Introduction
As the leading cause of cancer-associated mortality,
the incidence of lung cancer has been increasing worldwide
(1). Non small cell lung carcinoma
(NSCLC) is the term given to any type of epithelial lung cancer
that cannot be categorized as small cell lung carcinoma (SCLC), and
the most common types include squamous cell carcinoma, large cell
carcinoma and adenocarcinoma (2).
Despite improvements in surgery, radiotherapy and chemotherapy, the
overall 5-year survival rate remains poor, at <15% (1,3). As the
deregulation of oncogenes or tumor suppressors has been implicated
in the development and progression of NSCLC, investigations
regarding the molecular targets show promise for the treatment of
NSCLC (4,5).
MicroRNAs (miRs) are non-coding RNAs of 18–25
nucleotides in length that can result in the inhibition of gene
expression at a post-transcriptional stage, through binding to the
3′-untranslational region (UTR) of messenger RNAs (mRNAs) (6). Previously, the deregulation of miRs has
been reported to be associated with the development and advancement
of NSCLC (7,8). Among these miRs, miR-133b has been
indicated to inhibit NSCLC growth via targeting epidermal growth
factor receptor, suggesting that miR-133b may be a tumor suppressor
in NSCLC (9). However, the exact role
of miR-133b in the regulation of NSCLC cell migration and invasion
and the underlying mechanisms have not been previously
elucidated.
Fascin1 (FSCN1) is a member of the FSCN family of
actin-binding proteins, responsible for the organization of F-actin
into parallel bundles, and participates in the formation of
actin-based cellular projections (10). FSCN1 has been previously demonstrated
to be associated with lymph node metastasis and tumor node
metastasis (TNM) staging, but not tumor proliferation, in NSCLC,
and to promote NSCLC A549 cell migration and invasion in
vitro and in vivo (11).
However, the regulatory mechanism of FSCN1 in NSCLC cell migration
and invasion remains largely unknown.
The present study aimed to explore the molecular
mechanisms by which miR-133b regulates the migration and invasion
of NSCLC cells.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), Trizol reagent, MiRNA Reverse Transcription
kit, SYBR Green RT-PCR kit, BCA Protein Assay kit, ECL Western
Blotting kit, pMir-Report vector and Lipofectamine 2000 were
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
The plasmid of FSCN1, scramble miRNA mimics, miR-133b mimics,
miR-133b inhibitor and MiRNA Q-PCR Detection kit were purchased
from GeneCopoeia (FulenGen Co., Ltd., Guangzhou, China). The
Stratagene QuikChange site-directed mutagenesis kit was purchased
from Agilent Technologies, Inc. (Santa Clara, CA, USA). The
pRL-SV40 vector was purchased from Promega Corporation (Madison,
WI, USA). Mouse monoclonal anti-FSCN1 (dilution, 1:100; catalog
no., ab49815) and mouse monoclonal anti-glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; dilution, 1:100; catalog no., ab8245) primary
antibodies and rabbit anti-mouse polyclonal horseradish
peroxidase-conjugated secondary antibodies (dilution, 1:20,000;
catalog no., ab6728) were purchased from Abcam (Cambridge, MA,
USA). The Cell Invasion Assay kit was purchased from Merck
Millipore (Darmstadt, Germany).
Cell lines and cell culture
Five human NSCLC cell lines, H1229, A549, H358, H460
and SK-MES-1, and normal human lung epithelial BEAS-2B cells were
purchased from the Cell Bank of Central South University (Changsha,
China). All cells were cultured in DMEM supplemented with 10% FBS
at 37°C in 5% CO2.
RNA extraction and reverse
transcription (RT)-quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted using Trizol reagent,
according to the manufacturer's instructions. For the detection of
miR expression, the MiRNA Reverse Transcription kit was used to
convert 10 ng of total RNA into complementary DNA (cDNA), according
to the manufacturer's instructions. RT-qPCR was then performed
using a miRNA Q-PCR Detection kit on an Applied Biosystems 7500
Real-time PCR System (Thermo Fisher Scientific, Inc.). The U6 gene
was used as an internal reference. DNase I (1 unit) was used in the
reaction. The expression of mRNA was detected by RT-qPCR using the
SYBR Green RT-PCR kit, according to the manufacturer's
instructions. The specific primer pairs were as follows: FSCN1,
sense, 5′-ATTCTTGGACCACAAGGGAATAC-3′ and antisense,
5′-GCCATAAGAGCATAAGCCTCACA-3′; GAPDH (as an internal reference),
sense, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and antisense,
5′-GGCTGTTGTCATACTTCTCATGG-3′. The relative mRNA expression was
quantified using the GraphPad Prism 4.0 software (GraphPad
Software, Inc., La Jolla, CA, USA) and 2−ΔΔCq method
(12).
Western blotting
Cells were solubilized in cold
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Proteins were quantified using the
BCA Assay kit and then separated with 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and transferred onto a
polyvinylidene difluoride (PVDF) membrane (Thermo Fisher
Scientific, Inc.), which was then incubated with Tris-buffered
saline and Tween 20 (Beyotime Institute of Biotechnology)
containing 5% milk at room temperature for 3 h. The PVDF membrane
was then incubated with mouse anti-FSCN1 and mouse anti-GAPDH
primary antibodies at room temperature for 3 h, and then rabbit
anti-mouse secondary antibody at room temperature for 40 min.
Chemiluminent detection was performed using an ECL Western Blotting
kit. The relative protein expression was analyzed by Image-Pro Plus
software version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA)
and presented as the density ratio vs. GAPDH.
Transfection
Lipofectamine 2000 was used to perform transfection,
according to the manufacturer's instructions. Briefly, plasmid or
miRNA mimics and Lipofectamine 2000 were diluted with serum-free
medium (DMEM), respectively. The diluted Lipofectamine 2000 was
added into the diluted plasmid or miRNA mimics, incubated for 20
min at room temperature, and then added into the cell suspension.
The cells were then incubated at 37°C in 5% CO2 for 6 h.
Following incubation, the medium in each well was replaced with
DMEM supplemented with 10% FBS.
Dual luciferase reporter assays
A luciferase reporter assay was performed in order
to clarify whether FSCN1 is a direct target gene of miR-133b in
NSCLC cells. Total cDNA, obtained via the aforementioned RNA
extraction and RT-qPCR method, from NSCLC A549 cells was used to
amplify the 3′UTR of FSCN1, which was then cloned into a
pMir-Report vector. Mutations were introduced within the potential
seed sequences of the 3′UTR of FSCN1 using the QuikChange
site-directed mutagenesis kit. Using Lipofectamine 2000, cells were
transfected with the pMir-Report vectors, containing the wild type
(WT) or mutant type (MUT) of FSCN1 3′-UTR, and miR-133b mimics,
respectively. The pRL-SV40 vector carrying the Renilla luciferase
gene was used as an internal control. Luciferase activity was
determined after 48 h, using the Dual-Glo substrate system and
LD400 luminometer (Beckman Coulter, Inc., Brea, CA, USA). Data are
presented as the ratio of Renilla luciferase to firefly
luciferase.
Cell migration detection
A wound healing assay was performed in order to
assess the cell migratory capacity of NSCLC A549 cells in 4 groups:
Non transfected A549 cells were used as control; the miR-133b group
contained A549 cells transfected with the miR-133 mimic; the FSCN1
group contained A549 cells transfected with the FSCN1 plasmid; and
the miR-133b+FSCN1 group contained A549 cells co-transfected with
the miR-133 mimic and FSCN1 plasmids. In brief, 1×106
A549 cells were cultured to cultured in DMEM supplemented with 10%
FBS at 37°C in 5% CO2 for 24 h, until full confluence.
Wounds of ~1 mm in length were created on the cells with a plastic
scriber (BD Biosciences, Franklin Lakes, NJ, USA), and cells were
washed with PBS for 5 min and incubated in DMEM at 37°C in 5%
CO2 for 24 h. Subsequent to wounding, cells were
incubated in DMEM including 10% FBS. Following culture for 48 h,
cells were observed under a microscope (SMZ1000; Nikon Corporation,
Tokyo, Japan).
Cell invasion detection
Cells in each aforementioned group were starved in
serum-free medium for 24 h, and then resuspended in serum-free
medium. The cell suspension was added into the upper Transwell
chamber (BD Biosciences), while the lower chamber was filled with
DMEM containing 10% FBS. Following incubation for 24 h, cells
attached to the bottom were stained with crystal violet (Beyotime
Institute of Biotechnology) for 20 min, and then washed with PBS 3
times, each for 5 min, and dried in air. Invasive cells were
observed under a microscope (SMZ1000; Nikon Corporation).
Statistical methods
Data were expressed as mean ± standard deviation of
three independent experiments. Statistical analysis was performed
by using SPSS 17.0 statistical software (SPSS, Chicago, IL, USA).
The differences between groups were determined using the one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-133b is downregulated in NSCLC
cell lines
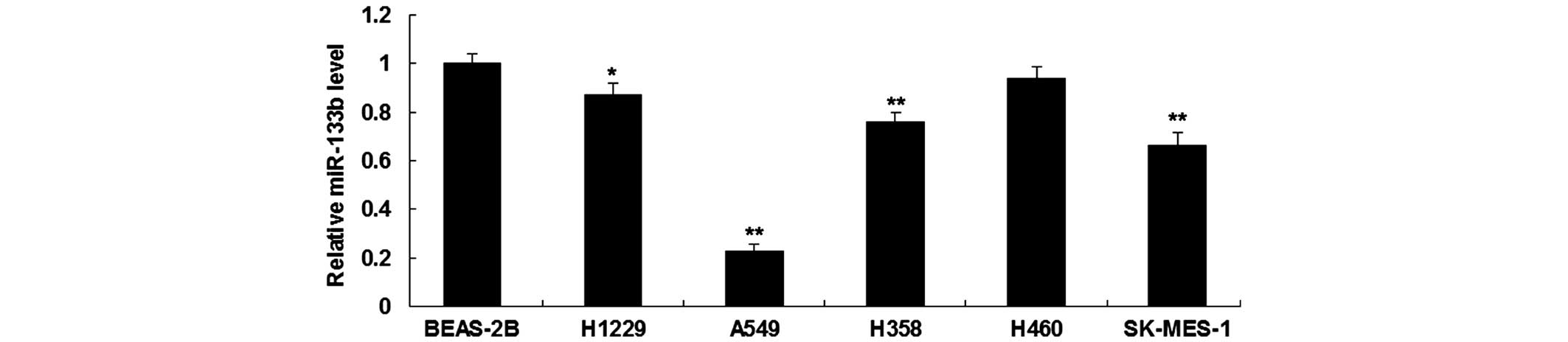
RT-qPCR was first performed in order to determine
the expression level of miR-133b in five human NSCLC cell lines,
H1229, A549, H358, H460 and SK-MES-1, and in normal human lung
epithelial BEAS-2B cells. As shown in Fig. 1, miR-133b was significantly
downregulated in NSCLC cell lines compared to BEAS-2B cells. As
A549 cells showed the most significant decrease in miR-133b
expression (Fig. 1), this cell line
was used in the following experiments.
FSCN1 is identified as a target gene
of miR-133b in NSCLC cells
According to bioinformatical prediction, FSCN1 is a
putative target gene of miR-133b (Fig.
2A). A luciferase reporter assay was performed in order to
clarify whether miR-133b can directly bind to seed sequences in the
FSCN1 3′-UTR in NSCLC A549 cells. As shown in Fig. 2B, the luciferase activity was
remarkably reduced in A549 cells co-transfected with the WT FSCN1
3′UTR and miR-133b mimics, but showed no difference in A549 cells
co-transfected with the MUT FSCN1 3′UTR and miR-133b mimics
(P>0.05), compared with the control group, indicating that FSCN1
is a target gene of miR-133b in NSCLC cells.
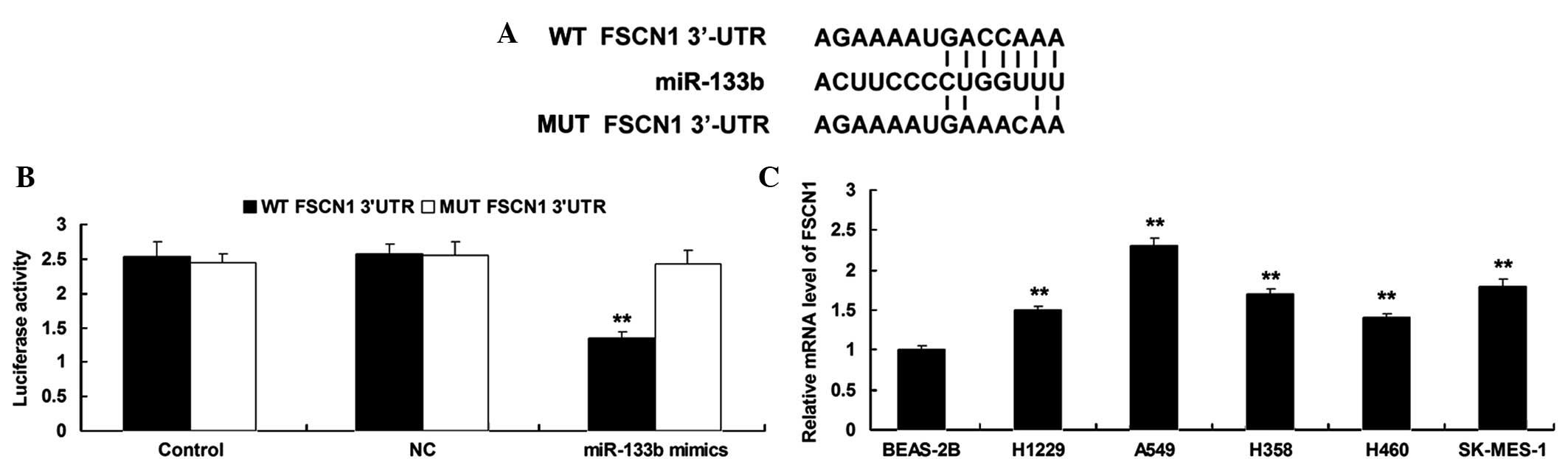 | Figure 2.(A) Seed sequences for miR-133b at the
WT or MT of FSCN1 3′UTR are shown. (B) Luciferase reporter assay
was performed to confirm whether FSCN1 is a target gene of
miR-133b. The luciferase activity was reduced only in NSCLC A549
cells co-transfected with miR-133b mimics and WT of FSCN1 3′UTR.
However, in other groups, the luciferase activity was unchanged.
**P<0.01 vs. Control. (C) Reverse transcription-polymerase chain
reaction was used to determine the mRNA expression level of FSCN1
in four NSCLC cell lines, H1229, A549, H358, H460 and SK-MES-1,
compared with normal human lung epithelial BEAS-2B cells.
**P<0.01 vs. BEAS-2B. miR, microRNA; WT, wild type; MUT, mutant
type; FSCN1, fascin1; UTR, untranslational region; NC, negative
control; mRNA, messenger RNA. |
The expression level of FSCN1 was then determined by
performing RT-qPCR on NSCLC cell lines, H1229, A549, H358, H460 and
SK-MES-1, and normal human lung epithelial BEAS-2B cells. As shown
in Fig. 2C, the mRNA levels of FSCN1
were significantly increased in NSCLC cell lines compared with
normal human lung epithelial BEAS-2B cells, respectively.
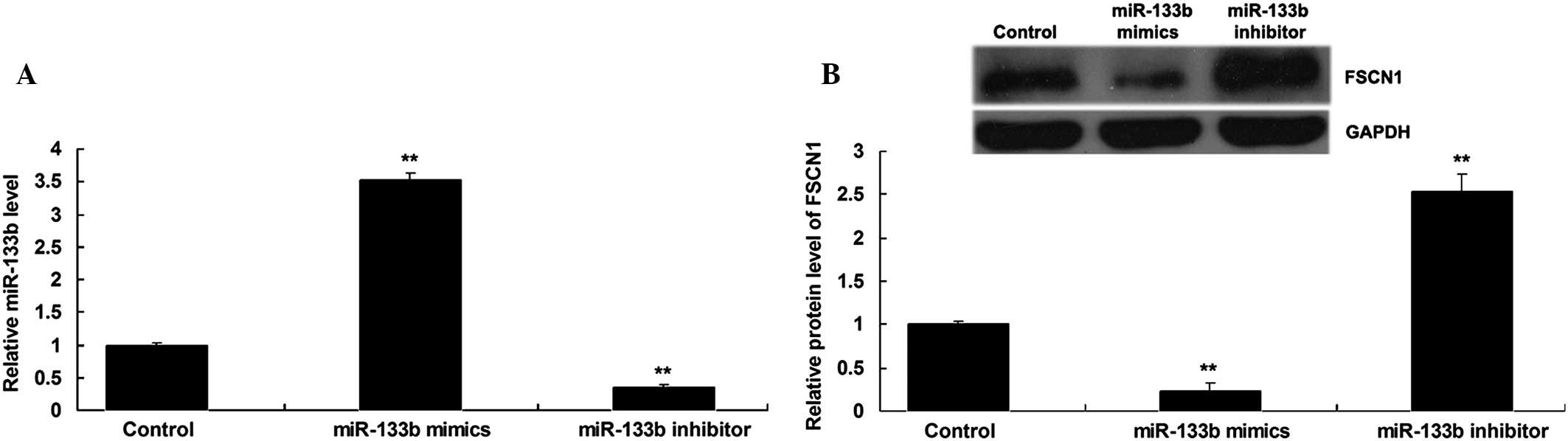
miR-133b negatively regulates FSCN1
expression in NSCLC cells
As miRs generally inhibit the expression of their
target genes at a post-transcriptional level (13), the present study further determined
whether miR-133b negatively regulated the FSCN1 expression in NSCLC
A549 cells. Following the transfection of NSCLC A549 cells with
miR-133b mimics or inhibitor, the expression level of miR-133b was
detected. As shown in Fig. 3A,
transfection with miR-133b mimics significantly enhanced the
expression level of miR-133b, while transfection with miR-133b
inhibitor significantly inhibited its expression. The protein
levels of FSCN1 were then determined by using western blot
analysis. The data showed that the overexpression of miR-133b
suppressed the protein level of FSCN1, while silencing of miR-133b
expression notably promoted the protein expression of FSCN1 in
NSCLC A549 cells (Fig. 3B).
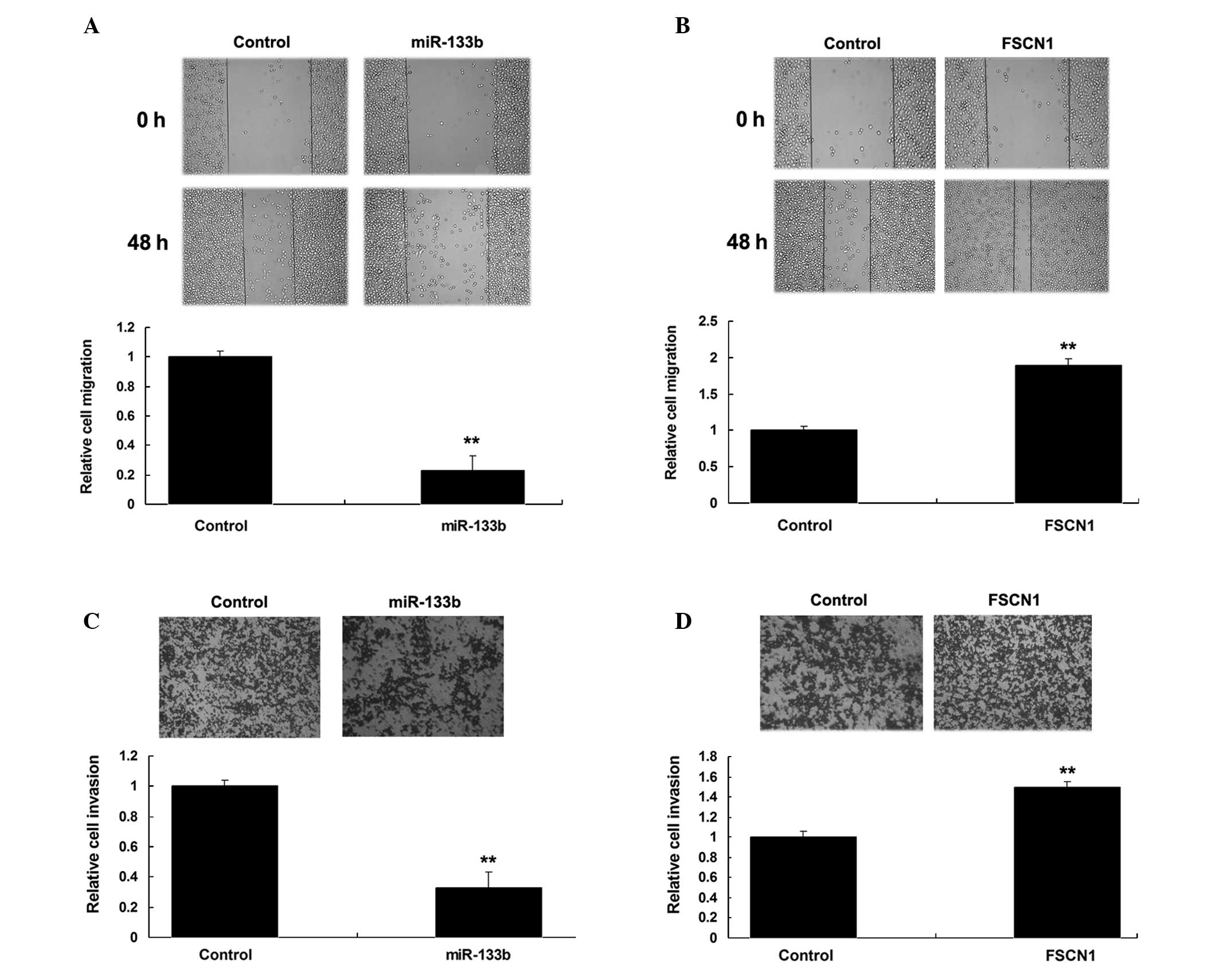
Roles of miR-133b and FSCN1 in the
regulation of migration and invasion of NSCLC cells
The wound heal assay and Transwell assay were then
performed to investigate the roles of FSCN1 and miR-133b in the
regulation of migration and invasion of NSCLC A549 cells. As shown
in Fig. 4A and B, overexpression of
miR-133b notably suppressed A549 cell migration and invasion.
Furthermore, FSCN1 plasmids were transfected into A549 cells. As
shown in Fig. 4C and D, the
overexpression of FSCN1 notably enhanced A549 cell migration and
invasion. Therefore, miR-133b and FSCN1 played contrary roles in
the regulation of migration and invasion of NSCLC A549 cells.
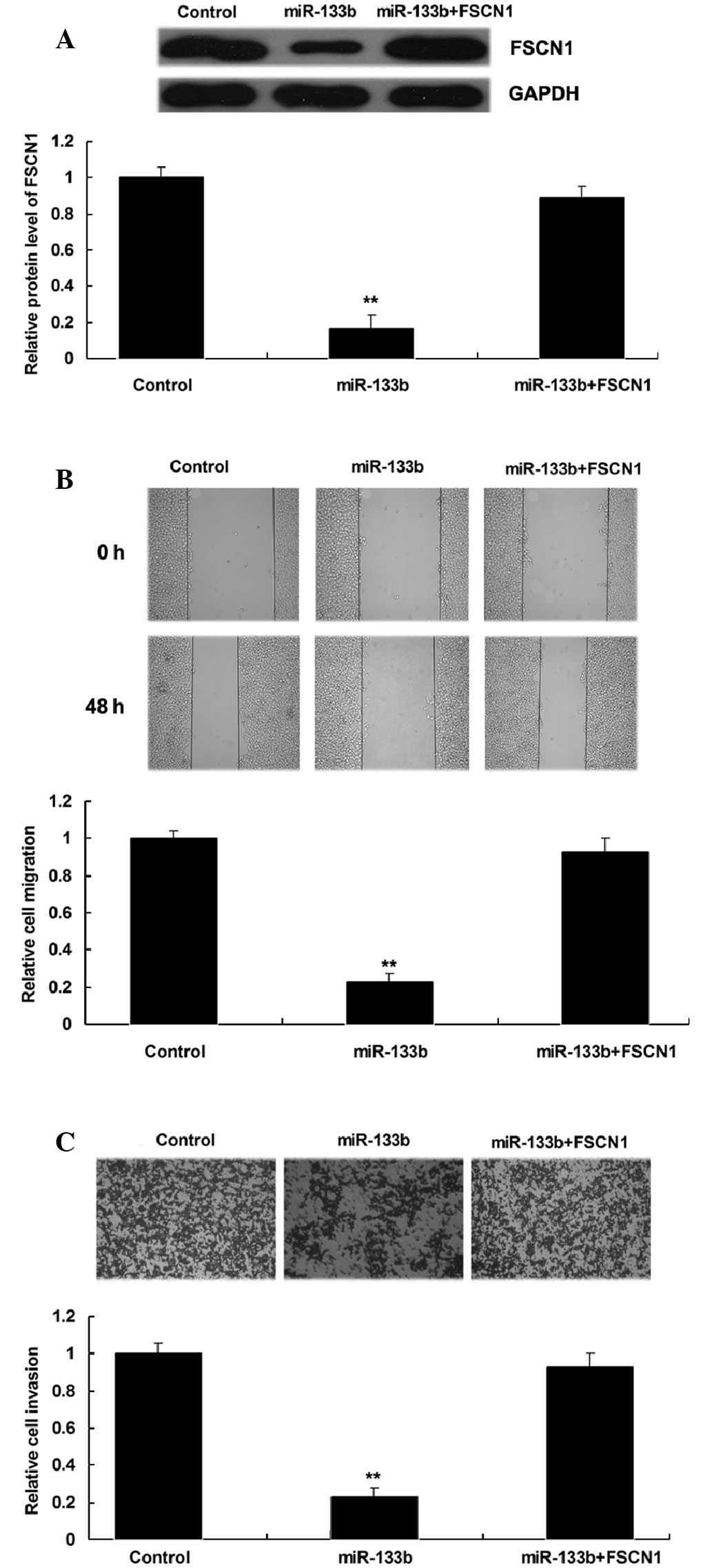
Upregulation of FSCN1 reverses the
inhibitory effect of miR-133b overexpression on NSCLC cell
migration and invasion
The present study additionally investigated whether
the role of miR-133b in the regulation of NSCLC cell migration and
invasion was through the modulation of FSCN1. NSCLC A549 cells were
transfected with miR-133b mimics, or co-transfected with miR-133b
mimics and a FSCN1 plasmid. The protein level of FSCN1 in each
group was then determined, which indicated that FSCN1 expression
was increased in A549 cells co-transfected with miR-133b mimics and
FSCN1 plasmids compared with A549 cells transfected with miR-133b
mimics only (Fig. 5A). The
overexpression of FSCN1 was also demonstrated to reverse the
inhibitory effect of miR-133b upregulation on A549 cell migration
and invasion (Fig. 5B and C). These
findings suggest that miR-133b inhibits the migration and invasion
of NSCLC A549 cells through targeting FSCN1.
Discussion
The present study showed that miR-133b was
significantly downregulated in NSCLC cell lines compared with
normal lung epithelial cells. Further investigation revealed that
FSCN1, upregulated in NSCLC cell lines, was a direct target of
miR-133b in NSCLC A549 cells, and that its protein expression was
negatively regulated by miR-133b in NSCLC cells. Upregulation of
miR-133b notably inhibited NSCLC cell migration and invasion, while
the overexpression of FSCN1 significantly promoted NSCLC cell
migration and invasion. Furthermore, overexpression of FSCN1
reversed the suppressive effect of miR-133b overexpression on NSCLC
cell migration and invasion.
The deregulation of miRs has been demonstrated to be
important for NSCLC growth and metastasis (14,15). For
instance, low miR-145 and high miR-367 expression are associated
with an unfavorable prognosis in patients with NSCLC (16). miR-145 can inhibit NSCLC cell
proliferation by targeting v-myc avian myelocytomatosis viral
oncogene homolog (17). In the
present study, miR-133b was frequently downregulated in NSCLC cell
lines compared with normal lung epithelial cells. It has been
previously reported that miR-133b is significantly downregulated in
lung tumor tissue compared with adjacent uninvolved tissue
(18). In addition, miR-133b was
indicated to be associated with tumor stage, the degree of regional
lymph node involvement, visceral pleura or vessel invasion and
epidermal growth factor receptor (EGFR) mRNA expression in Chinese
patients with NSCLC (9). Accordingly,
miR-133b may act as a tumor suppressor in NSCLC.
The regulatory mechanism of miR-133b in the
regulation of human cancers including NSCLC has been widely studied
(9,19,20).
Crawford et al suggested that two members of the B cell
lymphoma-2 (BCL-2) family, myeloid cell leukemia 1 (MCL-1) and
BCL-2 like 2, were targets of miR-133b, and that the overexpression
of miR-133b induced apoptosis following exposure to gemcitabine in
lung adenocarcinoma H2009 cells (18). In addition, EGFR was also found to be
a target of miR-133b, and the overexpression of miR-133b was
indicated to modulate apoptosis, invasion and sensitivity to
EGFR-tyrosine kinase inhibitor therapy through the EGFR signaling
pathways, particularly in EGFR-addicted NSCLC cells (9). Wu et al selected miR-133b as a
therapeutic target for lung cancer, and found that lipoplexes
delivered pre-miR-133b in a more efficient manner, with a ~2.3-fold
increase in mature miR-133b expression and ~1.8-fold difference in
MCL-1 protein downregulation in vitro, when compared with
siPORT NeoFX transfection agent (21). In addition, mice treated with
pre-miR-133b containing lipoplexes demonstrated mature miR-133b
expression in the lungs that was ~52-fold higher compared with
untreated mice (21). However, the
exact role of miR-133b in mediating NSCLC cell migration and
invasion and the detailed molecular mechanism, remains largely
unknown.
In the present study, the overexpression of miR-133b
notably inhibited NSCLC cell migration and invasion, suggesting
that miR-133b suppress the regulation of NSCLC metastasis.
Furthermore, FSCN1 was found to be a direct target of miR-133b in
NSCLC cells, and was involved in miR-133b-mediated NSCLC cell
migration and invasion. As a member of the FSCN family of
actin-binding proteins, FSCN1 is responsible for organization of
F-actin into parallel bundles and the formation of actin-based
cellular protrusions (22).
Therefore, FSCN1 is involved in the regulation of cell motility. In
the present study, FSCN1 was notably upregulated in NSCLC cell
lines, and the overexpression of FSCN1 reversed the suppressive
effect of miR-133b overexpression on NSCLC cell migration and
invasion, suggesting that FSCN1 plays an oncogenic role in NSCLC
(23–25). Similar findings have also been
reported in other types of human cancers; for instance, the
knockdown of FSCN1 was found to inhibit the proliferation and
invasion of gastric cancer cells (23). In addition, the inhibition of FSCN1
resulted in a reduced number of filopodia, an altered glioma cell
shape, and inhibited the migration and invasion of glioma cells
(24). FSCN1 was also found to be a
promoter of breast cancer invasion via the modification of
metastasis-associated molecules (25).
Previous studies have also reported that miR-133b
negatively regulates the expression of FSCN1 in other types of
human cancers, including esophageal squamous cell carcinoma (ESCC)
and gastric cancer (23,26,27). Kano
et al found that the overexpression of miR-133b inhibited
ESCC cell proliferation and invasion, that FSCN1 was a target of
miR-133b and that the knockdown of FSCN1 also suppressed ESCC cell
proliferation and invasion (26).
Yamamoto et al reported that FSCN1 was upregulated in
association with miR-133b downregulation in high-grade
gastrointestinal stromal tumors, and FSCN1 upregulation was
significantly correlated with a shorter disease-free survival time
and several aggressive pathological factors, which suggests that
the downregulation of miR-133b and overexpression of FSCN1 may be
important for the progression of gastrointestinal stromal tumor
(27). The data in the present study
expands the understanding of miR-133b and FSCN1 in human
cancers.
In summary, the present study indicates that
miR-133b suppresses the regulation of NSCLC cell migration and
invasion via targeting FSCN1, suggesting that miR-133b may be used
for the treatment of NSCLC.
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dimou A and Papadimitrakopoulou V:
Non-small cell lung cancer beyond biomarkers: The evolving
landscape of clinical trial design. J Pers Med. 4:386–401. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chouaid C, Crequit P, Borget I and
Vergnenegre A: Economic evaluation of first-line and maintenance
treatments for advanced non-small cell lung cancer: A systematic
review. Clinicoecon Outcomes Res. 7:9–15. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Landi L and Cappuzzo F: Pharmacotherapy
targeting the EGFR oncogene in NSCLC. Expert Opin Pharmacother.
15:2293–2305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Domvri K, Zarogoulidis P, Darwiche K,
Browning RF, Li Q, Turner JF, Kioumis I, Spyratos D, Porpodis K,
Papaiwannou A, et al: Molecular targeted drugs and biomarkers in
NSCLC, the evolving role of individualized therapy. J Cancer.
4:736–754. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Song Y, Wang Y, Luo J and Yu W:
MicroRNA-148a suppresses epithelial-to-mesenchymal transition by
targeting ROCK1 in non-small cell lung cancer cells. Mol Cell
Biochem. 380:277–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang M, Zhang P, Hu G, Xiao Z, Xu F,
Zhong T, Huang F, Kuang H and Zhang W: Relative expressions of
miR-205-5p, miR-205-3p and miR-21 in tissues and serum of non-small
cell lung cancer patients. Mol Cell Biochem. 383:67–75. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu L, Shao X, Gao W, Zhang Z, Liu P, Wang
R, Huang P, Yin Y and Shu Y: MicroRNA-133b inhibits the growth of
non-small-cell lung cancer by targeting the epidermal growth factor
receptor. FEBS J. 279:3800–3812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng K, Liu W, Liu Y, Jiang C and Qian Q:
MicroRNA-133a suppresses colorectal cancer cell invasion by
targeting Fascin1. Oncol Lett. 9:869–874. 2015.PubMed/NCBI
|
|
11
|
Zhao J, Zhou Y, Zhang Z, Tian F, Ma N, Liu
T, Gu Z and Wang Y: Upregulated fascin1 in non-small cell lung
cancer promotes the migration and invasiveness, but not
proliferation. Cancer Lett. 290:238–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boon RA and Vickers KC: Intercellular
transport of microRNAs. Arterioscler Thromb Vasc Biol. 33:186–192.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Yan S, Pei C and Cui Y: Decreased
microRNA-132 and its function in human non-small cell lung cancer.
Mol Med Rep. 11:3601–3608. 2015.PubMed/NCBI
|
|
15
|
Liu MX, Zhou KC and Cao Y: MCRS1
overexpression, which is specifically inhibited by miR-129*,
promotes the epithelial-mesenchymal transition and metastasis in
non-small cell lung cancer. Mol Cancer. 13:2452014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campayo M, Navarro A, Vinolas N, Diaz T,
Tejero R, Gimferrer JM, Molins L, Cabanas ML, Ramirez J, Monzo M
and Marrades R: Low miR-145 and high miR-367 are associated with
unfavourable prognosis in resected nonsmall cell lung cancer. Eur
Respir J. 41:1172–1178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Zeng H, Guo Y, Liu P, Pan H, Deng
A and Hu J: MiRNA-145 inhibits non-small cell lung cancer cell
proliferation by targeting c-Myc. J Exp Clin Cancer Res.
29:1512010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crawford M, Batte K, Yu L, Wu X, Nuovo GJ,
Marsh CB, Otterson GA and Nana-Sinkam SP: MicroRNA 133B targets
pro-survival molecules MCL-1 and BCL2L2 in lung cancer. Biochem
Biophys Res Commun. 388:483–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen XN, Wang KF, Xu ZQ, Li SJ, Liu Q, Fu
DH, Wang X and Wu B: MiR-133b regulates bladder cancer cell
proliferation and apoptosis by targeting Bcl-w and Akt1. Cancer
Cell Int. 14:702014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Li Y and Jiang C: MiR-133b
contributes to arsenic-induced apoptosis in U251 glioma cells by
targeting the hERG channel. J Mol Neurosci. 55:985–994. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Y, Crawford M, Yu B, Mao Y, Nana-Sinkam
SP and Lee LJ: MicroRNA delivery by cationic lipoplexes for lung
cancer therapy. Mol Pharm. 8:1381–1389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park SH, Song JY, Kim YK, Heo JH, Kang H,
Kim G, An HJ and Kim TH: Fascin1 expression in high-grade serous
ovarian carcinoma is a prognostic marker and knockdown of fascin1
suppresses the proliferation of ovarian cancer cells. Int J Oncol.
44:637–646. 2014.PubMed/NCBI
|
|
23
|
Guo L, Bai H, Zou D, Hong T, Liu J, Huang
J, He P, Zhou Q and He J: The role of microRNA-133b and its target
gene FSCN1 in gastric cancer. J Exp Clin Cancer Res. 33:992014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hwang JH, Smith CA, Salhia B and Rutka JT:
The role of fascin in the migration and invasiveness of malignant
glioma cells. Neoplasia. 10:149–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Al-Alwan M, Olabi S, Ghebeh H, Barhoush E,
Tulbah A, Al-Tweigeri T, Ajarim D and Adra C: Fascin is a key
regulator of breast cancer invasion that acts via the modification
of metastasis-associated molecules. PLoS One. 6:e273392011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kano M, Seki N, Kikkawa N, Fujimura L,
Hoshino I, Akutsu Y, Chiyomaru T, Enokida H, Nakagawa M and
Matsubara H: MiR-145, miR-133a and miR-133b: Tumor-suppressive
miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J
Cancer. 127:2804–2814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamamoto H, Kohashi K, Fujita A and Oda Y:
Fascin-1 overexpression and miR-133b downregulation in the
progression of gastrointestinal stromal tumor. Mod Pathol.
26:563–571. 2013. View Article : Google Scholar : PubMed/NCBI
|