Introduction
It is generally accepted that certain death effector
domain (DED)-containing proteins are involved in the progression of
apoptosis and cell activation (1).
Due to their similar protein structures, Sun et al (2) identified four members of the tumor
necrosis factor α-induced protein-8 (TNFAIP8, also known as TIPE)
family, named TIPE, TIPE1, TIPE2 and TIPE3. There are at least two
members of the protein family that have been identified to have a
DED-like domain (2,3). However, these two proteins separately
presented opposite functions in terms of cell death (2,3). Previous
studies have demonstrated that TIPE is an oncogene, and
overexpressed TIPE in cells can reduce cell death in vitro
and increase tumor growth in vivo (4,5). By
contrast, TIPE2, a negative regulator of innate and adaptive
immunity, has been demonstrated to be an inhibitor of Ras and to
have a pro-apoptotic ability (2,6,7).
However, few studies have been reported about the
functions of TIPE1, another member of the TIPE family. Cui et
al (8) observed that TIPE1 was
expressed in a wide variety of mouse tissues and human carcinoma
cell lines, suggesting that TIPE1 may be involved in cell secretion
and carcinogenesis. Hitomi et al (9) used a genome small interfering RNA
approach to predict that TIPE1 is required for
N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD, an
inhibitor of caspase)- or TNFα-induced necrosis in L929 cells, as
well as for TNFα/cycloheximide-induced apoptosis in NIH 3T3 cells.
Recently, Zhang et al (10)
demonstrated that TIPE1 overexpression in H22 cells could inhibit
tumor growth in vivo, and TIPE1 could inhibit the
Ras-related C3 botulinum toxin substrate 1 (Rac1)-p65-c-Jun
N-terminal kinase (JNK) pathway activation to promote apoptosis in
human hepatocellular carcinoma (HCC) cells. Since TIPE1 is
expressed in numerous tissues and cell lines, the physiological
function and the role in cell death of TIPE1 on the cells of
different tissue sources remain to be elucidated.
In present study, a murine macrophage cell line,
RAW264.7, was used to investigate the apoptotic function of TIPE1.
Our results demonstrated that TIPE1 could promote the apoptosis of
RAW264.7 cells by upregulating the pro-apoptotic members of the
B-cell leukemia/lymphoma (Bcl)-2 family of proteins, and that
sustained activation of the mitogen activated protein kinases
(MAPKs) signaling pathway was not involved in the process.
Materials and methods
Reagents
The Annexin V/PI Apoptosis Detection kit was
purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
Cisplatin [cis-diamminedichloroplatinum II (DDP)] was
obtained from Calbiochem (EMD Millipore, Billerica, MA, USA).
Dulbecco's modified Eagle medium (DMEM) and fetal bovine serum
(FBS) were acquired from HyClone (GE Healthcare Life Sciences,
Logan, UT, USA). TaKaRa Taq DNA Polymerase, RNAiso Plus and
PrimeScript Reverse Transcriptase were purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). Rabbit anti-mouse
antibodies against (Bcl)-2 associated X protein (Bax; #2722;
1:1,000), Bcl-2 interacting killer (Bik; #4592; 1:1,000), p53
upregulated modulator of apoptosis (Puma; #7467; 1:1,000),
Bcl-extra large (xl; #2764; 1:3,000), Bcl-2 (#3498; 1:1,000),
phospho-extracellular signal-regulated kinase (Erk) 1/2 (#4370;
1:1,000), phospho-p38 (#9212; 1:1,000) and phospho-JNK (#9251;
1:1,000), as well as U0126 (#9903), SB203580 (#5633) and SP600125
(#8177), were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Rabbit anti-mouse antibody against TIPE1
(#SAB2102488; 1:1,000) was purchased from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany).
Cell line and culture
RAW264.7 cells with stably transfected MigR1 or
MigR1-TIPE1 vector were obtained from Professor Yuhai Chen
(University of Pennsylvania School of Medicine, Philadelphia, PA,
USA). Cells were cultured in DMEM supplemented with 10% FBS, 100
µg/ml streptomycin and 100 U/ml penicillin. All cell cultures were
maintained at 37°C under 95% relative humidity and 5% carbon
dioxide.
Animals
Athymic mice (female, 6-week-old, ~20 g, n=20) were
purchased from the Shanghai Laboratory Animal Center of Chinese
Academy of Sciences (Shanghai, China) and kept at the Animal Center
of Xiamen University (Xiamen, China). Mice were housed in a
controlled environment and provided with standard rodent food and
water. The present study was approved by the Review Board of
Medical College at Xiamen University and was performed in
compliance with regulations of Xiamen University on experimental
animals.
Analysis of gene expression
Total RNA of cells was isolated using RNAiso Plus.
For each sample, 2 µg of RNA was subjected to reverse transcription
(RT) using PrimeScript Reverse Transcriptase. The complementary DNA
was amplified by polymerase chain reaction (PCR) with TaKaRa Taq
DNA Polymerase according to the manufacturer's protocol (95°C for 5
min followed by 35 cycles of 95°C for 30 sec, 60°C for 30 sec and
72°C for 30 sec, and 72°C for 5 min), using a QX200 Droplet Digital
PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The PCR
products were analyzed on 2% agarose gel electrophoresis and
visualized with ethidium bromide. β-actin was used as an internal
control. The sequences of the primers used are as follows: β-actin
sense 5′-CATCCGTAAAGACCTCTATGCCAAC-3′ and antisense
5′-ATGGAGCCACCGATCCACA-3′; and TIPE1 sense
5′-GCCCTGCAGGCCCAGAAGAAG-3′ and antisense
5′-GCTTCACAGCCACCTTCACCAGG-3′.
Apoptosis assay
Cell apoptosis assays were conducted as previously
reported (11). Briefly, for
determining the apoptosis rates of TIPE1-overexpressing cells,
8×104 RAW264.7 cells were seeded in 24-well plates and
treated with or without 1 µg/ml cisplatin (DDP) for 20 h. Next, all
cell samples were removed by trypsinization, and the removed cells
were rinsed with phosphate-buffered saline (PBS) and stained with
annexin V-allophycocyanin and propidium iodide (PI) in binding
buffer for 20 min at room temperature. Then, the cells were washed
once with PBS, and flow cytometry was performed with BD FACSCalibur
(BD Biosciences, San Jose, CA, USA). Data were analyzed with
CellQuest Pro 5.1 software (BD Biosciences).
Tumor growth assay
Tumor implantation experiments were conducted as
described previously (12). Briefly,
every mouse was challenged with 2×106 RAW264.7-TIPE1 or
RAW264.7-MigR1 cells through subcutaneous injection in the back. In
total, 10 mice were injected with RAW264.7-MigR1 cells (control
cells) and 10 mice were injected with RAW264.7-TIPE1 cells
(TIPE1-overexpressing cells). The tumor volume was measured every 2
days from day 5 to day 15. The three major axes (a, b and c) of the
tumors were recorded, and tumor volumes were then calculated using
the formula abc/2 as reported (4).
The mice were sacrificed on day 15, when the tumors' longest
dimension reached a length of 1.0 cm. Then, tumor weights were
recorded.
Western blot analysis
Proteins were extracted in lysis buffer as
previously described (11). Briefly,
cells were lysed with radioimmunoprecipitation assay buffer, and
protein lysates were electrophoresed on 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis prior to be transferred
to polyvinylidene difluoride (PVDF) membranes (Merck Millipore).
The PVDF membranes were incubated with the corresponding primary
monoclonal antibodies overnight at 4°C, followed by incubation with
the secondary antibodies for 2 h at room temperature and detection
with enhanced chemiluminescence (Merck Millipore). β-actin was used
as a loading control.
Statistical analysis
Unpaired two-tailed Student's t-test was performed
with GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
TIPE1 overexpression promotes the
apoptosis of RAW264.7 cells
Endogenous and exogenous expression of TIPE1 in
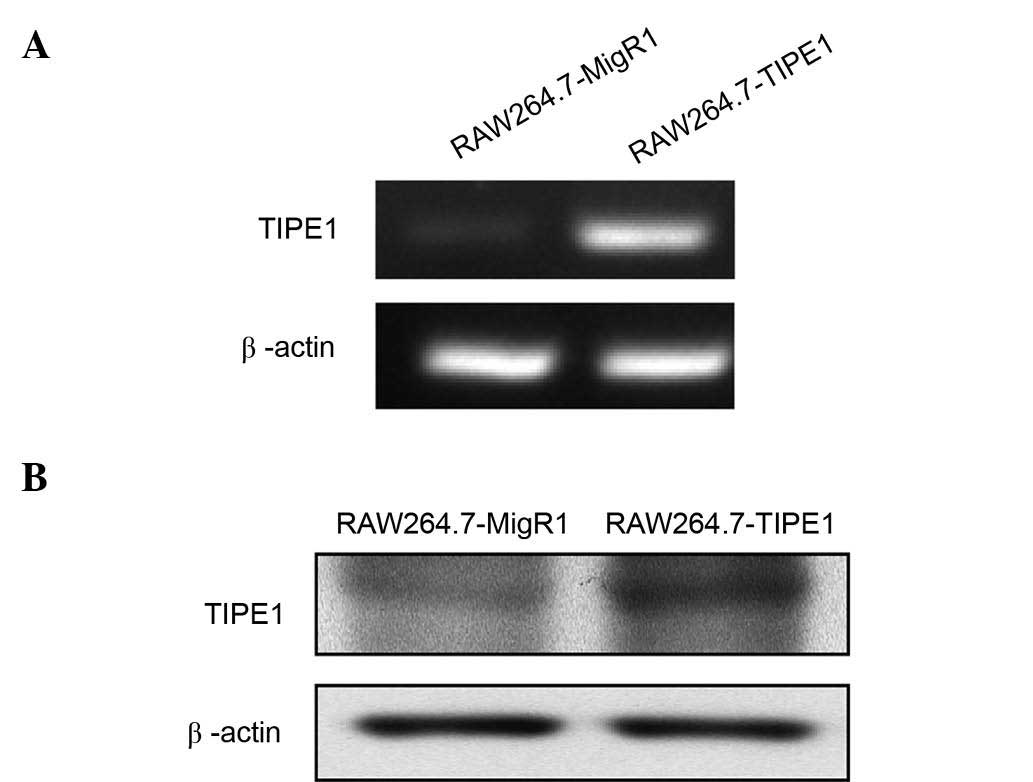
RAW264.7 cells was verified by RT-PCR. As shown in Fig. 1, RAW264.7 cells could express
endogenous TIPE1, and the messenger RNA level of TIPE1 was much
higher in TIPE1-overexpressing cells (RAW264.7-TIPE1) than in
control vector-expressing cells (RAW264.7-MigR1). Overexpression of
TIPE1 was also verified by western blotting (Fig. 1B). Since interference of TIPE1 RNA
expression prevents NIH 3T3 cells (a standard fibroblast cell line)
from zVAD-induced apoptosis (9), and
TIPE1 overexpression could induce apoptosis of Bel7402 cells (an
HCC cell line) (10), the present
study investigated whether TIPE1 overexpression could directly
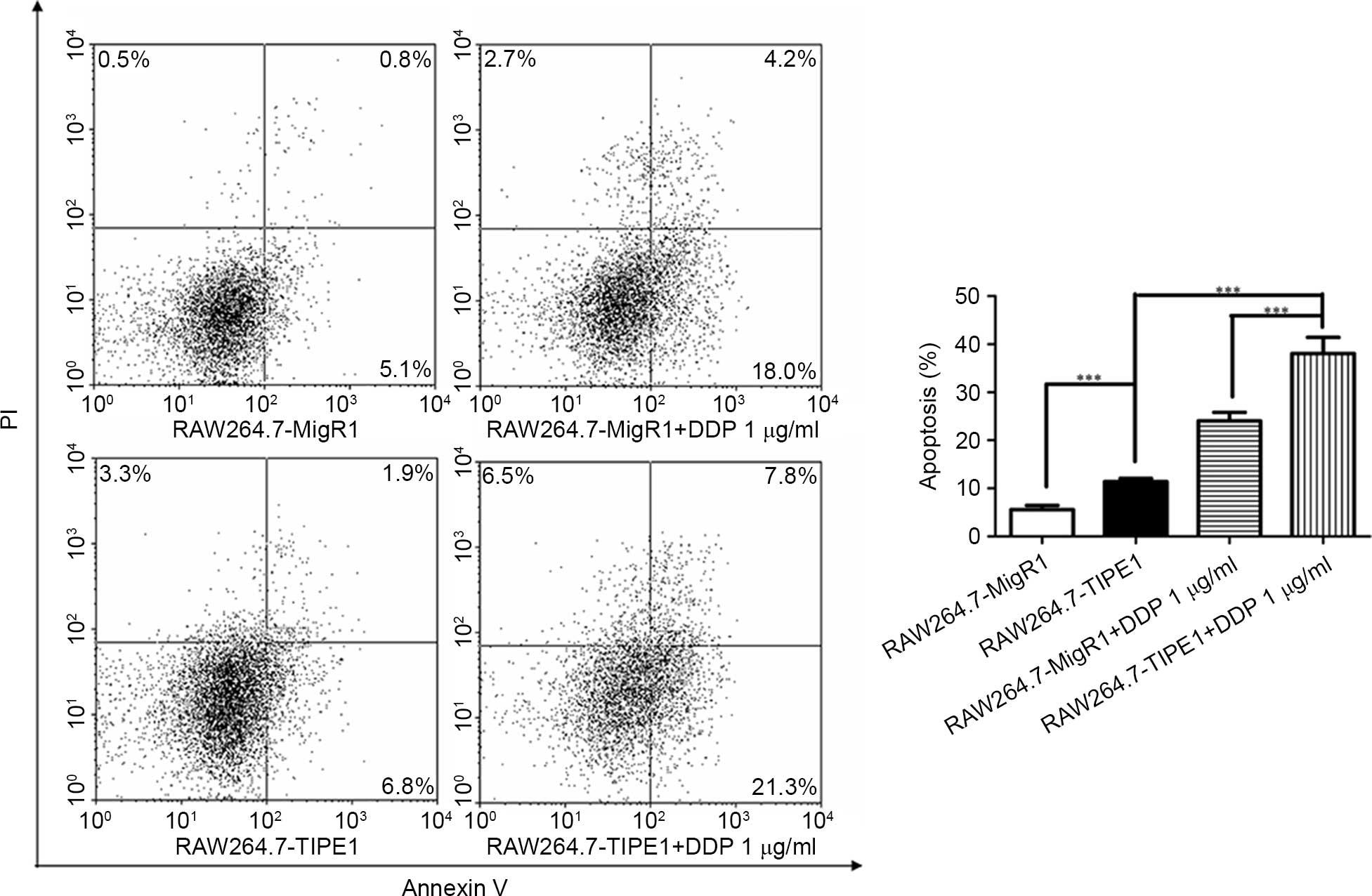
affect RAW264.7 cell apoptosis. Flow cytometry analysis with
annexin V and PI was used to detect the apoptosis of cells. The
results indicated that the apoptosis rates were relatively enhanced
by TIPE1, from 5.6 to 11.4% (Fig. 2,
P<0.001). Meanwhile, 1 µg/ml cisplatin (DDP), an anti-tumor
medicine involving the mitochondrial pathway of apoptosis and
causing DNA damage (13), was used to
induce cell death in RAW264.7 cells. TIPE1-overexpressing cells
also exhibited significantly increased apoptosis rates (38.1%)
compared with control cells (24.0%) upon DDP stimulation (Fig. 2, P<0.001). These results suggested
that overexpressed TIPE1 could induce apoptosis and enhance the
sensitivity of DDP-induced cell death in RAW264.7 cells in
vitro.
TIPE1 overexpression suppresses tumor
growth in vivo
Since TIPE1 was capable of affecting apoptosis in
RAW264.7 cells in vitro, it was hypothesized that the growth
of tumors in vivo could be suppressed by TIPE1
overexpression. To test this hypothesis, RAW264.7 cells with or
without TIPE1 overexpression were injected subcutaneously into
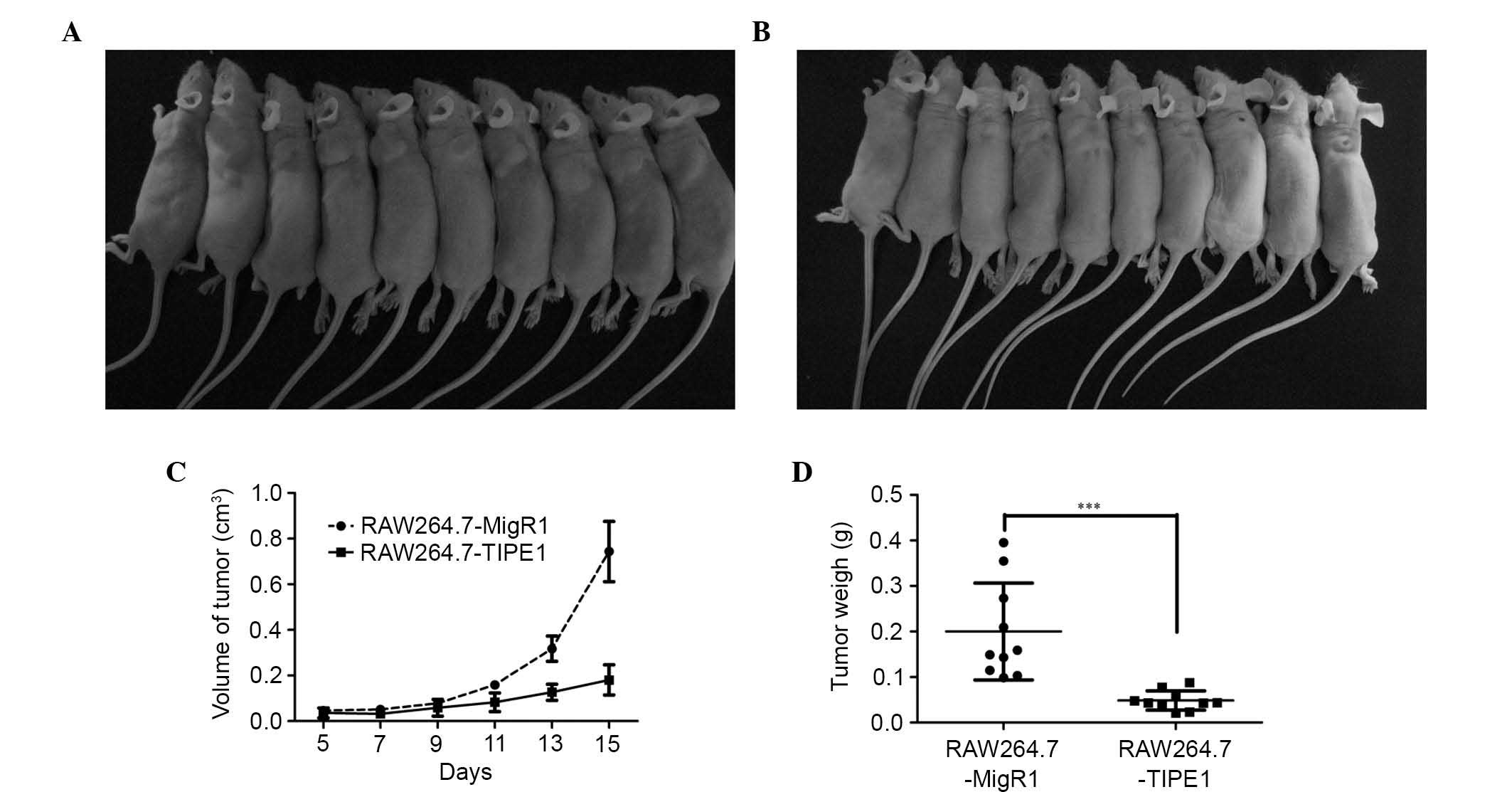
athymic mice. As shown in Fig. 3,
TIPE1-overexpressing RAW264.7 cells exhibited a remarkably
decreased tumor growth as compared with control cells from day 11
to day 15. In addition, the tumor weights were also significantly
suppressed by TIPE1 overexpression (day 15: RAW264.7-MigR1,
0.20±0.10 g vs. RAW264.7-TIPE1, 0.05±0.02 g, P<0.001). These
results indicated that TIPE1 could inhibit tumor growth in
vivo by inducing apoptosis.
TIPE1 overexpression influences the
protein expression levels of the Bcl-2 family to promote
apoptosis
Since the intrinsic apoptotic pathway could be
initiated by DNA damage (14,15), our in vitro studies (Fig. 2) strongly suggest that TIPE1 promotes
apoptosis of RAW264.7 cells by regulating the mitochondrial
pathway. It could be assumed that Bcl-2 family proteins, which are
important regulators of the mitochondrial pathway of apoptosis, may
be involved in the overexpressed TIPE1-induced apoptosis of
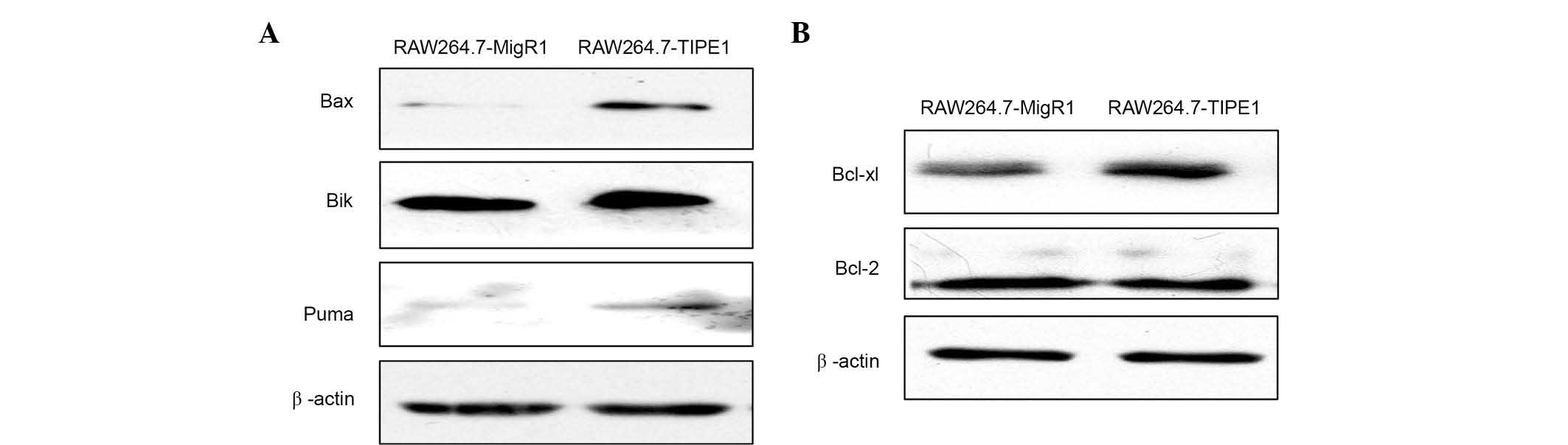
RAW264.7 cells (16). Bax, Bik and
Puma, the pro-apoptotic members of the Bcl-2 family of proteins
(16–19), were analyzed by western blotting.
Fig. 4A indicates, as expected, that
when TIPE1 was overexpressed in RAW264.7 cells, the expression of
Bax, Bik and Puma increased distinctly. According to a previous
report (10), TIPE1 could inhibit the
expression of Bcl-2, an important proliferation regulator of the
Bcl-2 family (16), in HCC cells.
Considering that Bcl-xl is another key regulator of anti-apoptotic
members of the Bcl-2 family (20),
both Bcl-2 and Bcl-xl levels were analyzed by western blotting. As
show in Fig. 4B, contrarily to
previous results (10), overexpressed
TIPE1 did not influence the level of Bcl-2 in RAW246.7 cells, but
slightly enhanced Bcl-xl expression. These results indicated that
TIPE1 overexpression could affect the mitochondrial pathway of
apoptosis by regulating the pro- and anti-apoptotic proteins of the
Bcl-2 family.
TIPE1 overexpression activates the
MAPKs signaling pathway
A previous study reported that TIPE1 could inhibit
the JNK phosphorylation induced by Rac1 (10). The activation of MAPKs is involved in
numerous aspects of the regulation of cellular proliferation and
apoptosis (21,22). Therefore, the effects of TIPE1
overexpression on the MAPKs signaling pathway was examined. Western
blot analysis revealed that the phosphorylation levels of Erk1/2,
p38 and JNK were all enhanced by TIPE1 overexpression (Fig. 5A). To gain insight into the
association between the activated MAPKs pathway and upregulated
Bcl-2 family proteins, TIPE1-overexpressing RAW264.7 cells were
treated with the inhibitor of the Erk1/2 (U0126), p38 (SB203580) or
JNK (SP600125) signaling pathway, respectively, and then Bax, Bik,
Puma and Bcl-xl levels were assessed by western blotting. As shown
in Fig. 5B, unexpectedly, the
expression of Bax, Bik and Puma was not decreased but slightly
enhanced by U0126, SB203580 and SP600125, respectively. Meanwhile,
Bcl-xl expression was also slightly increased by SB203580 and
SP600125, while U0126 did not influence its expression. These
findings suggested that the sustained MAPKs activation did not
contribute to the upregulation of the Bcl-2 family proteins induced
by TIPE1 overexpression.
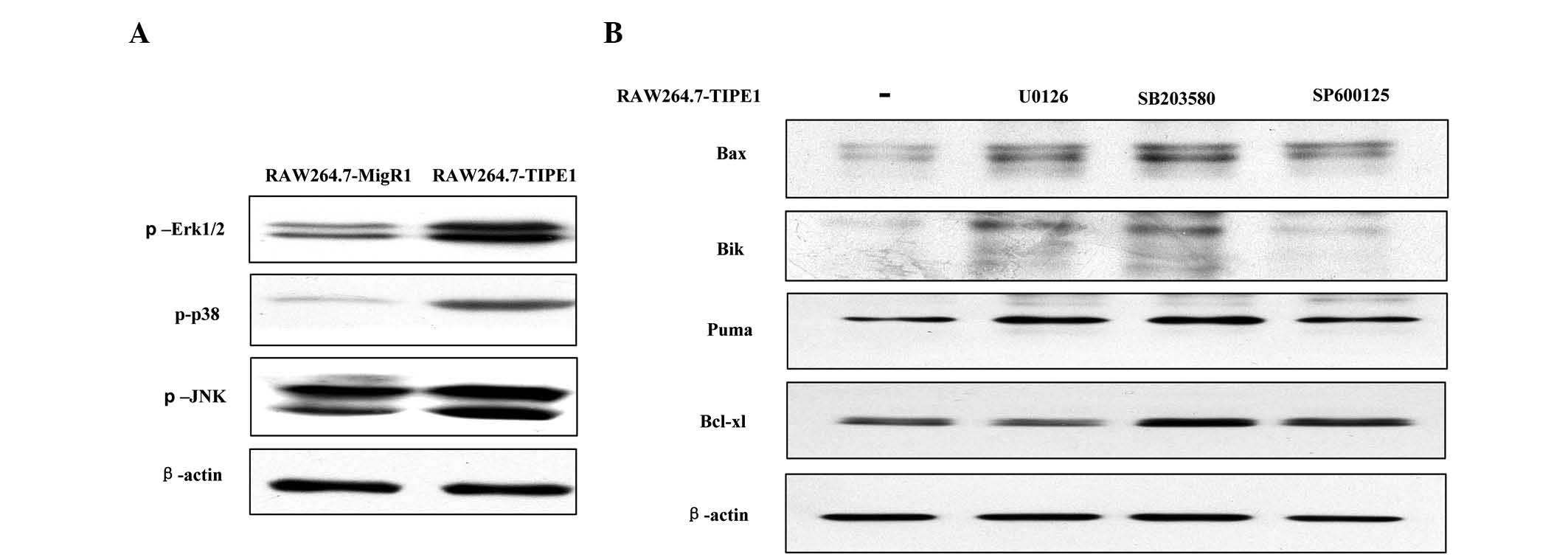 | Figure 5.Continuously activated mitogen
activated protein kinases signaling pathway did not contribute to
the upregulation of the Bcl-2 family of proteins induced by
overexpressed TIPE1. (A) Overexpressed TIPE1 enhanced the
phosphorylation levels of Erk1/2, p38 and JNK in RAW264.7 cells.
(B) RAW264.7-TIPE1 cells were treated with U0126 (10 µM), SB203580
(10 µM) or SP600125 (5 µM) for 15 h, and the levels of Bax, Bik,
Puma or Bcl-xl were not reduced by the inhibitors. β-actin was used
as a loading control. TIPE1, tumor necrosis factor α-induced
protein 8-like 1; p, phosphorylated; Erk, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; Bax, Bcl-2
associated X protein; Bik, Bcl-2 interacting killer; Puma, p53
upregulated modulator of apoptosis; Bcl-xl, Bcl-extra large; Bcl,
B-cell leukemia/lymphoma. |
Discussion
The present study has demonstrated that
overexpressed TIPE1 could promote RAW264.7 cell apoptosis in
vitro and inhibit tumor growth in vivo. These phenomena
are consistent with the fact that TIPE1 overexpression can induce
apoptosis and suppress tumor growth in human HCC cells (10). However, compared with HCC cells, TIPE1
overexpression in RAW264.7 cells did not reduce the Bcl-2 levels
and had no influence on its expression. At the same time, Bcl-xl
was slightly increased by TIPE1. Bcl-xl and Bcl-2 are multi-Bcl-2
homology (BH) domain proteins of the Bcl-2 family (16). Considering that Bcl-xl but not Bcl-2
level was changed by TIPE1 overexpression, Bcl-xl may be the most
important anti-apoptotic protein in RAW264.7 cells. On the other
hand, it was observed that TIPE1 upregulated pro-apoptotic
proteins, including Bax, Bik and Puma, in RAW264.7 cells. These
BH-only domain Bcl-2 family proteins, Bik and Puma, could bind and
neutralize the pro-survival function of Bcl-xl and Bcl-2 (16,23,24).
Compared with Bcl-2, Bik has a higher binding affinity for Bcl-xl,
while Puma has nearly similar abilities to bind these two
pro-survival Bcl-2 family proteins (23). In addition, Puma and Bik can also
directly activate Bax, a multi-BH domain Bcl-2 family protein and a
key pro-apoptotic effector in the mitochondrial pathway to trigger
apoptosis (17,25,26). It
has been reported that when Bax and Bcl-xl are both upregulated,
the cells exhibit a higher apoptosis rate than control cells
(17). Thus, we propose that
upregulated Bik and Puma could neutralize the pro-survival function
of increased Bcl-xl, and that increased Bax could also overcome the
anti-apoptotic ability of Bcl-xl while being activated by Bik and
Puma to promote apoptosis in TIPE1-overexpressing RAW264.7
cells.
It has been reported that TIPE1 could inhibit the
Rac1-induced JNK activation in HEK293 cells (10). Contrarily to this report, the present
study revealed that MAPKs, including JNK, were constantly activated
by TIPE1 overexpression in RAW264.7 cells. Erk, p38 and JNK could
regulate mitochondrial functions, including the levels of Bcl-2
family proteins, to influence apoptosis progression (27–30).
However, the present study demonstrated that the expression of
pro-apoptotic proteins (Bax, Bik and Puma) could not be reduced by
inhibiting Erk1/2, JNK or p38 activation, and the pro-survival
factor Bcl-xl was also enhanced. These phenomena indicated that
MAPKs activation is not involved in the TIPE1 pro-apoptotic
function. Notably, it is known from a previous study that
overexpressed TIPE2, another pro-apoptotic member of the TNFAIP8
family, could also constantly activate p38 (31). Besides its pro-apoptotic function,
TIPE2 is also an important negative regulator in innate and
adaptive immunity, which could influence the expression of several
cytokines by negatively regulating JNK and p38 activation in
various types of immune cells, including the macrophage cell line
RAW264.7 (2,7,32). TIPE,
another member of the TNFAIP8 family, has also been reported as a
regulator of the inflammatory cytokine interleukin-1β in RAW264.7
cells (33). Similarly, total or
partial activation of Erk1/2, JNK and p38 could be observed in
immune-related functions in macrophages (including RAW264.7 cells);
thus, inhibiting the activation of these MAPKs proteins could
decrease the expression of various cytokines (34–37). In
the present study, TIPE1 was overexpressed in RAW264.7 cells; thus,
the phenomenon of sustained MAPKs activation could possibly
indicate certain immune-related function of TIPE1.
In conclusion, the present study demonstrated that
TIPE1 overexpression could promote apoptosis in RAW264.7 cells and
inhibit tumor growth in athymic mice. Overexpressed TIPE1
influenced the intrinsic apoptosis pathway by regulating the
expression levels of pro- and anti-apoptotic factors of the Bcl-2
family of proteins, and sustained MAPKs activation did not
contribute to the pro-apoptotic progression. Our study supports the
idea that TIPE1 is a pro-apoptotic factor. Further studies are
required to understand how TIPE1 regulates cell death.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Fujian Province of China (Fuzhou, China;
grant no. 2013J05124). We thank Professor Yuhai Chen (University of
Pennsylvania School of Medicine, Philadelphia, PA, USA) for kindly
providing the RAW264.7 cells with stably transfected MigR1 or
MigR1-TIPE1 vector. In addition, we thank Professor Fengguang Gao
(Xiamen University, Fujian, China) for his technical advice.
References
|
1
|
Tibbetts MD, Zheng L and Lenardo MJ: The
death effector domain protein family: Regulators of cellular
homeostasis. Nat Immunol. 4:404–409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun H, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al: TIPE2, a negative
regulator of innate and adaptive immunity that maintains immune
homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar D, Whiteside TL and Kasid U:
Identification of a novel tumor necrosis factor-alpha-inducible
gene, SCC-S2, containing the consensus sequence of a death effector
domain of fas-associated death domain-like
interleukin-1beta-converting enzyme-inhibitory protein. J Biol
Chem. 275:2973–2978. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar D, Gokhale P, Broustas C,
Chakravarty D, Ahmad I and Kasid U: Expression of SCC-S2, an
antiapoptotic molecule, correlates with enhanced proliferation and
tumorigenicity of MDA-MB 435 cells. Oncogene. 23:612–616. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miao Z, Zhao T, Wang Z, Xu Y, Song Y, Wu J
and Xu H: SCC-S2 is overexpressed in colon cancers and regulates
cell proliferation. Tumour Biol. 33:2099–2106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gus-Brautbar Y, Johnson D, Zhang L, Sun H,
Wang P, Zhang S, Zhang L and Chen YH: The anti-inflammatory TIPE2
is an inhibitor of the oncogenic Ras. Mol Cell. 45:610–618. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang W, Zhang J, Zhao L, Shao J, Cui J,
Guo C, Zhu F, Chen YH and Liu S: TIPE2 protein negatively regulates
HBV-specific CD8+ T lymphocyte functions in humans. Mol Immunol.
64:204–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cui J, Zhang G, Hao C, Wang Y, Lou Y,
Zhang W, Wang J and Liu S: The expression of TIPE1 in murine
tissues and human cell lines. Mol Immunol. 48:1548–1555. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hitomi J, Christofferson DE, Ng A, Yao J,
Degterev A, Xavier RJ and Yuan J: Identification of a molecular
signaling network that regulates a cellular necrotic cell death
pathway. Cell. 135:1311–1323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Z, Liang X, Gao L, Ma H, Liu X, Pan
Y, Yan W, Shan H, Wang Z, Chen YH and Ma C: TIPE1 induces apoptosis
by negatively regulating Rac1 activation in hepatocellular
carcinoma cells. Oncogene. 34:2566–2574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang YY, Liu Y, Ni XY, Bai ZH, Chen QY,
Zhang Y and Gao FG: Nicotine promotes cell proliferation and
induces resistance to cisplatin by α7 nicotinic acetylcholine
receptor-mediated activation in Raw264.7 and El4 cells. Oncol Rep.
31:1480–1488. 2014.PubMed/NCBI
|
|
12
|
Gao FG, Wan da F and Gu JR: Ex vivo
nicotine stimulation augments the efficacy of therapeutic bone
marrow-derived dendritic cell vaccination. Clin Cancer Res.
13:3706–3712. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Loewer A, Batchelor E, Gaglia G and Lahav
G: Basal dynamics of p53 reveal transcriptionally attenuated pulses
in cycling cells. Cell. 142:89–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khan KH, Blanco-Codesido M and Molife LR:
Cancer therapeutics: Targeting the apoptotic pathway. Crit Rev
Oncol Hematol. 90:200–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Finucane DM, Bossy-Wetzel E, Waterhouse
NJ, Cotter TG and Green DR: Bax-induced caspase activation and
apoptosis via cytochrome c release from mitochondria is inhibitable
by Bcl-xL. J Biol Chem. 274:2225–2233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Viedma-Rodriguez R, Baiza-Gutman LA,
Garcia-Carrancá A, Moreno-Fierros L, Salamanca-Gómez F and
Arenas-Aranda D: Suppression of the death gene BIK is a critical
factor for resistance to tamoxifen in MCF-7 breast cancer cells.
Int J Oncol. 43:1777–1786. 2013.PubMed/NCBI
|
|
19
|
Nakano K and Vousden KH: PUMA, a novel
proapoptotic gene, is induced by p53. Mol Cell. 7:683–694. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Michels J, Kepp O, Senovilla L, Lissa D,
Castedo M, Kroemer G and Galluzzi L: Functions of BCL-X L at the
interface between cell death and metabolism. Int J Cell Biol.
2013:7052942013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beug ST, Cheung HH, LaCasse EC and
Korneluk RG: Modulation of immune signalling by inhibitors of
apoptosis. Trends Immunol. 33:535–545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Willis SN, Wei A, Smith BJ,
Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM and Huang DC:
Differential targeting of prosurvival Bcl-2 proteins by their
BH3-only ligands allows complementary apoptotic function. Mol Cell.
17:393–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Willis SN, Fletcher JI, Kaufmann T, van
Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD,
Bouillet P, et al: Apoptosis initiated when BH3 ligands engage
multiple Bcl-2 homologs, not Bax or Bak. Science. 315:856–859.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gillissen B, Essmann F, Graupner V, Stärck
L, Radetzki S, Dörken B, Schulze-Osthoff K and Daniel PT: Induction
of cell death by the BH3-only Bcl-2 homolog Nbk/Bik is mediated by
an entirely Bax-dependent mitochondrial pathway. EMBO J.
22:3580–3590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gallenne T, Gautier F, Oliver L, Hervouet
E, Noël B, Hickman JA, Geneste O, Cartron PF, Vallette FM, Manon S
and Juin P: Bax activation by the BH3-only protein Puma promotes
cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol.
185:279–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sheridan C, Brumatti G, Elgendy M, Brunet
M and Martin SJ: An ERK-dependent pathway to Noxa expression
regulates apoptosis by platinum-based chemotherapeutic drugs.
Oncogene. 29:6428–6441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El Mchichi B, Hadji A, Vazquez A and Leca
G: p38 MAPK and MSK1 mediate caspase-8 activation in
manganese-induced mitochondria-dependent cell death. Cell Death
Differ. 14:1826–1836. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng Y, Ren X, Yang L, Lin Y and Wu X: A
JNK-dependent pathway is required for TNF alpha-induced apoptosis.
Cell. 115:61–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu WW, Chen SY, Cheng CH, Cheng HJ and
Huang PH: Blm-s, a BH3-only protein enriched in postmitotic
immature neurons, is transcriptionally upregulated by p53 during
DNA damage. Cell Rep. 9:166–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu QQ, Zhang FF, Wang F, Qiu JH, Luo CH,
Zhu GY and Liu YF: TIPE2 inhibits lung cancer growth attributing to
promotion of apoptosis by regulating some apoptotic molecules
expression. PLoS One. 10:e01261762015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Fayngerts S, Wang P, Sun H,
Johnson DS, Ruan Q, Guo W and Chen YH: TIPE2 protein serves as a
negative regulator of phagocytosis and oxidative burst during
infection. Proc Natl Acad Sci USA. 109:15413–15418. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahn SH, Deshmukh H, Johnson N, Cowell LG,
Rude TH, Scott WK, Nelson CL, Zaas AK, Marchuk DA, Keum S, et al:
Two genes on A/J chromosome 18 are associated with susceptibility
to Staphylococcus aureus infection by combined microarray and QTL
analyses. Plos Pathog. 6:e10010882010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dai H, Xu D, Su J, Jang J and Chen Y:
Transmembrane protein 106a activates mouse peritoneal macrophages
via the MAPK and NF-κB signaling pathways. Sci Rep. 5:124612015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang CR, Wang JH, Hsieh SL, Wang SM, Hsu
TL and Lin WW: Decoy receptor 3 (DcR3) induces osteoclast formation
from monocyte/macrophage lineage precursor cells. Cell Death
Differ. 11(Suppl 1): S97–S107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
An HZ, Zhao W, Hou J, Zhang Y, Xie Y,
Zheng Y, Xu H, Qian C, Zhou J, Yu Y, et al: SHP-2 phosphatase
negatively regulates the TRIF adaptor protein-dependent type I
interferon and proinflammatory cytokine production. Immunity.
25:919–928. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xia M, Liu J, Wu X, Liu S, Li G, Han C,
Song L, Li Z, Wang Q, Wang J, et al: Histone methyltransferase
Ash1l suppresses interleukin-6 production and inflammatory
autoimmune diseases by inducing the ubiquitin-editing enzyme A20.
Immunity. 39:470–481. 2013. View Article : Google Scholar : PubMed/NCBI
|