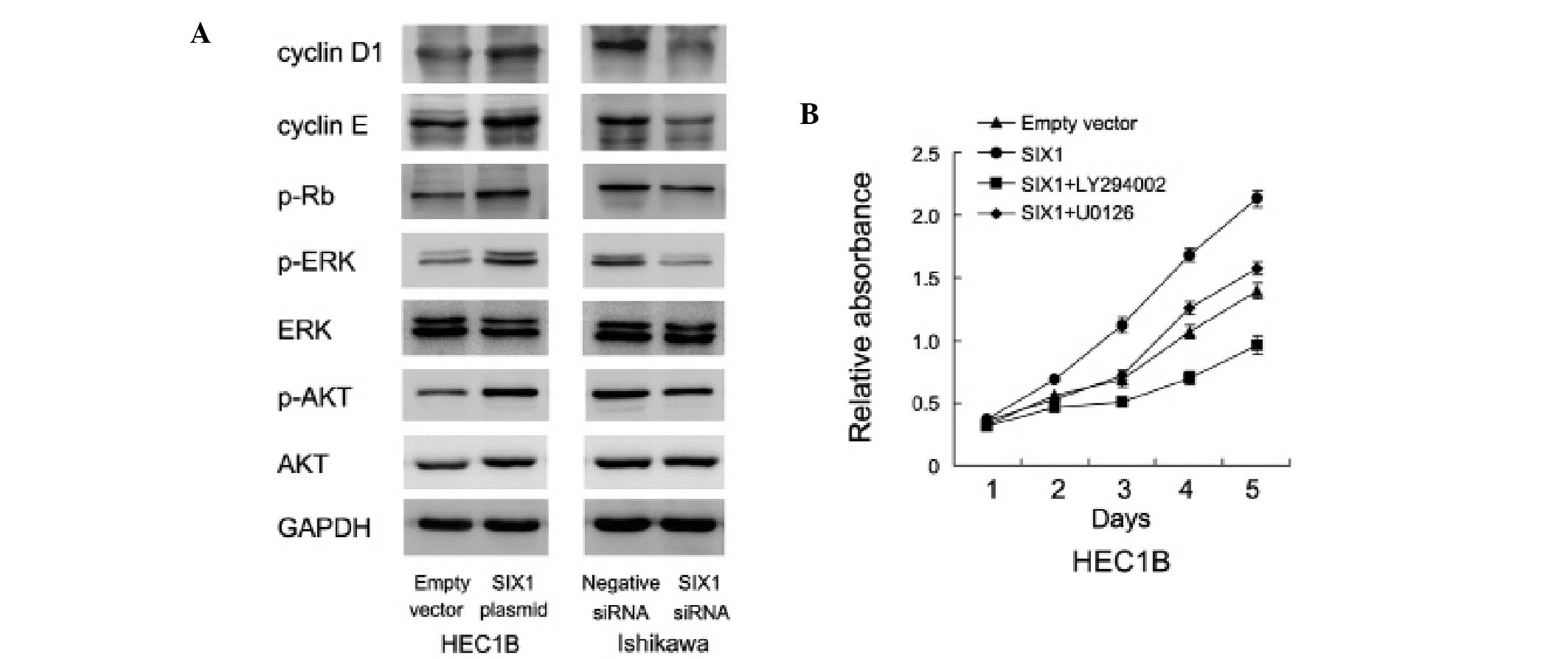 | Figure 4.SIX1 upregulates ERK and AKT activity.
(A) SIX1 transfection was performed in HEC1B cells and siRNA
knockdown was performed in Ishikawa cells. Western blot analysis
showed that SIX1 transfection upregulated, while SIX1 depletion
downregulated, cyclin D1, cyclin E, p-ERK and p-AKT expression. (B)
In SIX1 transfected HEC1B cells, treatment with AKT inhibitor,
LY294002 (20 µM), and ERK inhibitor, U0126 (10 µmol/l),
significantly blocked the role of SIX1 on cell growth rate. SIX1,
sineoculis homeobox homolog 1; siRNA, small interfering RNA; ERK,
extracellular signal-regulated kinase; AKT, protein kinase B;
p-ERK, phosphorylated-ERK; p-AKT, phosphorylated-AKT; p-Rb,
phosphorylated-retinoblastoma protein. |
SIX1 promotes cell proliferation
through ERK and AKT signaling
In order to investigate the potential mechanisms of
SIX1-induced endometrial carcinoma cell proliferation, the level of
ERK and AKT signaling pathway activation was examined in cells with
SIX1 transfection and depletion. SIX1 transfection was found to
upregulate AKT and ERK phosphorylation, whereas SIX1 depletion was
found to decrease the level of pathway activation (Fig. 4A). In addition, treatment with the AKT
inhibitor, LY294002 (20 µM), and ERK inhibitor, U0126 (10 µmol/l),
for 6 h significantly blocked the role of SIX1 on endometrial
cancer growth (Fig. 4B), suggesting
that the ERK and AKT signaling cascades were involved in
SIX1-induced endometrial cancer growth.
Discussion
Endometrial carcinoma is a common gynecological
cancer. Although studies have indicated that numerous oncogenes in
endometrial carcinoma promote cancer progression, novel markers to
predict aggressive phenotypes are urgently required. The
upregulation of SIX1 expression has been reported in human
gynecological cancers, including breast, cervical and ovarian
carcinoma, where it leads to increased proliferation and metastasis
(13–15,17). One
study reported that SIX1 was aberrantly upregulated in human
colorectal cancer and promoted epithelial-mesenchymal transition
via zinc finger E-box binding homeobox 1 activation (26). It was recently reported that the SIX1
protein is upregulated in human gastric carcinoma and associated
with clinical parameters (27).
However, the biological roles and potential molecular mechanism of
SIX1 in human endometrial carcinoma cells remains unexplored.
The present study found that SIX1 was overexpressed
in 43/84 endometrial carcinoma samples and associated with advanced
tumor grade. These findings indicate an association between SIX1
overexpression and the malignant endometrial carcinoma phenotype.
In addition, SIX1 overexpression promoted cell proliferation, while
SIX1 depletion inhibited cell growth. These data are in accordance
with previous reports, which confirmed the role of SIX1 as an
important oncogene in human endometrial carcinomas (15,26,28,29).
The regulation effect of SIX1 on cell cycle proteins
has been previously reported in other human cancers (30,31). The
present study examined the effect of SIX1 on cell cycle progression
and found that SIX1 overexpression upregulated, while SIX1
depletion downregulated, cyclin D1 and cyclin E expression; a
finding that was in accordance with previous reports (15,32,33). The
present study further investigated the molecular pathways involved
in SIX1-induced cancer progression. Previous reports indicated that
SIX1 could induce EMT, which is closely associated with cancer cell
invasion and metastasis (16,17). Previous studies indicated that SIX1
could induce ERK activation (34).
The present study investigated the change of ERK status in cells
transfected with SIX1 plasmids/siRNA. The results showed that SIX1
could increase the level of ERK phosphorylation. The present study
also examined the change of AKT phosphorylation in endometrial
carcinoma cells with the overexpression or knockdown of SIX1. SIX1
overexpression or knockdown resulted in the increased or decreased
Ser473 phosphorylation of AKT, respectively. In addition, the ERK
inhibitor, U0126, and AKT inhibitor, LY294002, could partially
reverse the upregulatory effect of SIX1 on proliferation, which
strongly supports the importance of SIX1 in regulating cell
proliferation via the ERK and AKT pathways.
In conclusion, the present study demonstrated that
increased SIX1 expression contributes to the malignant tumor growth
of endometrial carcinoma cells, possibly through the regulation of
the ERK and AKT signaling pathways. SIX1 may be considered as a
novel cancer marker for endometrial carcinoma.
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sivridis E, Giatromanolaki A, Gatter KC,
Harris AL and Koukourakis MI: Tumor and Angiogenesis Research
Group: Association of hypoxia-inducible factors 1alpha and 2alpha
with activated angiogenic pathways and prognosis in patients with
endometrial carcinoma. Cancer. 95:1055–1063. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakuragi N, Ohkouchi T, Hareyama H, Ikeda
K, Watari H, Fujimoto T, Kuwabara M, Yamamoto R, Sagawa T, Fujino T
and Fujimoto S: Bcl-2 expression and prognosis of patients with
endometrial carcinoma. Int J Cancer. 79:153–158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang S, Kang WD, Chung HH, Jeong DH, Seo
SS, Lee JM, Lee JK, Kim JW, Kim SM, Park SY and Kim KT:
Preoperative identification of a low-risk group for lymph node
metastasis in endometrial cancer: A Korean gynecologic oncology
group study. J Clin Oncol. 30:1329–1334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wright JD, Burke WM, Wilde ET, Lewin SN,
Charles AS, Kim JH, Goldman N, Neugut AI, Herzog TJ and Hershman
DL: Comparative effectiveness of robotic versus laparoscopic
hysterectomy for endometrial cancer. J Clin Oncol. 30:783–791.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Chakroun I, Yang D, Horner E, Liang
J, Aziz A, Chu A, De Repentigny Y, Dilworth FJ, Kothary R and Blais
A: Six1 regulates MyoD expression in adult muscle progenitor cells.
PLoS One. 8:e677622013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nord H, Skalman L Nygard and von Hofsten
J: Six1 regulates proliferation of Pax7-positive muscle progenitors
in zebrafish. J Cell Sci. 126:1868–1880. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ikeda K, Kageyama R, Suzuki Y and Kawakami
K: Six1 is indispensable for production of functional progenitor
cells during olfactory epithelial development. Int J Dev Biol.
54:1453–1464. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen B, Kim EH and Xu PX: Initiation of
olfactory placode development and neurogenesis is blocked in mice
lacking both Six1 and Six4. Dev Biol. 326:75–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar JP: The sine oculis homeobox (SIX)
family of transcription factors as regulators of development and
disease. Cell Mol Life Sci. 66:565–583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coletta RD, Christensen K, Reichenberger
KJ, Lamb J, Micomonaco D, Huang L, Wolf DM, Müller-Tidow C, Golub
TR, Kawakami K and Ford HL: The Six1 homeoprotein stimulates
tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci USA.
101:6478–6483. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reichenberger KJ, Coletta RD, Schulte AP,
Varella-Garcia M and Ford HL: Gene amplification is a mechanism of
Six1 overexpression in breast cancer. Cancer Res. 65:2668–2675.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng XH, Liang PH, Guo JX, Zheng YR, Han
J, Yu LL, Zhou YG and Li L: Expression and clinical implications of
homeobox gene Six1 in cervical cancer cell lines and cervical
epithelial tissues. Int J Gynecol Cancer. 20:1587–1592.
2010.PubMed/NCBI
|
|
14
|
Behbakht K, Qamar L, Aldridge CS, Coletta
RD, Davidson SA, Thorburn A and Ford H: Six1 overexpression in
ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and
is associated with poor survival. Cancer Res. 67:3036–3042. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Z, Tian T, Lv F, Chang Y, Wang X, Zhang
L, Li X, Li L, Ma W, Wu J and Zhang M: Six1 promotes proliferation
of pancreatic cancer cells via upregulation of cyclin D1
expression. PLoS One. 8:e592032013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith AL, Iwanaga R, Drasin DJ, Micalizzi
DS, Vartuli RL, Tan AC and Ford HL: The miR-106b-25 cluster targets
Smad7, activates TGF-β signaling and induces EMT and tumor
initiating cell characteristics downstream of Six1 in human breast
cancer. Oncogene. 31:5162–5171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Radisky DC: Defining a role for the
homeoprotein Six1 in EMT and mammary tumorigenesis. J Clin Invest.
119:2528–2531. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song JH, Kim SH, Lee JH, Cho HM, Kim DY,
Kim TH, Kim SY, Baek JY, Oh JH, Nam TK, et al: Significance of
histologic tumor grade in rectal cancer treated with preoperative
chemoradiotherapy followed by curative surgery: A
multi-institutional retrospective study. Radiother Oncol.
118:387–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davidson BA, Foote J, Clark LH, Broadwater
G, Ehrisman J, Gehrig P, Graybill W, Secord A Alvarez and
Havrilesky LJ: Tumor grade and chemotherapy response in
endometrioid endometrial cancer. Gynecol Oncol Rep. 27:3–6. 2016.
View Article : Google Scholar
|
|
20
|
Mittelbronn M, Schittenhelm J, Lemke D,
Ritz R, Nägele T, Weller M, Meyermann R and Beschorner R: Low grade
ganglioglioma rapidly progressing to a WHO grade IV tumor showing
malignant transformation in both astroglial and neuronal cell
components. Neuropathology. 27:463–467. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saida T, Tanaka YO, Matsumoto K, Satoh T,
Yoshikawa H and Minami M: Revised FIGO staging system for cancer of
the ovary, fallopian tube, and peritoneum: Important implications
for radiologists. Jpn J Radiol. 34:117–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barbosa H, Slater NK and Marcos JC:
Protein quantification in the presence of poly(ethylene glycol) and
dextran using the Bradford method. Anal Biochem. 395:108–110. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carlsson N, Borde A, Wölfel S, Kerman B
and Larsson A: Quantification of protein concentration by the
Bradford method in the presence of pharmaceutical polymers. Anal
Biochem. 411:116–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu TS, Yiao SY, Lim K, Jensen RV and Hsiao
LL: Interpretation of biological and mechanical variations between
the Lowry versus Bradford method for protein quantification. N Am J
Med Sci. 2:325–328. 2010.PubMed/NCBI
|
|
26
|
Ono H, Imoto I, Kozaki K, Tsuda H, Matsui
T, Kurasawa Y, Muramatsu T, Sugihara K and Inazawa J: SIX1 promotes
epithelial-mesenchymal transition in colorectal cancer through ZEB1
activation. Oncogene. 31:4923–4934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lv H, Cui A, Sun F, Zhang Y, Li Y, Li L
and Lin Z: Sineoculis homeobox homolog 1 protein as an independent
biomarker for gastric adenocarcinoma. Exp Mol Pathol. 97:74–80.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun SH, Liu D, Deng YT, Zhang XX, Wan DY,
Xi BX, Huang W, Chen Q, Li MC, Wang MW, et al: SIX1 coordinates
with TGFβ signals to induce epithelial-mesenchymal transition in
cervical cancer. Oncol Lett. 12:1271–1278. 2016.PubMed/NCBI
|
|
29
|
Wang CA, Jedlicka P, Patrick AN, Micalizzi
DS, Lemmer KC, Deitsch E, Casás-Selves M, Harrell JC and Ford HL:
SIX1 induces lymphangiogenesis and metastasis via upregulation of
VEGF-C in mouse models of breast cancer. J Clin Invest.
122:1895–1906. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu Y, Davicioni E, Triche TJ and Merlino
G: The homeoprotein six1 transcriptionally activates multiple
protumorigenic genes but requires ezrin to promote metastasis.
Cancer Res. 66:1982–1989. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu K, Li Z, Cai S, Tian L, Chen K, Wang J,
Hu J, Sun Y, Li X, Ertel A and Pestell RG: EYA1 phosphatase
function is essential to drive breast cancer cell proliferation
through cyclin D1. Cancer Res. 73:4488–4499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Christensen KL, Brennan JD, Aldridge CS
and Ford HL: Cell cycle regulation of the human Six1 homeoprotein
is mediated by APC(Cdh1). Oncogene. 26:3406–3414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ford HL, Landesman-Bollag E, Dacwag CS,
Stukenberg PT, Pardee AB and Seldin DC: Cell cycle-regulated
phosphorylation of the human SIX1 homeodomain protein. J Biol Chem.
275:22245–22254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iwanaga R, Wang CA, Micalizzi DS, Harrell
JC, Jedlicka P, Sartorius CA, Kabos P, Farabaugh SM, Bradford AP
and Ford HL: Expression of Six1 in luminal breast cancers predicts
poor prognosis and promotes increases in tumor initiating cells by
activation of extracellular signal-regulated kinase and
transforming growth factor-beta signaling pathways. Breast Cancer
Res. 14:R1002012. View
Article : Google Scholar : PubMed/NCBI
|