Introduction
In spite of a major decline in incidence and
mortality over several decades, pancreatic cancer (PC) is still one
of the most common causes of cancer mortality worldwide (1–3). Since PC
is a tumor with a high potential of invasion and metastasis, the
majority of patients with PC are in advanced stage when they are
diagnosed (4). Although numerous
studies indicate that tumor invasion and metastasis are attributed
to the increase in cell motility and loss of cell-cell adhesion
(5), the molecular mechanisms of PC
invasion and metastasis are not completely understood.
Notch4, which belongs to a family of transmembrane
receptor proteins (Notch1 to Notch4), is upregulated in numerous
malignant tumors and plays critical roles in various cell fate
decisions, including proliferation, apoptosis and differentiation
(6–9).
Previous studies demonstrated that Notch4 is overexpressed in human
hepatocellular carcinoma and is associated with poor overall
survival (8), and that inactivation
of Notch4 by small interfering RNA (siRNA) sensitizes breast tumor
cells to TNF-related apoptosis-inducing ligand-induced apoptosis
(7). Furthermore, our previous study
demonstrated that Notch4 contributes to gastric cancer cell growth
associated with Wnt1/β-catenin activation (6). These data suggest that Notch4 is
important in tumorigenesis, and that Notch4 may be a potential
target for cancer prevention and therapy.
The serine/threonine kinase Akt, also known as
protein kinase B, is a central node in cell signaling downstream of
growth factors, cytokines and other cellular stimuli (10). Aberrant Akt activation is one of the
most common molecular alterations in cancer and contributes to
tumorigenesis (10), as well as to
promote resistance to chemotherapy (11–13). In
addition, Akt is a potential target for enhancing DNA damage or
apoptosis in response to chemotherapeutic agents in cancer therapy
(11–13). Since there may be cross-talk between
Notch4 and Akt (14), we expect that
Notch4 may regulate chemosensitivity via Akt in PC cells.
However, to date, there are no additional studies
with respect to the role of Notch4 in pancreatic carcinogenesis. In
order to investigate the role of Notch4 in pancreatic
carcinogenesis, siRNA was used in the present study to specifically
inhibit Notch4 expression in several PC cell lines, and cell
viability, migration and invasion of PC cells were detected in
vitro. Furthermore, the potential induction of chemosensitivity
in PC cells by a chemotherapeutic agent under treatment with Notch4
siRNA was also investigated.
Materials and methods
Cell culture
The human PC cell lines MiaPaCa-2, BxPC3, PANC-1 and
Capan-1, as well as HPDE6c7, an immortalized but not transformed
pancreatic epithelial cell line, were cultured in 90% RPMI 1640
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with antibiotics (1X penicillin/streptomycin 100 U/ml)
and 10% fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific,
Inc.). Cells were incubated in a humidified atmosphere containing
5% CO2 at 37°C.
Western blot analysis
Western blot analysis was performed to evaluate the
protein levels of Notch4, phosphorylated (p)-Akt, Akt, glycogen
synthase kinase 3 (GSK3), p-GSK3 and fascin. Proteins were
harvested from cells using 2X electrophoresis sample buffer [125
mmol/l Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), 5%
glycerol and 1% β-mercaptoethanol]. Equal amounts of denatured
proteins (35 µg) were resolved by 10% SDS-polyacrylamide gel
electrophoresis and then blotted onto polyvinylidene fluoride
membranes (Pall Life Sciences, Port Washington, NY, USA). After
blocking for 2 h with phosphate-buffered saline containing 0.1%
Tween 20 (PBST) and 5% powdered skim milk, the blots were incubated
with primary Notch4 (cat. no. ab134831; 1:1,000), p-Akt (cat. no.
ab8933; 1:500), Akt (cat. no. ab8805; 1:1,000), GSK3 (cat. no.
ab131344; 1:500), p-GSK3 (cat. no. ab28808; 1:500) and fascin (cat.
no. ab97753; 1:1,000) antibodies (Abcam, Cambridge, UK) overnight
in 5% powdered skim milk buffer, washed thrice with PBST and
incubated with horseradish peroxidase-conjugated anti-rabbit (cat.
no. 7074) and anti-mouse (cat. no. 7076) IgG secondary antibodies
(1:2,000; Cell Signaling Technology, Beverly, MA, USA). An
anti-glyceraldehyde 3-phosphate dehydrogenase antibody (cat. no.
sc-25778; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
was used as a control. All bands were detected using ECL Western
Blotting (GE Healthcare Life Sciences, Chalfont, UK).
siRNA transfection
The siRNAs targeting specific sequences of Notch4
and a negative control scrambled siRNA (not homologous to any gene)
were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China)
(6). The PC cells were seeded into
6-well plates (4–5×104 cells/well) and cultured in 2 ml
of basic culture medium containing 10% FBS until the cells were 70%
confluent. Cells were transfected with control (non-specific siRNA)
or specific siRNA (100–200 nM) for 48 h.
3-(4,5-Dimethylthiazol-2-yl)
2,5-diphenyltetrazolium bromide (MTT) assay
Untransfected PC cells (blank control) and PC cells
(1×106) transfected with Notch4 siRNA or control siRNA
in 200 µl RPMI 1640 were seeded in duplicate into each well of a
96-well culture plate, and 100 µl MTT (5 mg/ml; Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) was added at 24, 48, 72, 96,
120, 144 and 168 h. After 4-h incubation at 37°C in 5%
CO2, 100 µl dimethyl sulfoxide was added to solubilize
the formazan product for 30 min at room temperature. The absorbance
at 570 nm was determined using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Cell migration and invasion
assays
Cell migration was assessed using transwell
permeable supports (Corning Inc., Corning, NY, USA). Briefly, cells
were allowed to grow to confluence. In total, 5×104
cells/well were resuspended in 100 µl serum-free medium and plated
onto uncoated 8-µm transwell filter inserts in 24-well plates in
triplicate. The lower chambers contained 600 µl of medium
containing 15% FBS as a chemoattractant. Non-migratory cells in the
upper chamber were removed with a cotton swab following incubation
for 16 h. Cells on the bottom side were fixed in 100% methanol and
stained with 0.5 µg/ml 4′,6-diamidino-2-phenylindole for 5 min.
Cells were counted using a fluorescence microscope (Eclipse 80i;
Nikon Corporation, Tokyo, Japan) in five random fields. For
evaluation of cell invasion, cells were allowed to invade
matrigel-coated transwell filters. At the end of the experiments,
the invaded cells on the bottom of the membrane were incubated with
0.1% crystal violet solution and dissolved in 20% acetic acid.
Finally, 100 µl dye mixture was transferred to a 96-well plate for
absorbance readings at 560 nm.
Drug sensitivity assay
To assess the chemosensitivity to docetaxel
(Sigma-Aldrich; Merck Millipore), untransfected PC cells (blank
control) and PC cells (1×104) transfected with Notch4
siRNA or control siRNA and cultured for 24 h were incubated with
different concentrations of docetaxel (0, 100, 200 and 400 nM) for
additional 48 h. Then, cells were treated with MTT as described
earlier. Each group contained five wells.
Statistical analysis
SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) was
used for statistical analysis. The data were expressed as the mean
± standard deviation, and the Student's t-test was used to
determine the significance of differences between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of Notch4 in PC cell
lines
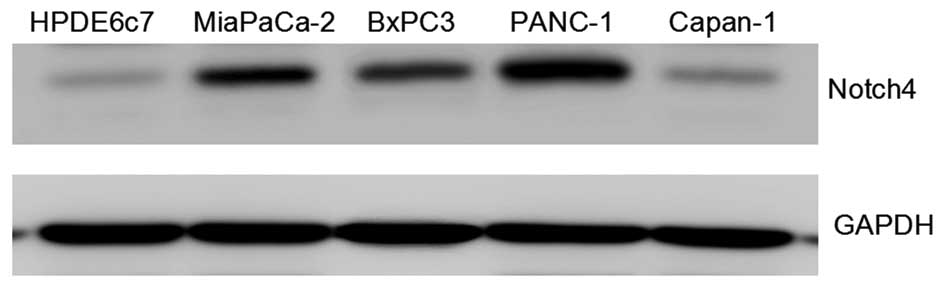
The expression of Notch4 in the PC cell lines
MiaPaCa-2, BxPC3, PANC-1 and Capan-1, as well as in HPDE6c7, an
immortalized but not transformed pancreatic epithelial cell line,
was determined, and it was observed that, although Notch4 is
differentially expressed in PC cell lines, Notch4 expression was
significantly upregulated in PC cell lines compared with the normal
pancreatic epithelial cell line HPDE6c7, suggesting a correlation
of Notch4 expression with pancreatic tumorigenesis and malignancy.
In addition, Notch4 expression was higher in MiaPaCa-2, PANC-1 and
BxPC3 cells than that in HPDE6c7 and Capan-1 cells (Fig. 1).
Silencing of Notch4 in PC cells by RNA
interference (RNAi) approach
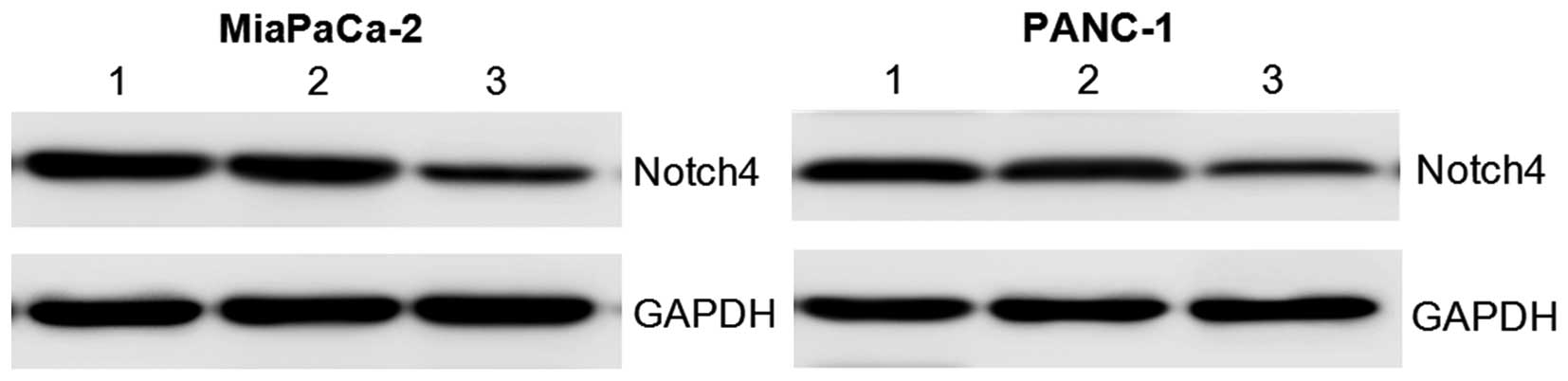
To directly test whether silencing of Notch4 was
responsible for the decreased proliferative and invasive
capabilities of PC cells, Notch4 expression was downregulated in
MiaPaCa-2 and PANC-1 cells using an RNAi approach. The protein
levels of Notch4 were significantly reduced in Notch4
siRNA-transfected cells after transfection for 48 h compared with
those in the control siRNA or blank control groups (Fig. 2). These results confirmed that Notch4
expression was indeed knocked down by Notch4 siRNA in the PC
cells.
Silencing of Notch4 reduces PC cell
viability, migration and invasion in vitro
The cell viability of Notch4-deficient cells was
determined by MTT assay. The results indicated that silencing of
Notch4 significantly suppressed cell viability as compared with the
controls (Fig. 3A). Meanwhile, the
cell viability of MiaPaCa-2 and PANC-1 cells transfected with
control siRNA was not affected (Fig.
3A). Thus, silencing of Notch4 could inhibit the cell viability
of MiaPaCa-2 and PANC-1 cells in vitro.
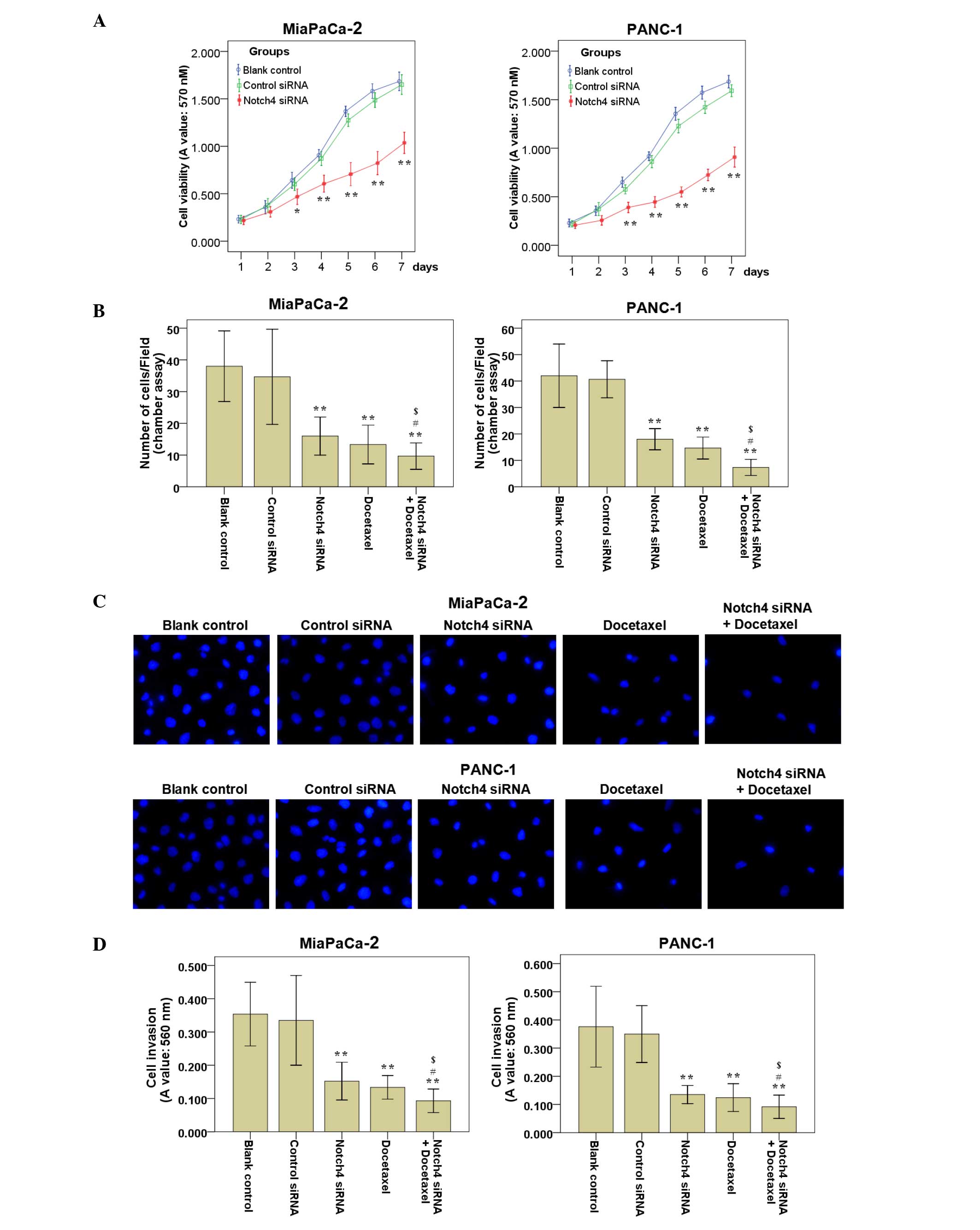 | Figure 3.Silencing of Notch4 reduces the cell
viability, cell migration and cell invasion abilities of PC cells
in vitro. (A) Cells were harvested at days 1–7
post-transfection, and cell viability was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
Decreased cell viability was detected in Notch4 siRNA-transfected
cells compared with that of the blank control and control siRNA
cells. (B) PC cells were treated as described, and cell migration
assays were performed. The migrated cells on the lower side of the
filter were fixed and stained with 4′,6-diamidino-2-phenylindole.
The mean number of migrated cells in ≥5 visual fields of three
independent experiments was calculated. (C) Representative
experiments of cell migration assays are shown (magnification,
×200). (D) PC cells were treated as described, and cell invasion
assays were performed. The invaded cells were stained and eluted
for absorbance readings at 560 nm. Data are the mean ± standard
deviation of ≥2 independent experiments (*P<0.05, **P<0.01
vs. control siRNA; #P<0.05 vs. Notch4 siRNA alone; $P<0.05
vs. docetaxel alone). PC, pancreatic cancer; siRNA, small
interfering RNA; A, absorbance. |
To examine whether the targeted downregulation of
Notch4 in MiaPaCa-2 and PANC-1 cells affected the cell migration
and invasion abilities, cell migration and invasion assays were
performed. The results of the cell migration (Fig. 3B and C) and invasion (Fig. 3D) assays revealed that the blank
control and the control siRNA cells had a similar migration and
invasion ability, while cells subjected to Notch4 siRNA
transfection migrated and invaded less efficiently compared with
the blank control and control siRNA cells. Representative results
of cell migration assays are shown in Fig. 3C.
Silencing of Notch4 enhances the
sensitivity of PC cells to docetaxel treatment
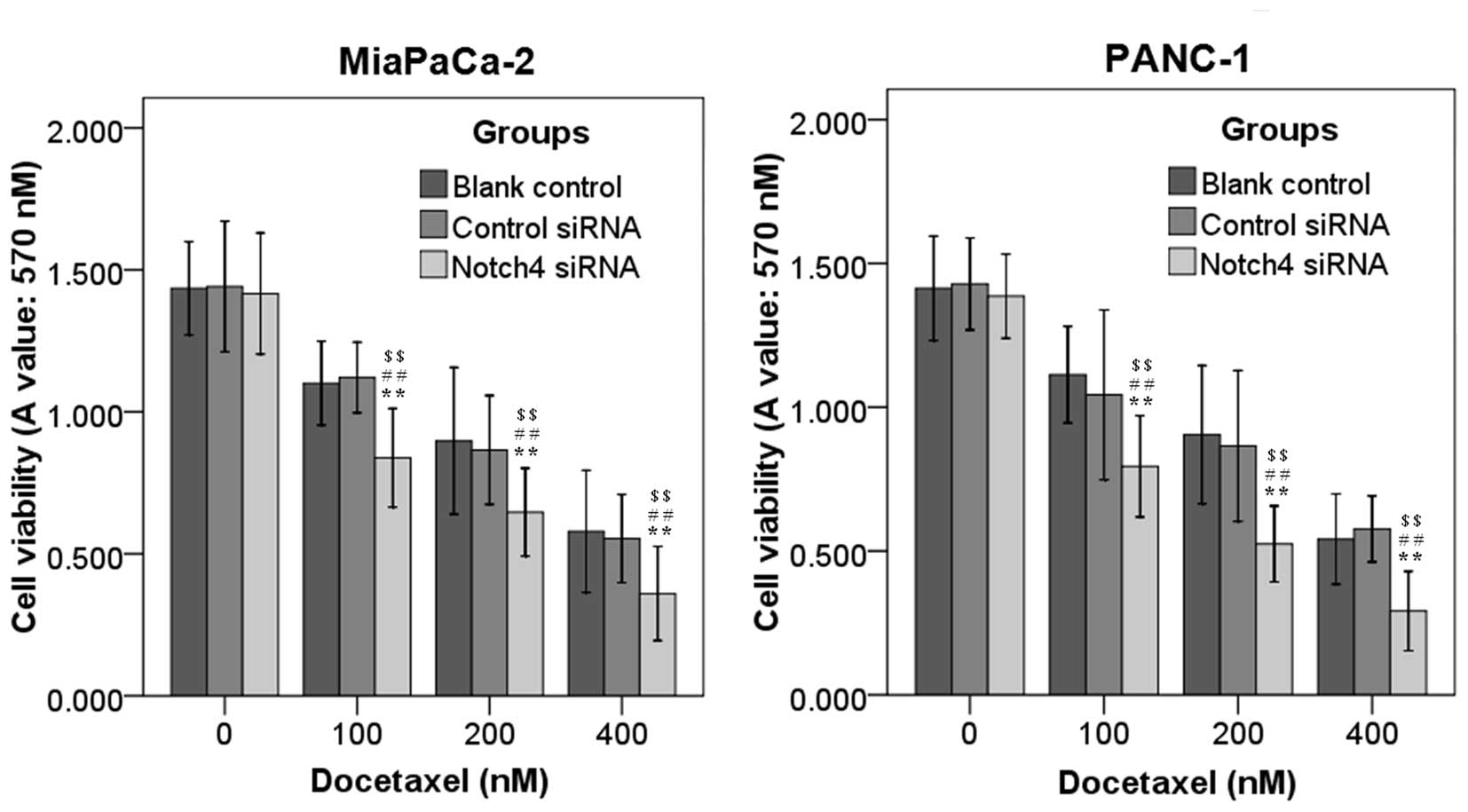
To examine whether blockade of Notch4 signaling with
Notch4 siRNA potentiated the ability of docetaxel to inhibit the
cell viability of PC cells, MiaPaCa-2 and PANC-1 cells transfected
with Notch4 siRNA or control siRNA cultured for 24 h were incubated
with different concentrations of docetaxel (0, 100, 200 and 400 nM)
for additional 48 h. It was observed that treatment with Notch4
siRNA alone resulted in no or moderate anti-tumor effects (Fig. 4). The cell viability of Notch4
siRNA-transfected cells was markedly reduced with the addition of
docetaxel relative to that of the controls, and this reduction was
concentration dependent (Fig. 4). In
addition, Notch4 siRNA significantly enhanced the inhibition of
cell migration (Fig. 3B and C) and
invasion (Fig. 3D) induced by
docetaxel in MiaPaCa-2 and PANC-1 cells (Fig. 3). Thus, silencing of Notch4 could
increase the sensitivity of PC cells to docetaxel treatment.
Downregulation of Notch4 enhances the
inhibition of fascin expression and the activation of Akt in PC
cells subjected to docetaxel treatment
To elucidate the molecular mechanism by which Notch4
silencing induces cells invasion, western blot analysis was used to
examine the expression of fascin, a critical hallmark of the
invasive phenotype of cancer cells (15). In MiaPaCa-2 and PANC-1 cells,
docetaxel or Notch4 siRNA led to a significant decrease in the
expression of fascin, an actin-binding protein that provides
mechanical support and cell motility and is involved in PC
metastasis (15), which was
consistent with the inhibition of cell invasion. Furthermore, the
combination of Notch4 siRNA and docetaxel treatment decreased the
expression of fascin in MiaPaCa-2 and PANC-1 cells to a much lower
level than that in cells treated with Notch4 siRNA or docetaxel
alone (Fig. 5).
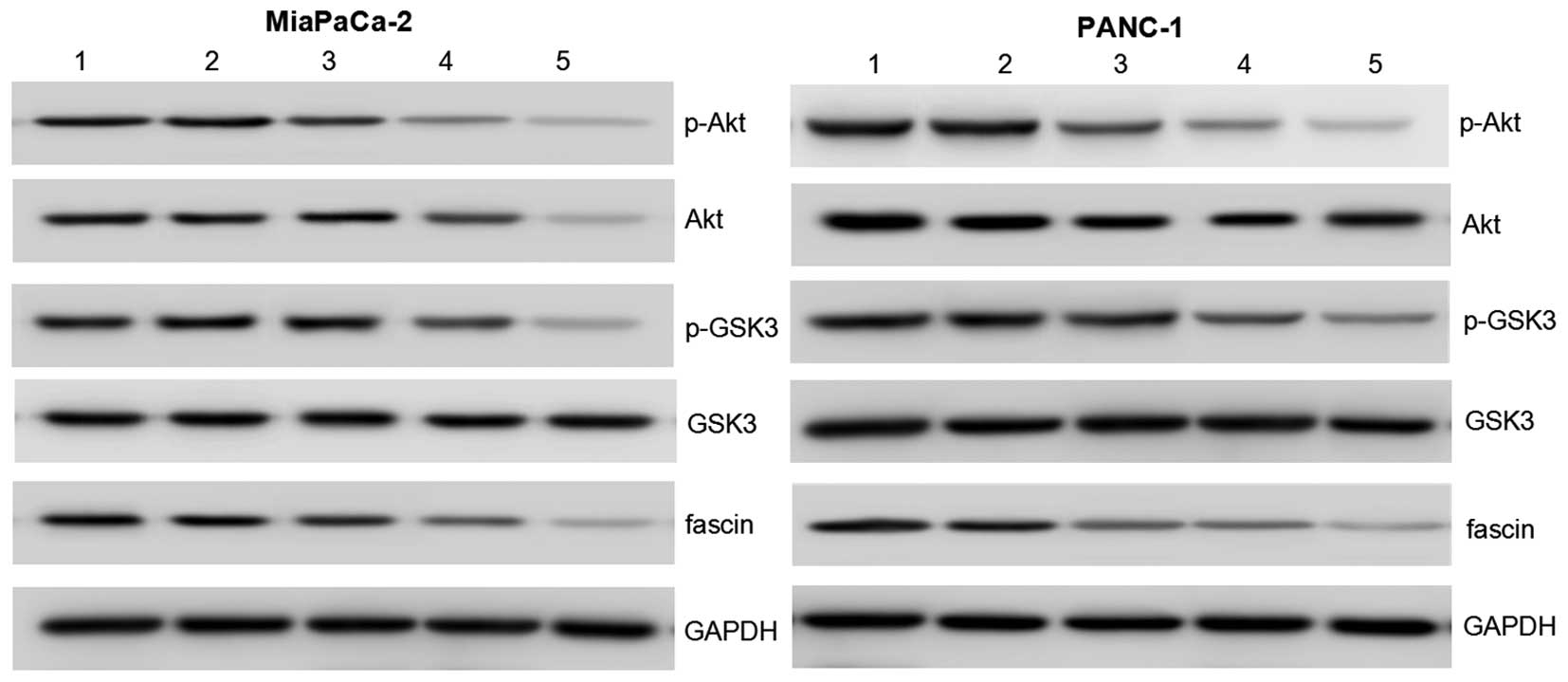 | Figure 5.Notch4 siRNA regulates the expression
of p-Akt, Akt, GSK3, p-GSK3 and fascin in pancreatic cancer cells.
The protein expression levels were determined by western blot
analysis. Akt, p-Akt and fascin protein expression were markedly
decreased in the Notch4 siRNA + docetaxel group compared with that
in the Notch4 siRNA and docetaxel groups. GAPDH was used as an
internal control to demonstrate equal protein loading. Lane 1,
blank control; lane 2, control siRNA; lane 3, Notch4 siRNA; lane 4,
docetaxel; lane 5, Notch4 siRNA + docetaxel. GSK3, glycogen
synthase kinase 3; GAPDH, glyceraldehyde 3-phosphate dehydrogenase;
p, phosphorylated; siRNA, small interfering RNA. |
The Akt family of proteins integrates a wide array
of diverse upstream survival and distress signals to decide cell
fate (16). GSK3 is a primary target
of Akt (17). To investigate the
mechanism by which Notch4 silencing regulates cell viability,
migration and invasion in MiaPaCa-2 and PANC-1 cells, the protein
expression and activation levels of Akt and GSK3 were examined.
Compared with cells treated with Notch4 siRNA or docetaxel alone,
the results of protein expression of Akt and GSK3 and activation of
Akt and GSK3 indicated that p-Akt and p-GSK were significantly
downregulated in cells treated with both Notch4 siRNA and docetaxel
(Fig. 5). These data suggest that
Notch4 siRNA may enhance the docetaxel-induced anti-tumor effects
by modulating Akt, GSK3 and fascin in PC cells.
Discussion
One of the primary aims of tumor research is to
identify signaling pathways and genetic factors that contribute to
tumorigenesis and to elucidate the mechanism of tumor development
(18). In this way, specific proteins
can be selected as potential markers for tumor development or as
potential targets for tumor therapy. Notch4 signaling has gained
attention due to its activation in a number of different tumors
(6–8),
and Notch4 signaling is involved in the progression of tumors;
thus, modulation of this cascade has potential for targeted therapy
(6–8).
The present study clearly demonstrated that Notch4 expression was
higher in human PC cell lines compared with that in a
non-transformed pancreatic epithelial cell line. Thus, it is likely
that Notch4 is related to the malignant behavior of PC.
Substantial research has been conducted at the
molecular level, which is expected to identify clues for markers of
PC progression, predictors of outcome and targets for
pharmacological therapy (1–3). Notch4 has been shown to influence the
cell proliferation of salivary adenoid cystic carcinoma,
triple-negative breast cancer and gastric carcinoma (GC) cells
(6,19,20). In
addition, our previous findings revealed that the activation of
Notch4 promoted GC cell growth in vitro and in vivo,
while the inactivation of Notch4 by siRNA had the opposite effects
(6). In the present study, it was
demonstrated that Notch4 expression was significantly upregulated
in PC cell lines compared with that in an immortalized but not
transformed pancreatic epithelial cell line, and RNAi was used to
directly downregulate the expression of Notch4 in PC cells.
Consistently, further observations revealed that silencing of
Notch4 could impede PC cell viability in vitro.
Notch expression in PC was correlated with serosal
invasion, lymph node metastasis, histopathological grading,
tumor-node-metastasis stage and recurrence (21–24).
Enhanced Notch4 expression in salivary adenoid cystic carcinoma may
stand as a novel mechanism for promoting metastasis (25). Consistently, the present study
demonstrated that Notch4 depletion significantly decreased the cell
migration and invasion abilities of PC cells. These findings
support a regulatory function for the Notch4 molecule in PC cell
tumorigenesis through modulating cell migration and invasion in
vitro.
Docetaxel is a monocyclic monoterpene with a
lemon-like odor and is a major constituent in several citrus oils
(26). Previous studies have
demonstrated that docetaxel has a chemoprotective effect on several
types of tumors (27,28). However, drug resistance is one of the
main causes of docetaxel treatment failure (29,30). In
the present study, Notch4 siRNA significantly enhanced the
inhibition of cell migration and the invasion ability induced by
docetaxel in PC cells. This result indicated that the
downregulation of Notch4 enhanced the sensitivity of PC cells to
docetaxel treatment, suggesting that Notch4 may be an adjuvant gene
therapy target to chemotherapy of PC.
Akt is a downstream target of Notch1 signaling, and
a significant reduction in cell viability and an increase in
apoptosis in PC cells have been correlated with the downregulation
of Notch1 and Akt (31). In the
present study, it was observed that the downregulation of Notch4
inhibited the activation of Akt, suggesting that Akt signaling may
be involved in the anti-tumor effects induced by Notch4 siRNA.
Dysregulation of Akt was involved in docetaxel resistance (32). In the present study, it was
demonstrated that the downregulation of Notch4 enhanced the
inhibition of Akt induced by docetaxel. Therefore, it is possible
that Akt activation could contribute to the effects of Notch4
knockdown on cell survival and chemotherapy sensitivity. GSK3 is
one of the downstream targets of Akt, and the activation of Akt
leads to the phosphorylation of GSK3 (31,32). In
the present study, in agreement with the decreased phosphorylation
of Akt upon Notch4 knockdown, GSK3 phosphorylation was also
decreased by Notch4 knockdown.
Of note, it was observed that the downregulation of
Notch4 reduced the expression of fascin. Several studies reported
that fascin significantly increases cell migration by enhancing the
directional motility of cells (33).
The present study demonstrated that the downregulation of Notch4
decreased the expression of fascin, suggesting that Notch4 may
regulate cell migration, at least by fascin. In addition, fascin is
involved in the chemotherapeutic resistance of breast cancer cells
via the Akt pathway (34). However,
the mutual association between Akt and fascin in PC is still
unclear, and requires in-depth study.
In summary, by using RNAi technology, the expression
of the Notch4 gene was successfully downregulated, and Notch4
depletion effectively suppressed PC cell viability, migration and
invasion abilities, suggesting that silencing of Notch4 inhibits PC
growth and metastasis. It was also demonstrated that Notch4
depletion increased the sensitivity to docetaxel treatment of PC
cells, and increased the expression and/or activation of Akt and
fascin, which may be related to the sensitivity of PC cells to
docetaxel treatment. These findings may at least partially imply
that Notch4 may represent an appropriate target for tumor therapy
of PC. Further studies are necessary to define the exact role of
Notch4 on metastasis and chemotherapy resistance in PC.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of Zhejiang Province (Zhejiang, China;
grant no. LY16H160033), Public Welfare Technical Applied Research
Project of Zhejiang Province (Zhejiang, China; grant no.
2016C33189), Science and Technology Plans of Taizhou City (Taizhou,
China; grant no. 1301KY39), Science and Technology Plans of
Jiaojiang District of Taizhou City (Taizhou, China; grant no.
132061) and National Innovation and Entrepreneurship Training
Project for University Students (Beijing, China; grant no.
201410350020).
Glossary
Abbreviations
Abbreviations:
|
PC
|
pancreatic cancer
|
|
RNAi
|
RNA interference
|
|
siRNA
|
small interfering RNA
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-
2,5-diphenyltetrazolium bromide
|
References
|
1
|
Teague A, Lim KH and Wang-Gillam A:
Advanced pancreatic adenocarcinoma: A review of current treatment
strategies and developing therapies. Ther Adv Med Oncol. 7:68–84.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeo TP: Demographics, epidemiology, and
inheritance of pancreatic ductal adenocarcinoma. Semin Oncol.
42:8–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He XY and Yuan YZ: Advances in pancreatic
cancer research: Moving towards early detection. World J
Gastroenterol. 20:11241–11248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yilmaz M and Christofori G: Mechanisms of
motility in metastasizing cells. Mol Cancer Res. 8:629–642. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qian C, Liu F, Ye B, Zhang X, Liang Y and
Yao J: Notch4 promotes pancreatic cancer growth through activation
of Wnt1/β-catenin signaling. Mol Cell Biochem. 401:165–174. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naik S, MacFarlane M and Sarin A: Notch4
signaling confers susceptibility to TRAIL induced apoptosis in
breast cancer cells. J Cell Biochem. 116:1371–1380. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahn S, Hyeon J and Park CK: Notch1 and
Notch4 are markers for poor prognosis of hepatocellular carcinoma.
Hepatobiliary Pancreat Dis Int. 12:286–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SH and Singh SV: The role of polycomb
group protein Bmi-1 and Notch4 in breast cancer stem cell
inhibition by benzyl isothiocyanate. Breast Cancer Res Treat.
149:681–692. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stegeman H, Span PN, Kaanders JH and
Bussink J: Improving chemoradiation efficacy by PI3-K/AKT
inhibition. Cancer Treat Rev. 40:1182–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hafsi S, Pezzino FM, Candido S, Ligresti
G, Spandidos DA, Soua Z, McCubrey JA, Travali S and Libra M: Gene
alterations in the PI3K/PTEN/AKT pathway as a mechanism of
drug-resistance (review). Int J Oncol. 40:639–644. 2012.PubMed/NCBI
|
|
13
|
Brown KK, Montaser-Kouhsari L, Beck AH and
Toker A: MERIT40 is an Akt substrate that promotes resolution of
DNA damage induced by chemotherapy. Cell Rep. 11:1358–1366. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ramakrishnan G, Davaakhuu G, Chung WC, Zhu
H, Rana A, Filipovic A, Green AR, Atfi A, Pannuti A, Miele L and
Tzivion G: AKT and 14-3-3 regulate Notch4 nuclear localization. Sci
Rep. 5:87822015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao X, Gao S, Ren H, Sun W, Zhang H, Sun
J, Yang S and Hao J: Hypoxia-inducible factor-1 promotes pancreatic
ductal adenocarcinoma invasion and metastasis by activating
transcription of the actin-bundling protein fascin. Cancer Res.
74:2455–2464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sheppard K, Kinross KM, Solomon B, Pearson
RB and Phillips WA: Targeting PI3 kinase/AKT/mTOR signaling in
cancer. Crit Rev Oncog. 17:69–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW,
Wang Z, Fan J, Dai Z and Zhou J: CXCR2/CXCL5 axis contributes to
epithelial-mesenchymal transition of HCC cells through activating
PI3K/Akt/GSK-3β/Snail signaling. Cancer Lett. 358:124–135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Booth AK and Gutierrez-Hartmann A:
Signaling pathways regulating pituitary lactotrope homeostasis and
tumorigenesis. Adv Exp Med Biol. 846:37–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen W, Zhang H, Wang J, Cao G, Dong Z, Su
H, Zhou X and Zhang S: Lentiviral-mediated gene silencing of
Notch-4 inhibits in vitro proliferation and perineural invasion of
ACC-M cells. Oncol Rep. 29:1797–1804. 2013.PubMed/NCBI
|
|
20
|
Nagamatsu I, Onishi H, Matsushita S, Kubo
M, Kai M, Imaizumi A, Nakano K, Hattori M, Oda Y, Tanaka M and
Katano M: NOTCH4 is a potential therapeutic target for
triple-negative breast cancer. Anticancer Res. 34:69–80.
2014.PubMed/NCBI
|
|
21
|
Singh D, Upadhyay G, Srivastava RK and
Shankar S: Recent advances in pancreatic cancer: Biology,
treatment, and prevention. Biochim Biophys Acta. 1856:13–27.
2015.PubMed/NCBI
|
|
22
|
Damaskos C, Karatzas T, Kostakis ID,
Nikolidakis L, Kostakis A and Kouraklis G: Nuclear receptors in
pancreatic tumor cells. Anticancer Res. 34:6897–6911.
2014.PubMed/NCBI
|
|
23
|
Tang SC and Chen YC: Novel therapeutic
targets for pancreatic cancer. World J Gastroenterol.
20:10825–10844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mann CD, Bastianpillai C, Neal CP, Masood
MM, Jones DJ, Teichert F, Singh R, Karpova E, Berry DP and Manson
MM: Notch3 and HEY-1 as prognostic biomarkers in pancreatic
adenocarcinoma. PLoS One. 7:e511192012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding LC, She L, Zheng DL, Huang QL, Wang
JF, Zheng FF and Lu YG: Notch-4 contributes to the metastasis of
salivary adenoid cystic carcinoma. Oncol Rep. 24:363–368.
2010.PubMed/NCBI
|
|
26
|
Jakate AS, Einhaus CM, DeAnglis AP,
Retzinger GS and Desai PB: Preparation, characterization, and
preliminary application of fibrinogen-coated olive oil droplets for
the targeted delivery of docetaxel to solid malignancies. Cancer
Res. 63:7314–7320. 2003.PubMed/NCBI
|
|
27
|
Iqbal S, Lenz HJ, Gandara DR, Shibata SI,
Groshen S, Synold TW and Newman EM: A phase I trial of oxaliplatin
in combination with docetaxel in patients with advanced solid
tumors. Cancer Chemother Pharmacol. 72:85–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Salazar R, Morales S, Gil-Martín M,
Aguirre E, Oaknin A, Garcia M, Callies S, Wickremsinhe ER, Benhadji
KA and Llombart A: Phase 1 dose escalation and pharmacokinetic
evaluation of oral gemcitabine prodrug (LY2334737) in combination
with docetaxel in patients with advanced solid tumors. Cancer
Chemother Pharmacol. 73:1205–1215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zu S, Ma W, Xiao P, Cui Y, Ma T, Zhou C
and Zhang H: Evaluation of docetaxel-sensitive and
docetaxel-resistant proteomes in PC-3 cells. Urol Int. 95:114–119.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mahon KL, Lin HM, Castillo L, Lee BY,
Lee-Ng M, Chatfield MD, Chiam K, Breit SN, Brown DA, Molloy MP, et
al: Cytokine profiling of docetaxel-resistant castration-resistant
prostate cancer. Br J Cancer. 112:1340–1348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Li Y, Ahmad A, Banerjee S, Azmi
AS, Kong D, Wojewoda C, Miele L and Sarkar FH: Downregulation of
Notch-1 is associated with Akt and FoxM1 in inducing cell growth
inhibition and apoptosis in prostate cancer cells. J Cell Biochem.
112:78–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hour TC, Chung SD, Kang WY, Lin YC, Chuang
SJ, Huang AM, Wu WJ, Huang SP, Huang CY and Pu YS: EGFR mediates
docetaxel resistance in human castration-resistant prostate cancer
through the Akt-dependent expression of ABCB1 (MDR1). Arch Toxicol.
89:591–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sonego M, Gajendra S, Parsons M, Ma Y,
Hobbs C, Zentar MP, Williams G, Machesky LM, Doherty P and Lalli G:
Fascin regulates the migration of subventricular zone-derived
neuroblasts in the postnatal brain. J Neurosci. 33:12171–12185.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ghebeh H, Al-Khaldi S, Olabi S, Al-Dhfyan
A, Al-Mohanna F, Barnawi R, Tulbah A, Al-Tweigeri T, Ajarim D and
Al-Alwan M: Fascin is involved in the chemotherapeutic resistance
of breast cancer cells predominantly via the PI3K/Akt pathway. Br J
Cancer. 111:1552–1561. 2014. View Article : Google Scholar : PubMed/NCBI
|