Introduction
Colon cancer is the world's third most common
malignant tumor (1). In 2002, only in
Europe, >370,000 people suffered from it, and ~200,000 people
succumbed to the disease, which accounted for 12% of all cancer
mortalities (2). In China, at
present, colon cancer is ranked as the fifth most common tumor,
whose growth speed is very fast, particularly in certain large
cities (3). However, colon cancer's
lack of specificity in terms of clinical symptoms leads to patients
being often misdiagnosed, thus delaying treatment (4). Therefore, research has focused on early
detection and treatment of the tumor (5). The occurrence and development of colon
cancer are associated with numerous factors, including activation
of a variety of oncogenes and deactivation of tumor-suppressor
genes, which are important in these processes (5,6).
Induction of cell apoptosis is the main type of
tumor treatment (7). Regulation of
the intracellular redox state determines the cell's survival fate
(8). In normal cells, two redox
systems exist, the peroxide enzyme system and resistance against
superoxide (9). The former includes
the oxygen reduction sulfur protein, while the latter includes
superoxide dismutase, catalase and glutathione peroxidase (10). These systems can remove reactive
oxygen species (ROS) produced in the process of metabolism, which
results in relatively stable levels of intracellular ROS (11). Multiple studies have confirmed that
the level of intracellular ROS and the redox state are closely
associated with cell apoptosis (12).
By far, the B-cell lymphoma (Bcl)-2 gene family is
the most important gene family involved in the regulation of
apoptosis (13). The family is
divided into two categories: The first one comprises antiapoptotic
proteins, mainly Bcl-2, Bcl-extra large, Bcl-W, A1/Bfl-1, myeloid
cell leukemia 1 and Boo, while the second one comprises
proapoptotic proteins, including Bcl-2 associated X protein (Bax),
Bcl-2 homologous antagonist/killer, Bcl-2 related ovarian killer,
Bcl-x, BH3 interacting-domain death agonist and Bcl-2-associated
death promoter (14). The Bcl-2
protein can inhibit cell apoptosis, but is unable to promote cell
proliferation, in cancer cells (13).
Pharmacological studies have demonstrated that the
active ingredients of icaritin can inhibit the proliferation of
HL-60 and U-937 leukemia cells (15).
Previous studies identified that the mechanism of inhibition of
cell proliferation is mainly due to the induction of apoptosis and
inhibition of telomerase activity (16). Icaritin was demonstrated to be able to
alter the cell cycle of HL-60 cells (15). In addition, by increasing p21
tumor-suppressor genes and reducing c-Myc oncogene expression,
antitumor effects were achieved (17). Previous reports have also demonstrated
that icaritin's active ingredients can induce G1 phase retardation
in prostate cancer cells, and cell growth was significantly
inhibited as a consequence (17).
Extensive research demonstrated that the active ingredients of
icaritin can alter the extracellular signal-regulated kinase
signaling pathway of liver cancer cells (16,18). All
the above results confirmed that icaritin has certain antitumor
effects. The present study demonstrated that the anticancer effect
of icaritin inhibits the growth of colon cancer cells through ROS,
Bcl-2 and cyclin D1/E signaling.
Materials and methods
Chemicals and reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), 1-(4,5-dimethylthiazol- 2-yl)-3,5-diphenyl
formazan (MTT) and icaritin (whose chemical structure is indicated
in Fig. 1) were provided by
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Propidium
iodide (PI) and the Annexin V-FITC Apoptosis Detection kit were
purchased from BD Biosciences (Franklin Lakes, NJ, USA). The DC
Protein Assay was purchased from Bio-Rad Laboratories, Inc.
(Hercules, CA, USA).
Cell lines
The human colon cancer cell line COLO-205 was
provided by the experimental center of China-Japan Friendship
Hospital (Beijing, China). Cells were cultured in DMEM with 10%
FBS, supplemented with 100 U/ml penicillin G and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C and 5% CO2/95% air atmosphere.
Cell viability assay
COLO-205 cells were seeded at a density of
1–2×103 cells/well in a 96-well plate and cultured with
icaritin (0, 1, 2.5, 5, 7.5 and 10 µM) for 1 or 2 days (19). Subsequently, MTT reagent was added to
a final concentration of 0.5 mg/ml to each well, and the cells were
cultured for additional 5 h at 37°C and 5% CO2/95% air
atmosphere. Upon incubation, the medium was removed, and 100 µl
dimethyl sulfoxide was added into each well. The absorbance was
then read at 570 nm in a microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA).
Apoptosis assay
COLO-205 cells were seeded at a density of
1–2×106 cells/well in a 6-well plate and cultured with
icaritin (0, 1, 5 and 10 µM) for 2 days. Subsequently, the cells
were collected with fluorescence-activated cell sorting wash buffer
(BD Biosciences) and stained with annexin V-fluorescein
isothiocyanate and PI (BD Biosciences) according to the
manufacturer's protocol. Cell apoptosis was analyzed by flow
cytometry (Accuri™ C6; BD Biosciences).
ROS level detection
COLO-205 cells were seeded at a density of
1–2×106 cells/well in a 6-well plate and cultured with
icaritin (0, 1, 5 and 10 µM) for 2 days. Subsequently, the cells
were incubated with 50 µM 2′,7′-dichlorofluorescein-diacetate
(Sigma-Aldrich; Merck Millipore) for 10 min at 37°C and 5%
CO2/95% air atmosphere. ROS level was detected with a
FACSCanto™ flow cytometer (BD Biosciences).
Western blot assay
COLO-205 cells were seeded at a density of
1–2×106 cells/well in a 6-well plate and cultured with
icaritin (0, 1, 5 and 10 µM) for 2 days. Subsequently, the cells
were collected with Cell Lysis Buffer (Cell Signaling Technology,
Inc., Danvers, MA, USA) by plate scraping on ice. Protein
concentrations were evaluated by DC Protein Assay. A total of 20 mg
of protein was resolved on a 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred to
polyvinylidene fluoride membranes (GE Healthcare Life Sciences,
Chalfont, UK). Membranes were blocked with 10% non-fat dry milk in
Tris-buffered saline with Tween 20 (TBST) for 1 h at room
temperature, prior to be incubated with primary rabbit anti-human
antibodies against Bcl-2 (1:1,000; sc-492; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), cyclin D1 (1:500; sc-753;
Santa Cruz Biotechnology, Inc.), cyclin E (1:2,000; sc-481; Santa
Cruz Biotechnology, Inc.) and β-actin (1:2,000; sc-130656; Santa
Cruz Biotechnology, Inc.) in TBST with 5% bovine serum albumin at
4°C overnight, followed by incubation with a peroxidase-conjugated
secondary antibody (D110056; Sangon Biotech Co., Ltd., Shanghai,
China) according to the manufacturer's protocol. Detection was
performed with a highly sensitive chemiluminescent detection kit
(C500044; Sangon Biotech Co., Ltd.).
Measurement of caspase-3 activity
COLO-205 cells were seeded at a density of
1–2×106 cells/well in a 6-well plate and cultured with
icaritin (0, 1, 5 and 10 µM) for 2 days. Subsequently, the cells
were collected with Cell Lysis Buffer by plate scraping on ice.
Protein concentrations were evaluated by DC Protein Assay. Equal
quantities of protein were added to the reaction buffer containing
Asp-Glu-Val-Asp-p-nitroaniline, and were then incubated at 37°C for
6 h at room temperature. Caspase-3 activity was measured at an
absorbance of 405 nm in a microplate reader (Molecular Devices,
LLC).
Statistical analysis
Data are represented as the mean ± standard error of
the mean. Statistical analysis was conducted using with SPSS 11.5
statistical software (SPSS, Inc., Chicago, IL, USA) with the paired
samples t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Anticancer effect of icaritin on cell
growth in COLO-205 cells
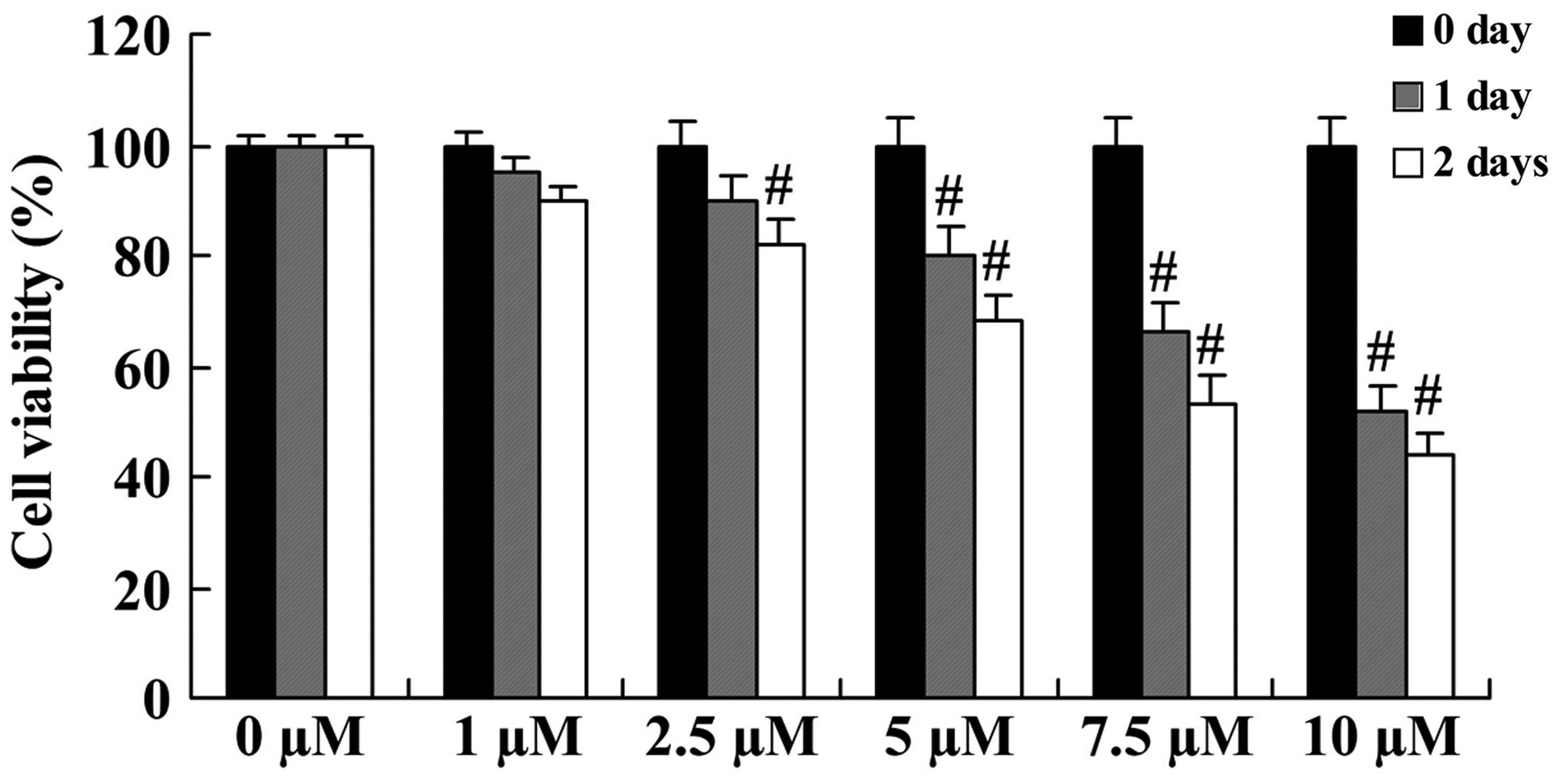
The anticancer effect of icaritin on cell growth was
initially evaluated in COLO-205 cells using a standard MTT assay.
Compared with COLO-205 cells without icaritin treatment, the cell
growth of treated COLO-205 cells was inhibited in a dose- and
time-dependent manner (Fig. 2). The
inhibition of cell growth was significant upon treatment with 5,
7.5 and 10 µM icaritin for 1 day, or with 2.5, 5, 7.5 and 10 µM for
2 days (Fig. 2).
Anticancer effect of icaritin on cell
apoptosis in COLO-205 cells
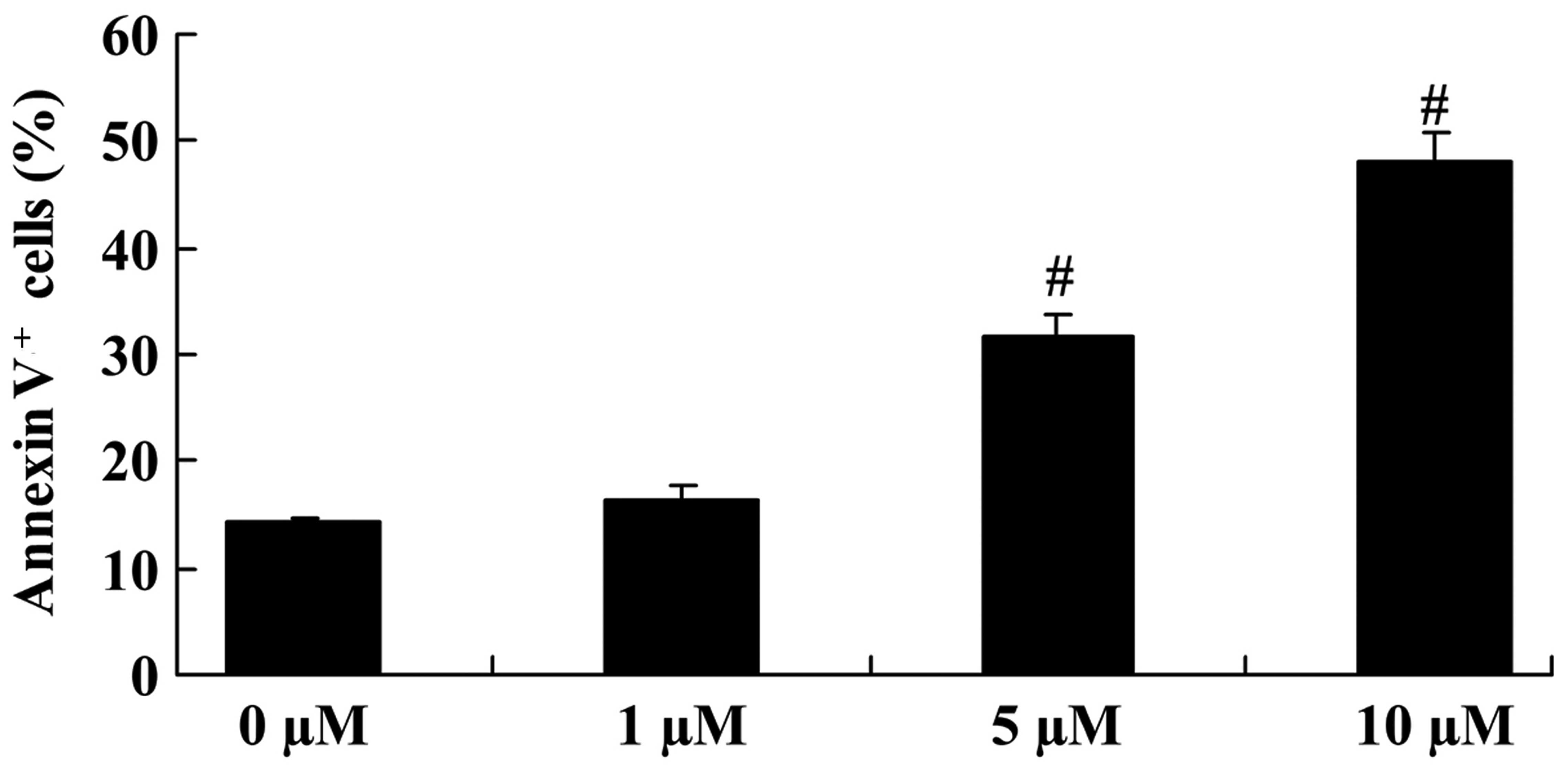
The apoptosis of COLO-205 cells was analyzed using
PI and the Annexin V-FITC Apoptosis Detection kit. Upon treatment
with 5 and 10 µM icaritin for 2 days, cell apoptosis was
significantly increased, compared with that of COLO-205 cells
without icaritin treatment (Fig.
3).
Anticancer effect of icaritin on ROS
level in COLO-205 cells
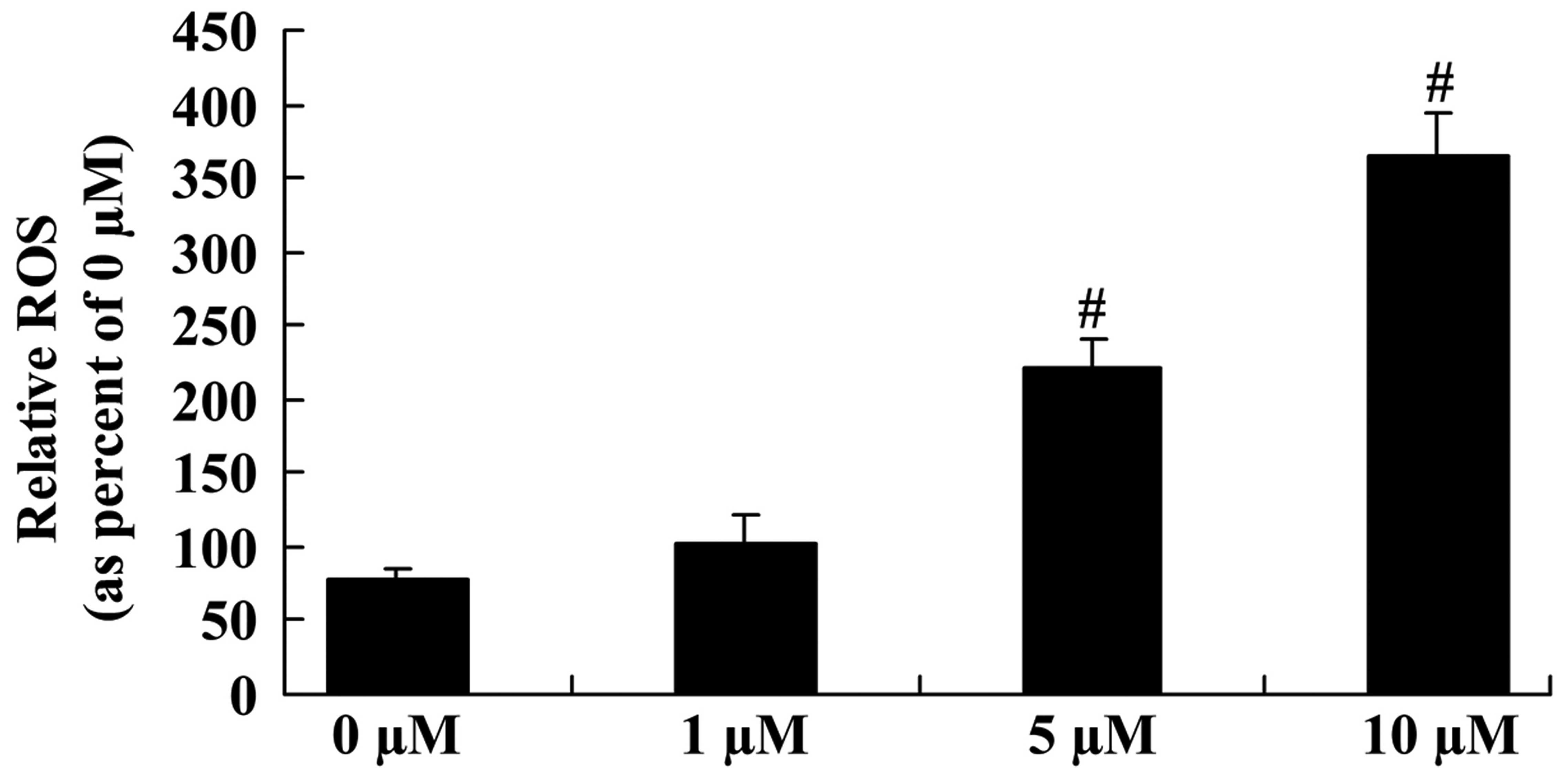
To explore the underlying mechanisms regulating the
anticancer effect of icaritin on the ROS level of COLO-205 cells,
the ROS level was measured in different groups of COLO-205 cells.
The ROS level was significantly enhanced following 5- and 10-µM
icaritin treatment for 2 days in COLO-205 cells (Fig. 4).
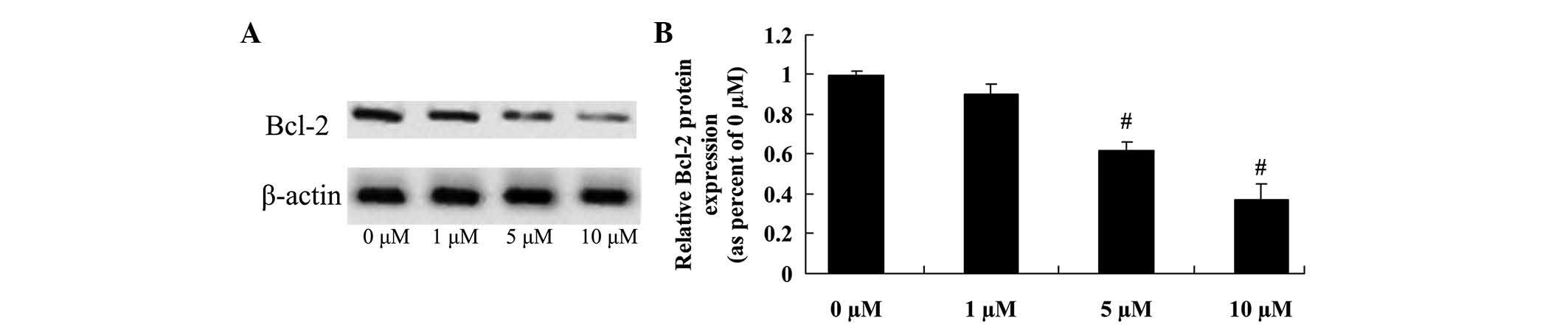
Anticancer effect of icaritin on Bcl-2
expression in COLO-205 cells
The anticancer effect of icaritin on COLO-205 cells,
which is frequently suppressed by Bcl-2 expression, was further
assessed in COLO-205 cells. The inhibition of Bcl-2 protein
expression correlated with the 5- and 10-µM icaritin-induced cell
apoptosis of COLO-205 cells treated for 2 days (Fig. 5).
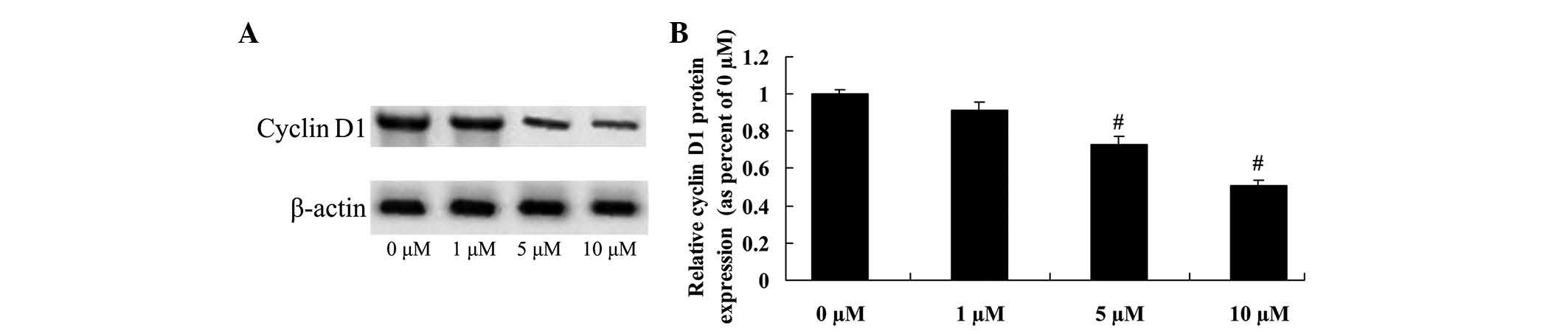
Anticancer effect of icaritin on
cyclin D1 expression in COLO-205 cells
To determine the anticancer effect of icaritin on
cyclin D1 expression in COLO-205 cells, cyclin D1 protein
expression was analyzed using a western blot assay. The results
suggested that cyclin D1 protein expression of COLO-205 cells was
effectively reduced by treatment with 5 and 10 µM icaritin
(Fig. 6).
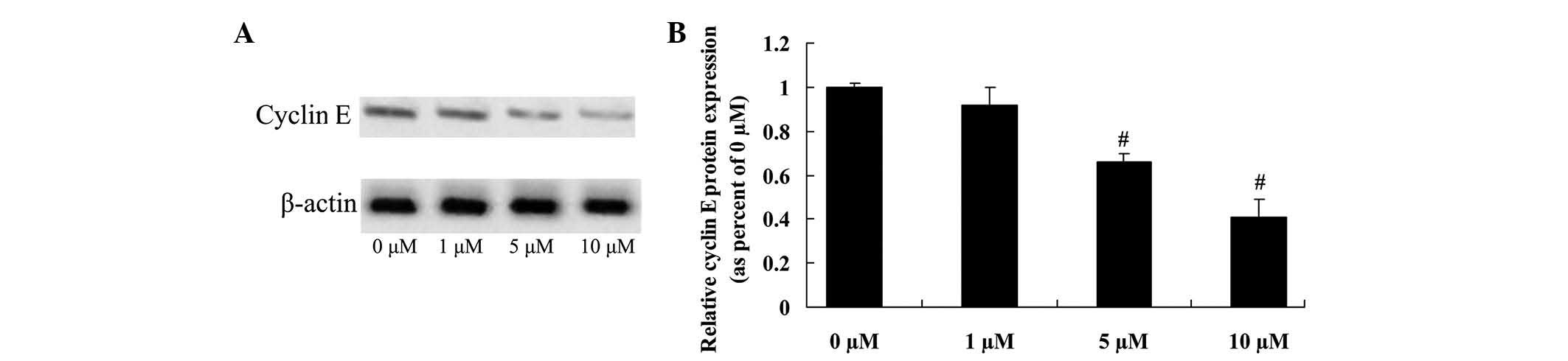
Anticancer effect of icaritin on
cyclin E expression in COLO-205 cells
To further investigate whether cyclin E directly
influences the anticancer effects of icaritin on COLO-205 cells,
cyclin E protein expression was confirmed by western blot analysis.
The initial results revealed that 5 and 10 µM of icaritin
significantly inhibited cyclin E protein expression, which may be
important for the anticancer effects of icaritin on COLO-205 cells
(Fig. 7).
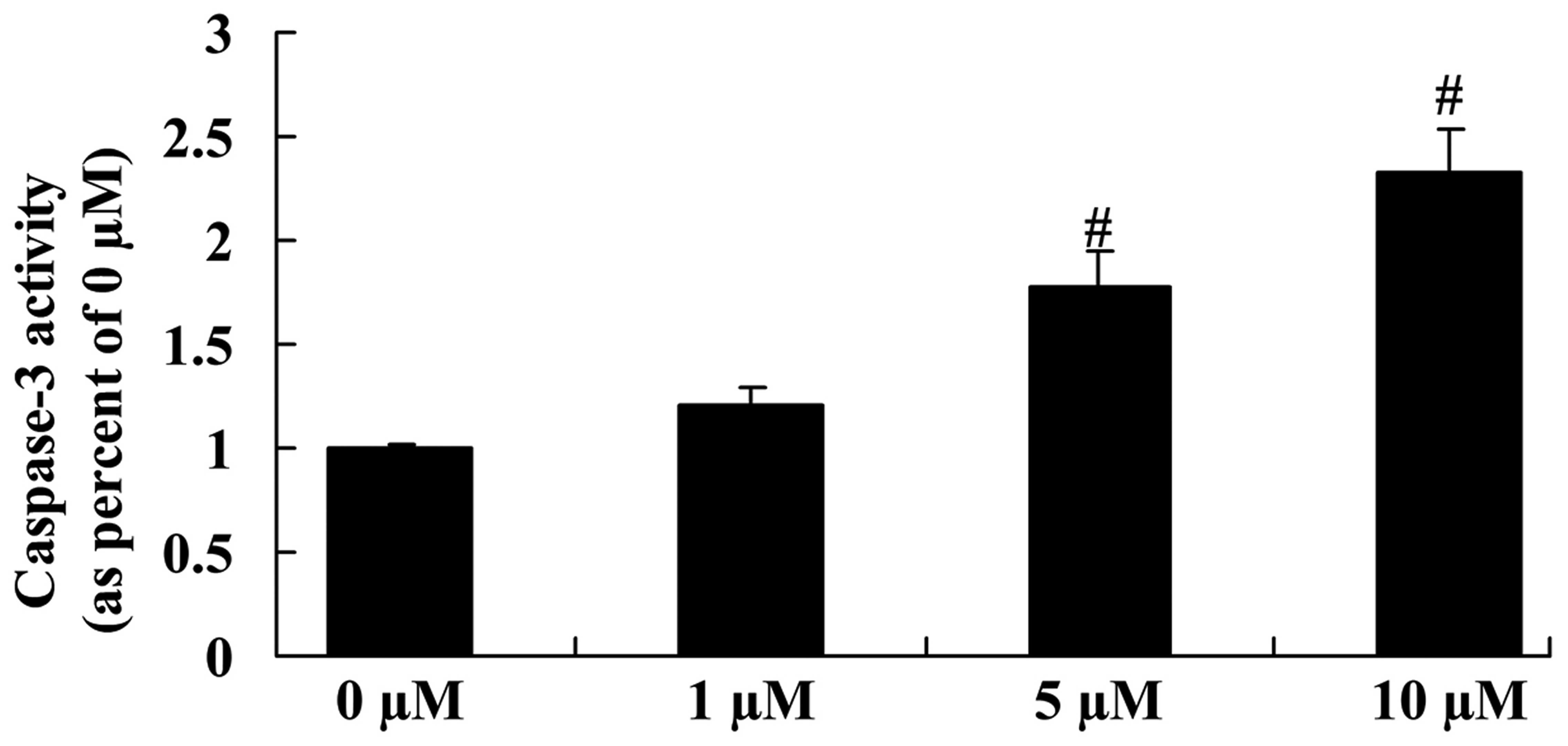
Anticancer effect of icaritin on
caspase-3 activity in COLO-205 cells
The anticancer effect of icaritin on caspase-3
activity was next assessed in COLO-205 cells. As represented in
Fig. 8, a significant reduction in
caspase-3 activity was observed in COLO-205 cells treated with 5
and 10 µM icaritin, compared with vehicle control.
Discussion
As one of the most common malignant tumors of the
digestive system, colon cancer is severely harmful to humans
(1). In recent years, with the
continuous improvement of people's living standards in China and a
gradual westernized diet, the incidence of colon cancer has
increased year by year, which is a matter of concern (20). Large-scale population census studies
have demonstrated that, among people older than 35 years, the
incidence of colon cancer is 24–32 cases/100,000 individuals
(4). However, colon cancer prognosis
is not optimistic, and the 5-year survival rate is ~50% (21). All these observations enabled colon
cancer to become a hot topic in tumor research. The present results
demonstrate that icaritin significantly inhibited cell growth and
induced cell apoptosis of COLO-205 cells. In addition, recent
studies suggested that icaritin induced cell apoptosis in human
lung cancer cells (22), renal cell
carcinoma (19) and human
osteosarcoma cells (23).
Collectively, these data indicate that icaritin may exert potent
antitumor and proapoptotic effects on human colon cancer.
ROS is a general term for reactive oxygen compounds,
which are produced in the process of biological aerobic metabolism
in the form of O-2, H2O2, HO- and nitric oxide (10). Besides, it is important in cell
apoptosis through various different pathways (24). Numerous studies have demonstrated that
ROS is important for killing tumor cells in all types of living
organisms (25,26). Besides, it may act as a second
messenger to regulate cell proliferation, differentiation and
apoptosis, which are associated with signal transduction pathways
(10). A previous study has
demonstrated that the antioxidant enzyme activity within the tumor
cells was lower than that within normal cells, and that the removal
efficiency of ROS was also low in tumor cells, suggesting that the
intracellular ROS levels are more likely to kill tumor cells
(27). Furthermore, recent studies
have demonstrated that the excess of ROS in the cell can kill tumor
cells, thus suggesting a function for ROS on the treatment of
tumors (12). In the present study,
it was observed that icaritin treatment enhanced the ROS level of
COLO-205 cells. Zheng et al suggested that icaritin induced
the apoptosis of lung cancer cells through increasing their ROS
level (22). The current results
demonstrated that icaritin also modestly promoted the ROS level of
COLO-205 cells, which may be attributed to the different signaling
pathways that mediate the anticancer effect of icaritin on human
colon cancer.
The Bcl-2 protein, which is encoded by the Bcl-2
gene, can inhibit apoptosis and prolong the life of the cell, but
cannot promote cell proliferation (13). The protein encoded by the Bax gene,
together with other antiapoptotic proteins, can be formed during
the formation of Bcl-2 heterologous dimers, with the function of
antagonizing the function of Bcl-2 in order to promote cell
apoptosis (14). Besides, structural
changes in various organelles and in the cytoplasmic membrane,
particularly in the mitochondrial outer membrane, as well as
changes in the interaction between the antiapoptotic Bcl-2 protein
and the membrane, may cause the loss of antiapoptotic proteins
responsible for apoptosis inhibition, resulting in the loss of
function of these organelles and the release of several
apoptosis-promoting factors, eventually leading to cell apoptosis
(14). The present study demonstrated
that icaritin inhibits Bcl-2 protein expression in COLO-205 cells.
A previous study also reported that icaritin induces the apoptosis
of HepG2 cells through modulating the Bax/Bcl-2 ratio (18) and that of breast cancer cells through
the downregulation of Bcl-2 expression (16). In present study, icaritin
significantly inhibited Bcl-2 expression in COLO-205 cells.
The normal growth of cells in the body depends on
the balance of various regulatory factors involved in the
regulation of the cell cycle. Therefore, any type of occurring
disorder involving regulatory factors will lead to abnormal cell
proliferation, thus inducing tumor formation and progression.
Cyclin D1 can be combined with cyclin-dependent kinase 4 in the
cell cycle to promote cell cycle progression, and therefore, is
regarded as a type of proto-oncogene (28). Cyclin D1 protein expression is
significantly correlated with colon cancer histology, presence of
lymph node metastasis and staging, and colon cancer progression and
metastasis (29).
Cyclin E has been confirmed to regulate the cell
cycle in cancer cells. Besides, it is important in the process of
tumor development. Previous studies have demonstrated that cyclin E
is the main protein involved in mediating G1/S-phase
transformation, thus being important in cell proliferation
(30). Cyclin E expression can
shorten the G1 phase of the cell cycle, cause centrosome
proliferation, disrupt mitosis and lead to the formation of
chromosome instability, thus inducing tumor formation (30). Cyclin E overexpression could increase
the number of colon cancer cells in the G1/S phase. Furthermore,
cyclin E overexpression has been associated with tumor progression
and metastasis (31,32). The present study revealed that
icaritin effectively reduced cyclin D1 and cyclin E protein
expression in COLO-205 cells. Li et al suggested that
icaritin inhibits the cell growth of renal cell carcinoma through
cyclin D1 and cyclin E (19).
In conclusion, to the best of our knowledge, the
present study has reported for the first time that the anticancer
effect of icaritin inhibits cell growth and induces apoptosis of
human colon cancer. The underlying mechanisms of icaritin may be
associated with the promotion of ROS, and the inhibition of Bcl-2
and cyclin D1/E signaling. The current study highlights icaritin as
a potential anticancer target for treating metastatic cancer
through the regulation of ROS, Bcl-2 and cyclin D1/E signaling.
References
|
1
|
Lee WS, Yun JW, Nagappan A, Park HS, Lu
JN, Kim HJ, Chang SH, Kim DC, Lee JH, Jung JM, et al: Tetraarsenic
hexoxide demonstrates anticancer activity at least in part through
suppression of NF-kB activity in SW620 human colon cancer cells.
Oncol Rep. 33:2940–2946. 2015.PubMed/NCBI
|
|
2
|
Quaglia A, Tavilla A, Shack L, Brenner H,
Janssen-Heijnen M, Allemani C, Colonna M, Grande E, Grosclaude P
and Vercelli M: EUROCARE Working Group: The cancer survival gap
between elderly and middle-aged patients in Europe is widening. Eur
J Cancer. 45:1006–1016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang W, Wei Y, Ren L, Zhong Y, Yu Y, Chen
J, Zhu D, Ye L, Qin C, Zhao N, et al: Randomized controlled trial
of intraportal chemotherapy combined with adjuvant chemotherapy
(mFOLFOX6) for stage II and III colon cancer. Ann Surg.
263:434–439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aleksandrova K, Pischon T, Buijsse B, May
AM, Peeters PH, Bueno-de-Mesquita HB, Jenab M, Fedirko V, Dahm CC,
Siersema PD, et al: Adult weight change and risk of colorectal
cancer in the European prospective investigation into cancer and
nutrition. Eur J Cancer. 49:3526–3536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li F, Chen Z, Yang Y, Yi X, Yang Y and
Zhang L: Characterization of the pathologic and endoscopic
measurements of colorectal polyp sizes with a focus on sessile
serrated adenoma and high-grade dysplasia. Int J Clin Exp Pathol.
7:1635–1643. 2014.PubMed/NCBI
|
|
6
|
Sahm M, Wesselmann S, Kube R, Schöffel N,
Pross M, Lippert H and Kahl S: The development process of colon
cancer centres. Zentralbl Chir. 138:33–37. 2013.(In German).
PubMed/NCBI
|
|
7
|
Rivoltini L, Chiodoni C, Squarcina P,
Tortoreto M, Villa A, Vergani B, Bürdek M, Botti L, Arioli I, Cova
A, et al: TNF-related apoptosis-inducing ligand (TRAIL)-armed
exosomes deliver proapoptotic signals to tumor site. Clin Cancer
Res. 22:3499–3512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhattacharyya S, Pal PB and Sil PC: A 35
kD Phyllanthus niruri protein modulates iron mediated oxidative
impairment to hepatocytes via the inhibition of ERKs, p38 MAPKs and
activation of PI3k/Akt pathway. Food Chem Toxicol. 56:119–130.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arts WF, Scholte HR, Loonen MC, Przyrembel
H, Fernandes J, Trijbels JM and Luyt-Houwen IE: Cytochrome c
oxidase deficiency in subacute necrotizing encephalomyelopathy. J
Neurol Sci. 77:103–115. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Araki K and Nagata K: Functional in vitro
analysis of the ERO1 protein and protein-disulfide isomerase
pathway. J Biol Chem. 286:32705–32712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Satapathy SR, Mohapatra P, Das D,
Siddharth S and Kundu CN: The apoptotic effect of plant based
nanosilver in colon cancer cells is a p53 dependent process
involving ROS and JNK cascade. Pathol Oncol Res. 21:405–411. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng X and Gandhi V: ROS-activated
anticancer prodrugs: A new strategy for tumor-specific damage. Ther
Deliv. 3:823–833. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Y, Liu QH, Liu CL and Lin L:
Calycosin induces apoptosis in human ovarian cancer SKOV3 cells by
activating caspases and Bcl-2 family proteins. Tumour Biol.
36:5333–5339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alibek K, Irving S, Sautbayeva Z,
Kakpenova A, Bekmurzayeva A, Baiken Y, Imangali N, Shaimerdenova M,
Mektepbayeva D, Balabiyev A and Chinybayeva A: Disruption of Bcl-2
and Bcl-xL by viral proteins as a possible cause of cancer. Infect
Agent Cancer. 9:442014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q, Huai L, Zhang C, Wang C, Jia Y, Chen
Y, Yu P, Wang H, Rao Q, Wang M and Wang J: Icaritin induces AML
cell apoptosis via the MAPK/ERK and PI3K/AKT signal pathways. Int J
Hematol. 97:617–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo Y, Zhang X, Meng J and Wang ZY: An
anticancer agent icaritin induces sustained activation of the
extracellular signal-regulated kinase (ERK) pathway and inhibits
growth of breast cancer cells. Eur J Pharmacol. 658:114–122. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tong JS, Zhang QH, Huang X, Fu XQ, Qi ST,
Wang YP, Hou Y, Sheng J and Sun QY: Icaritin causes sustained
ERK1/2 activation and induces apoptosis in human endometrial cancer
cells. PLoS One. 6:e167812011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He J, Wang Y, Duan F, Jiang H, Chen MF and
Tang SY: Icaritin induces apoptosis of HepG2 cells via the JNK1
signaling pathway independent of the estrogen receptor. Planta Med.
76:1834–1839. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li S, Priceman SJ, Xin H, Zhang W, Deng J,
Liu Y, Huang J, Zhu W, Chen M, Hu W, et al: Icaritin inhibits
JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS One.
8:e816572013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vemulapalli R, Lara LF, Sreenarasimhaiah
J, Harford WV and Siddiqui AA: A comparison of palliative stenting
or emergent surgery for obstructing incurable colon cancer. Dig Dis
Sci. 55:1732–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haghighi MM, Vahedi M, Mohebbi SR,
Pourhoseingholi MA, Fatemi SR and Zali MR: Comparison of survival
between patients with hereditary non polyposis colorectal cancer
(HNPCC) and sporadic colorectal cancer. Asian Pac J Cancer Prev.
10:209–212. 2009.PubMed/NCBI
|
|
22
|
Zheng Q, Liu WW, Li B, Chen HJ, Zhu WS,
Yang GX, Chen MJ and He GY: Anticancer effect of icaritin on human
lung cancer cells through inducing S phase cell cycle arrest and
apoptosis. J Huazhong Univ Sci Technolog Med Sci. 34:497–503. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang XF and Wang J: Icaritin suppresses
the proliferation of human osteosarcoma cells in vitro by
increasing apoptosis and decreasing MMP expression. Acta Pharmacol
Sin. 35:531–539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trachootham D, Zhou Y, Zhang H, Demizu Y,
Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J and
Huang P: Selective killing of oncogenically transformed cells
through a ROS-mediated mechanism by beta-phenylethyl
isothiocyanate. Cancer Cell. 10:241–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gong EY, Shin YJ, Hwang IY, Kim JH, Kim
SM, Moon JH, Shin JS, Lee DH, Hur DY, Jin DH, et al: Combined
treatment with vitamin C and sulindac synergistically induces p53-
and ROS-dependent apoptosis in human colon cancer cells. Toxicol
Lett. 258:126–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park KW, Kundu J, Chae IG, Bachar SC, Bae
JW and Chun KS: Methanol extract of Flacourtia indica aerial parts
induces apoptosis via generation of ROS and activation of caspases
in human colon cancer HCT116 cells. Asian Pac J Cancer Prev.
15:7291–7296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhai W, Xu YF, Peng B, Zhang HM, Huang JH,
Liu M, Wang GC and Zheng JH: Effect of free radical scavenger on
c-jun activation in rats with crush syndrome. Int J Clin Pharmacol
Ther. 51:600–605. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gopalakrishnan N, Saravanakumar M,
Madankumar P, Thiyagu M and Devaraj H: Colocalization of β-catenin
with Notch intracellular domain in colon cancer: A possible role of
Notch1 signaling in activation of CyclinD1-mediated cell
proliferation. Mol Cell Biochem. 396:281–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin A, Yu Q, Gao Y, Tan J, Huang H, Qiao Z
and Qian W: Inhibition of STAT3/cyclinD1 pathway promotes
chemotherapeutic sensitivity of colorectal caner. Biochem Biophys
Res Commun. 457:681–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prosnitz RG, Patwardhan MB, Samsa GP,
Mantyh CR, Fisher DA, McCrory DC, Cline KE, Gray RN and Morse MA:
Quality measures for the use of adjuvant chemotherapy and radiation
therapy in patients with colorectal cancer: A systematic review.
Cancer. 107:2352–2360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patwardhan MB, Samsa GP, McCrory DC,
Fisher DA, Mantyh CR, Morse MA, Prosnitz RG, Cline KE and Gray RN:
Cancer care quality measures: Diagnosis and treatment of colorectal
cancer. Evid Rep Technol Assess (Full Rep). 1–116. 2006.PubMed/NCBI
|
|
32
|
Rahmutulla B, Matsushita K, Satoh M,
Seimiya M, Tsuchida S, Kubo S, Shimada H, Ohtsuka M, Miyazaki M and
Nomura F: Alternative splicing of FBP-interacting repressor
coordinates c-Myc, P27Kip1/cyclinE and Ku86/XRCC5 expression as a
molecular sensor for bleomycin-induced DNA damage pathway.
Oncotarget. 5:2404–2417. 2014. View Article : Google Scholar : PubMed/NCBI
|