Introduction
Cervical cancer is one of most common gynecological
malignancies (1) and the incidence is
increasing in China, where the age-specific incidence rate
increased from 8.76 to 23.1 cases per 100,000 individuals between
1993 and 2008 (2). Despite the
treatment of cervical cancer patients with surgery and adjuvant
therapy, such as radiotherapy and chemotherapy, the effectiveness
of treatment has not improved significantly over the past decades
(3,4).
In China, annual incidence and mortality rates have increased from
10.4 cases and 1.22 mortalities per 100,000 individuals,
respectively, to 13.4 cases and 2.59 mortalities per 100,000
individuals between 2003 and 2011 (5). Thus, it is important to identify
molecular markers that are able to predict the malignant phenotype
of cervical carcinoma (6,7).
Crk-like (CrkL) adapter protein has been reported to
be involved in numerous biological activities, such as cell
proliferation and migration, and plays an important role in
leukemia (8–11). CrkL proteins contain two Src homology
(SH) 3 domains and one N-terminal SH2 domain, which could bind
various docking proteins, including p130Cas, paxillin and Bcr-Abl
(9,12,13).
Recently, CrkL protein has been demonstrated to be overexpressed in
a number of types of human cancer, and to induce cancer cell
proliferation and invasion (14–18).
Overexpression of CrkL in fibroblast cells promotes
anchorage-independent growth (19).
Additionally, activating mutations of anaplastic lymphoma kinase
have been shown to exert this protein's downstream effects through
CrkL (20). Collectively, these
findings implicate CrkL as an important oncoprotein in human
cancers. However, the expression pattern and biological roles of
CrkL in cervical carcinoma remain unexplored.
In the present study, CrkL protein expression was
examined in specimens from 92 cases of cervical carcinoma. In
addition, CrkL expression was upregulated in HeLa and CaSki cell
lines and its effect on cell proliferation and apoptosis assessed.
Furthermore, the molecular signaling pathways underlying the
biological effects of CrkL were investigated.
Materials and methods
Patients and specimens
The study protocol was approved by the Institutional
Review Board of Shengjing Hospital of China Medical University
(Shenyang, China). Primary tumor specimens were obtained from
patients diagnosed with cervical carcinoma who underwent resection
at the First Affiliated Hospital of Jinzhou Medical University
(Jinzhou, China) and Shengjing Hospital of China Medical University
between January 2009 and December 2012. Normal endocervical tissues
were obtained from patients with benign uterine disease without
cervical dysplasia. The histological diagnosis was evaluated in
sections stained with hematoxylin and eosin, according to the World
Health Organization classification guidelines. Clinical and
histopathological data were obtained from medical records.
Immunohistochemistry
Surgically excised tumor specimens were fixed with
10% neutral formalin and embedded in paraffin, and 4 µm-thick
sections were prepared. Immunostaining was performed using the
Elivision Plus Polyer HRP (Mouse/Rabbit) IHC kit (Fuzhou Maixin
Biotech. Co., Ltd., Fuzhou, China). The sections were
deparaffinized in xylene, rehydrated with graded alcohol and then
boiled in 0.01 M citrate buffer (pH 6.0) for 2 min in an autoclave.
Hydrogen peroxide (0.3%) was applied to block endogenous peroxide
activity and the sections were incubated with normal goat serum to
reduce nonspecific binding. Tissue sections were incubated with an
anti-CrkL rabbit polyclonal antibody (dilution, 1:600; cat. no.
ABC242; EMD Millipore, Billerica, MA, USA;) at 4°C overnight. A
biotinylated goat anti-rabbit horseradish peroxidase polymer
(dilution, 1:800; cat. no. KIT-9902B; Fuzhou Maixin Biotech. Co.,
Ltd.) was used as a secondary antibody at room temperature for 30
min. After washing, the peroxidase reaction was developed with DAB.
Counterstaining with hematoxylin was performed and the sections
were dehydrated in ethanol prior to mounting.
Two independent investigators, who were blinded to
the patient characteristics, examined all tumor slides randomly:
Five views were examined per slide, and 100 cells were observed per
view at ×400 magnification. In accordance with previous reports,
immunostaining of CrkL was scored on a semi-quantitative scale by
evaluating the intensity and percentage of tumor cells (18,21).
Cytoplasmic and membrane immunostaining was considered positive
staining. After counting 400 tumor cells, the percentage of
positively stained cells was calculated. The intensity of CrkL
staining was scored as 0 (no signal), 1 (moderate) or 2 (strong).
Percentage scores were assigned as 1 (1–25%), 2 (26–50%), 3
(51–75%) or 4 (76–100%). The scores of each tumor sample were
multiplied to give a final score of 0–8; tumor samples that scored
4–8 were considered to exhibit CrkL overexpression.
Cell culture and transfection
HeLa and CaSki cell lines were obtained from the
American Type Culture Collection (Manassas, VA, USA). Cells were
cultured in Invitrogen Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc., Carlsbad, CA, USA) containing 10%
fetal calf serum (FCS) (Invitrogen; Thermo Fisher Scientific,
Inc.), 100 IU/ml penicillin (Sigma-Aldrich, St. Louis, MO, USA),
and 100 µg/ml streptomycin (Sigma-Aldrich). Cells were grown in
sterilized culture dishes and were passaged every 2 days with 0.25%
trypsin.
The plasmid pCMV6-CrkL was purchased from OriGene
Technologies, Inc. (Rockville, MD, USA). Plasmid was transfected
into cells using Lipofectamine LTX reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). pCMV6 empty vector (pCMV6 EV) was used as
a negative control. Cisplatin (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA). was dissolved in dimethyl sulfoxide (DMSO)
and 10 µM cisplatin was used to treat cancer cells for 24 h. DMSO
was used as the negative control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) using SYBR Green method
Total RNA was extracted from HeLa and CaSki cells
using Trizol (Life Technologies; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. RNA was reverse
transcribed into cDNA using PrimerScript RT Master Mix kit (Takara
Bio, Dalian, China) at 85°C for 2 min and 37°C for 30 min. Briefly,
20 µl reverse-transcription reaction solution was prepared using 5X
RT Master Mix (4 µl) and 800 ng RNA. RT-qPCR was performed using
SYBR Select PCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) in a total volume of 20 µl on an Applied
Biosystems 7300 Real-Time PCR System (Thermo Fisher Scientific,
Inc.), with the following conditions: 95°C for 30 sec; and 40
cycles of 95°C for 5 sec and 60°C for 30 sec. The PCR solution (20
µl) consisted of 5 µl cDNA, 0.5 µl forward primer, 0.5 µl reverse
primer, 4 µl; H2O and 10 µl SYBRgreen Master mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.). A
dissociation step was performed to generate a melting curve to
confirm the specificity of the amplification. β-actin was used as
the reference gene. The relative levels of gene expression were
calculated by the 2−ΔΔCq method (22). The primer sequences were as follows:
CrkL forward, 5′-CCTTTGCCATCCACACAGAAT-3′, CrkL reverse,
5′-TTTCACGATGTCACCAACCTCTA-3′; β-actin forward,
5′-ATAGCACAGCCTGGATAGCAACGTAC-3′, and β-actin reverse,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′. All experiments were performed in
triplicate.
Western blot analysis
Total proteins from HeLa and CaSki cells were
extracted and quantified using the Bradford method, and 20 mg
protein was separated by SDS-PAGE. Samples were transferred to
polyvinylidene difluoride membranes (EMD Millipore) and incubated
overnight at 4°C with antibodies against CrkL (rabbit polyclonal;
dilution, 1:1,000; cat. no. ABC242; EMD Millipore), p-Src (rabbit
monoclonal; dilution, 1:1,000; cat. no. 12432; Cell Signaling
Technology, Inc., Boston, MA, USA), Src (rabbit monoclonal;
dilution, 1:1,000; cat. no. 2109; Cell Signaling Technology, Inc.),
p-Akt (rabbit polyclonal; dilution, 1:1,000; cat. no. 9271; Cell
Signaling Technology, Inc.), Akt (rabbit polyclonal; dilution,
1:1,000; cat. no. 9272; Cell Signaling Technology, Inc.; dilution,
1:1,000) and GAPDH (rabbit polyclonal; dilution, 1:1,000; cat. no.
G5262; Santa Cruz Biotechnology, Inc.). Following incubation with
peroxidase-coupled anti-mouse/rabbit IgG (dilution, 1:2,000; cat.
no. 5127; Cell Signaling Technology, Inc.) at 37°C for 2 h,
proteins were visualized using Pierce ECL Western Blotting
Substrate (Thermo Fisher Scientific, Inc.) and detected using a DNR
Bio-Imaging System (DNR Bio-Imaging Systems Ltd., Jerusalem,
Israel).
Methyl thiazolyl tetrazolium (MTT)
assay
For the MTT cell viability assay, at 24 h after
transfection, cells were plated in 96-well plates at a
concentration of ~2,000 cells/well and cultured for 5 days. To
quantitate cell viability, 20 µl of 5 mg/ml MTT (thiazolyl blue)
solution was added to each well and incubated for 4 h at 37°C. The
medium was removed from each well and the resulting MTT formazan
was solubilized in 150 µl of DMSO. Culture medium without cells was
used as the control. Each solution was measured
spectrophotometrically at 490 nm.
Cell invasion assay
A cell invasion assay was performed using a Costar
24-well Transwell chamber with a pore size of 8 µm. The inserts
were coated with 20 µl Matrigel (1:4dilution; BD Biosciences, San
Jose, CA, USA). At 48 h after the transfection, cells were
trypsinized and 3×105 cells in 100 µl of serum-free
medium were transferred to the upper Matrigel-coated chamber and
incubated for 16 h; the lower chamber contained medium supplemented
with 10% FCS as the chemoattractant. Following incubation, the
non-invaded cells on the upper membrane surface were removed with a
cotton tip, and the cells that passed through the filter were fixed
with 4% paraformaldehyde and stained with hematoxylin. The number
of invaded cells was counted in 10 randomly selected high-power
fields under a microscope (BX53; Olympus Corporation, Tokyo,
Japan). This experiment was performed in triplicate.
Statistical analysis
SPSS software version 11.5 for Windows (SPSS, Inc.,
Chicago, IL, USA) was used for all statistical analyses. A
χ2 test was used to examine the possible correlations
between CrkL expression and clinicopathological factors. A
Student's t-test was used to compare densitometry data
between control and CrkL-transfected cells. All P-values are based
on a two-sided statistical analysis, and P<0.05 was considered
to indicate statistical significance.
Results
Expression of CrkL in human cervical
carcinoma
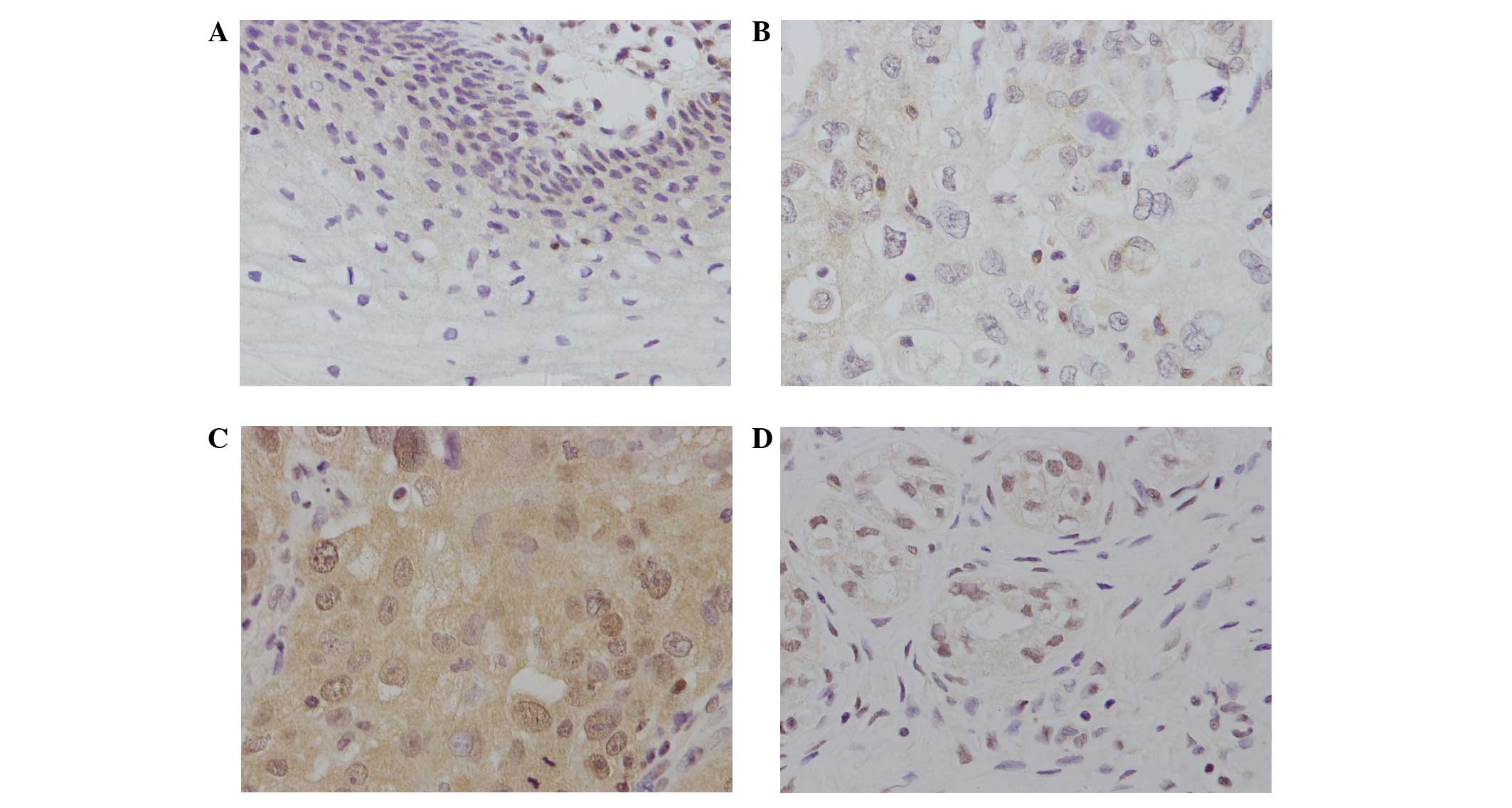
CrkL expression was located in the nucleus or
cytoplasm of cancer cells as brown staining, but could not be
easily detected in normal cervical tissues (Fig. 1A). Of the 92 cervical cancer tissues,
45 (48.9%) exhibited positive CrkL staining (Fig. 1B-D). The association of CrkL
overexpression with clinicopathological characteristics was
analyzed (Table I). The results
indicate that positive CrkL immunostaining in cervical carcinoma
was significantly associated with advanced TNM stage (stage II+III
vs. stage I, P=0.0165) and lymph node metastasis (negative vs.
positive, P=0.0212). No significant association was identified
between CrkL level and other parameters, including age (<50 vs.
≥50 years, P=0.2106), histological type (squamous cell carcinoma
vs. adenocarcinoma, P=0.9419) and differentiation (well/moderate
vs. poor, P=0.5647).
 | Table I.Distribution of CrkL status in
cervical carcinoma according to clinicopathological
characteristics. |
Table I.
Distribution of CrkL status in
cervical carcinoma according to clinicopathological
characteristics.
|
|
| CrkL status, n |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Total patients,
n | Weak/negative | Positive | P-value |
|---|
| Age, years |
|
|
| 0.2106 |
|
<50 | 61 | 34 | 27 |
|
| ≥50 | 31 | 13 | 18 |
|
| Histological
type |
|
|
| 0.9419 |
| Squamous
cell carcinoma | 82 | 42 | 40 |
|
|
Adenocarcinoma | 10 | 5 | 5 |
|
| Differentiation |
|
|
| 0.5647 |
|
Well/moderate | 67 | 33 | 34 |
|
|
Poor | 25 | 14 | 11 |
|
| TNM stage |
|
|
| 0.0165 |
| I | 36 | 24 | 12 |
|
|
II+III | 56 | 23 | 33 |
|
| T stage |
|
|
| 0.0972 |
| T1 | 49 | 29 | 20 |
|
|
T2+3 | 43 | 18 | 25 |
|
| Lymph node
metastasis |
|
|
| 0.0212 |
|
Negative | 56 | 34 | 22 |
|
|
Positive | 36 | 13 | 23 |
|
CrkL promotes cervical carcinoma cell
growth, invasion and chemoresistance
To determine the effects of CrkL on cervical cancer
cell lines, HeLa and CaSki cells were transfected with plasmids
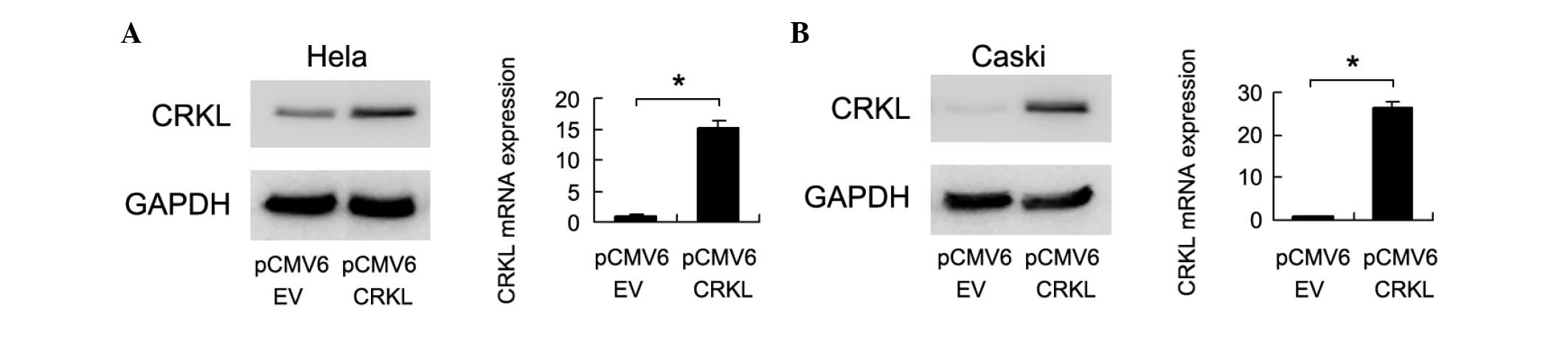
containing CrkL. As shown in Fig. 2,
CrkL transfection increased its protein and mRNA levels in the two
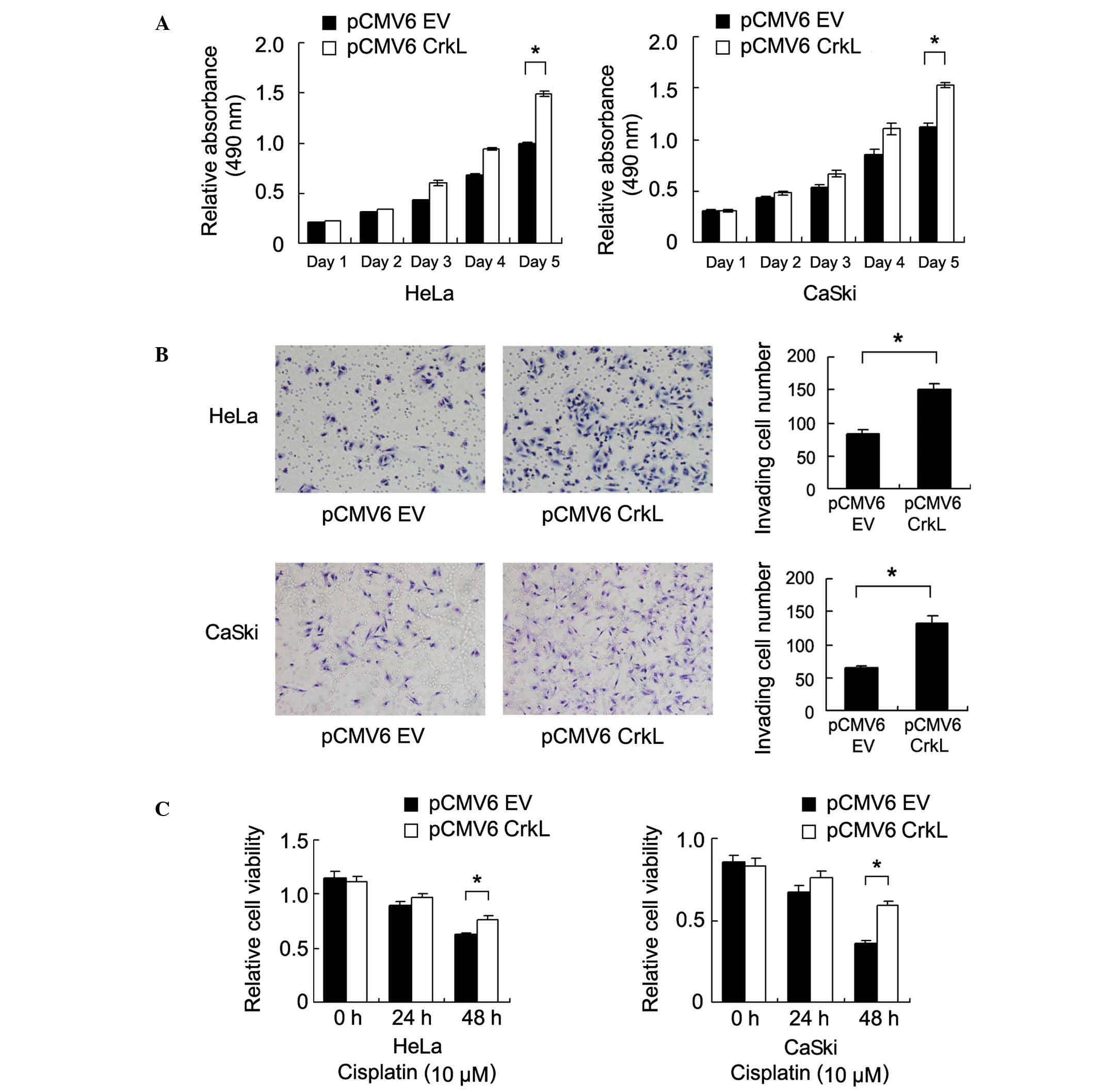
cell lines. An MTT assay revealed that CrkL upregulation increased
the rate of cell growth [Day 5, pCMV6 EV vs. CrkL: HeLa,
0.993±0.009 vs. 1.496±0.026 (P=0.002); CaSki, 1.126±0.028 vs.
1.527±0.038 (P=0.007)] (Fig. 3A).
A Matrigel invasion assay was also conducted to
assess the role of CrkL in cell invasion. As shown in Fig. 3B, CrkL transfection significantly
increased the invasion ability of HeLa and CaSki cells [pCMV6 EV
vs. CrkL: HeLa, 83±7 vs. 151±8 (P=0.013); CaSki, 65±3 vs. 132±11
(P=0.014)] (Fig. 3B).
In order to investigate the role of CrkL on the
chemoresistance of cervical carcinoma cells, control cells and
CrkL-transfected cells were treated with cisplatin (10 µM) followed
by analysis of cell viability using MTT. Compared with the control
group, CrkL transfection significantly increased cell viability
following 48 h of cisplatin treatment (Fig. 3C), suggesting that CrkL is important
in chemoresistance of cervical cancer cells.
CrkL promotes cell invasion through
Src-dependent pathways
In order to investigate the molecular pathways
underlying CrkL-induced cell growth and invasion, several signaling
pathways were examined. The levels of Src and Akt phosphorylation
were significantly increased following CrkL transfection (Fig. 4A).
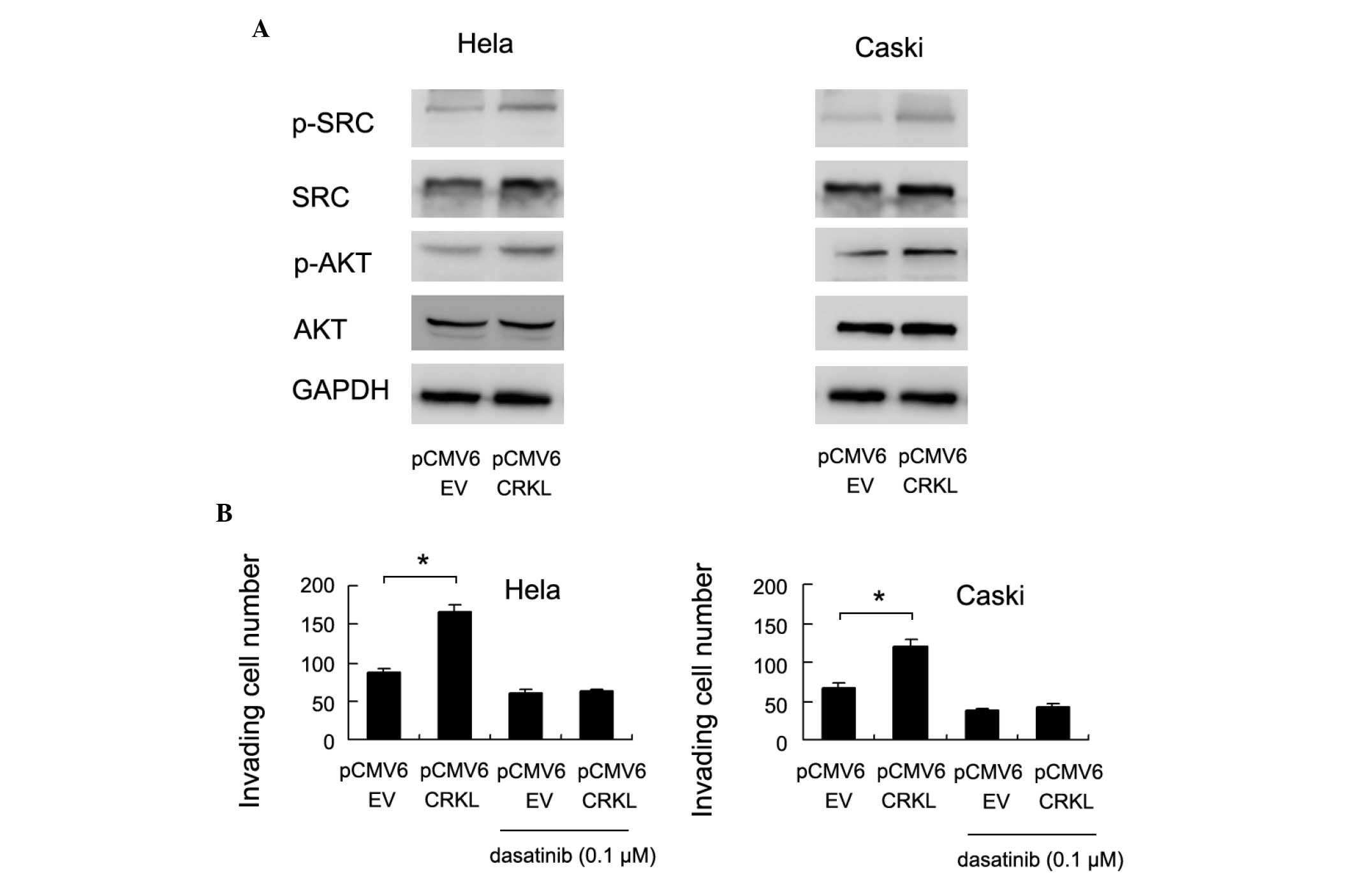 | Figure 4.CrkL promotes cervical cancer cell
invasion through Src signaling pathways. (A) Western blot analysis
revealed that transfection with the CrkL plasmid (pCMV6 CrkL)
significantly upregulated the level of p-Src and p-Akt, without
significant changes in the total amounts of Src and Akt. (B) The
Src inhibitor dasatinib (0.1 µM, 3 h) blocked the effects of CrkL
on cancer cell invasion (Hela, *P=0.003, CRKL plasmid vs. CRKL
plasmid + dasatinib; Caski, *P=0.006, CRKL plasmid vs. CRKL plasmid
+ dasatinib). CrkL, Crk-like; p-, phosphorylated; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; pCMV6 EV, empty
vector. |
Src activation has been reported to be associated
with cancer cell invasion (23). To
validate its involvement in CrkL-induced cervical cancer invasion,
the Src inhibitor dasatinib (0.1 µM, 3 h) was employed to treat
CrkL-transfected and control cells. As shown in Fig. 4B, dasatinib treatment eliminated the
effect of CrkL on cell invasion in HeLa and CaSki cell lines
(Fig. 4B).
Discussion
The expression and biological functions of CrkL have
been implicated in numerous types of human malignancy, including
breast, lung, pancreatic and colorectal carcinomas (17,18,21,24).
However, the involvement of CrkL in human cervical carcinoma has
not been reported. The present study examined CrkL protein
expression in 92 cases of cervical carcinoma and found that CrkL
was overexpressed in 48.9%. Statistical analysis indicated that
CrkL overexpression was correlated with advanced TNM grade and
lymph node metastasis, suggesting its association with cervical
cancer invasion. The present study revealed that CrkL is
overexpressed in human cervical carcinoma and is associated with an
advanced stage of disease, which is in accordance with previous
data that confirmed CrkL as an oncogene (24–26).
CrkL contains SH2 and SH3 domains that mediate
protein-protein interactions and regulate diverse cellular
processes (21,27–29).
Several studies have suggested that CrkL may be involved in the
progression of solid tumors by regulating cell proliferation and
invasion (28–30). In the current study, HeLa and CaSki
cells were transfected with a CrkL plasmid and the effects on cell
proliferation and invasion were examined. In support of the
immunohistochemical results, CrkL transfection significantly
upregulated the rate of cell growth and invasion ability in of the
two cell lines.
To the best of our knowledge, the association of
CrkL and chemoresistance has not been reported previously. In the
current study, CrkL transfection was found to increase cervical
cancer cell viability following cisplatin treatment, which suggests
that CrkL may function as an important modifier of chemoresistance
in cervical carcinoma cells.
The potential mechanism of CrkL in cervical cancer
cell invasion was also explored. Previous reports have indicated
that CrkL functions as an adaptor protein that links Src and C3G
proteins (31,32). Increased activity of Src is a frequent
occurrence in many types of human cancer, and there is growing
evidence of a prominent role of Src in invasion and in other tumor
progression-related events, such as the epithelial-mesenchymal
transition and development of metastasis (33–35). Thus,
the present study assessed the level of Src phosphorylation in
CrkL-overexpressing HeLa and CaSki cell lines, and determined that
CrkL transfection significantly upregulated Src phosphorylation,
suggesting that Src may be involved in CrkL-induced cell invasion.
To further validate this, the Src inhibitor dasatinib was employed.
Dasatinib treatment significantly downregulated the invasion
ability of cervical cancer cells and eliminated the
invasion-promoting function of CrkL. These results demonstrate that
CrkL may promote cervical cancer cell invasion through activation
of Src signaling pathways. In addition, CrkL was also found to
promote Akt phosphorylation; Akt signaling activation has been
reported to be involved in the chemoresistance of cancer cells
through antiapoptotic proteins such as Bcl-2 and Bcl-xL (36,37). Thus
it is possible that CrkL increases cervical cancer chemoresistance
through activating Akt signaling.
In conclusion, CrkL is overexpressed in human
cervical carcinomas and is associated with advanced stage and nodal
metastasis. CrkL may also increase cervical cancer cell
proliferation and chemoresistance, and promote tumor invasion
through activation of Src signaling. Thus, CrkL may potentially
serve as a novel therapeutic target for cervical carcinoma.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang T, Wu MH, Wu YM and Zhang WY: A
population-based study of invasive cervical cancer patients in
Beijing: 1993–2008. Chin Med J (Engl). 128:3298–3304. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Angioli R, Plotti F, Luvero D, Aloisi A,
Guzzo F, Capriglione S, Terranova C, De Cicco Nardone C and
Benedetti-Panici P: Feasibility and safety of carboplatin plus
paclitaxel as neoadjuvant chemotherapy for locally advanced
cervical cancer: A pilot study. Tumour Biol. 35:2741–2746. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Organista-Nava J, Gómez-Gómez Y and
Gariglio P: Embryonic stem cell-specific signature in cervical
cancer. Tumour Biol. 35:1727–1738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du PL, Wu KS, Fang JY, Zeng Y, Xu ZX, Tang
WR, Xu XL and Lin K: Cervical cancer mortality trends in China,
1991–2013, and predictions for the future. Asian Pac J Cancer Prev.
16:6391–6396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wentzensen N, Schwartz L, Zuna RE, Smith
K, Mathews C, Gold MA, Allen RA, Zhang R, Dunn ST, Walker JL and
Schiffman M: Performance of p16/Ki-67 immunostaining to detect
cervical cancer precursors in a colposcopy referral population.
Clin Cancer Res. 18:4154–4162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwarz JK, Payton JE, Rashmi R, Xiang T,
Jia Y, Huettner P, Rogers BE, Yang Q, Watson M, Rader JS and
Grigsby PW: Pathway-specific analysis of gene expression data
identifies the PI3K/Akt pathway as a novel therapeutic target in
cervical cancer. Clin Cancer Res. 18:1464–1471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rhodes J, York RD, Tara D, Tajinda K and
Druker BJ: CrkL functions as a nuclear adaptor and transcriptional
activator in Bcr-Abl-expressing cells. Exp Hematol. 28:305–310.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feller SM: Crk family adaptors-signalling
complex formation and biological roles. Oncogene. 20:6348–6371.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fidler IJ and Kripke ML: Genomic analysis
of primary tumors does not address the prevalence of metastatic
cells in the population. Nat Genet. 34(23): author reply 25.
2003.PubMed/NCBI
|
|
11
|
ten Hoeve J, Morris C, Heisterkamp N and
Groffen J: Isolation and chromosomal localization of CRKL, a human
crk-like gene. Oncogene. 8:2469–2474. 1993.PubMed/NCBI
|
|
12
|
Sattler M, Salgia R, Shrikhande G, Verma
S, Pisick E, Prasad KV and Griffin JD: Steel factor induces
tyrosine phosphorylation of CRKL and binding of CRKL to a complex
containing c-kit, phosphatidylinositol 3-kinase, and p120 (CBL). J
Biol Chem. 272:10248–10253. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oda T, Heaney C, Hagopian JR, Okuda K,
Griffin JD and Druker BJ: Crkl is the major tyrosine-phosphorylated
protein in neutrophils from patients with chronic myelogenous
leukemia. J Biol Chem. 269:22925–22928. 1994.PubMed/NCBI
|
|
14
|
Beroukhim R, Mermel CH, Porter D, Wei G,
Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J,
Urashima M, et al: The landscape of somatic copy-number alteration
across human cancers. Nature. 463:899–905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Senechal K, Halpern J and Sawyers CL: The
CRKL adaptor protein transforms fibroblasts and functions in
transformation by the BCR-ABL oncogene. J Biol Chem.
271:23255–23261. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van 't Veer LJ, Dai H, van de Vijver MJ,
He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ,
Witteveen AT, et al: Gene expression profiling predicts clinical
outcome of breast cancer. Nature. 415:530–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin F, Chengyao X, Qingchang L, Qianze D,
Enhua W and Yan W: CRKL promotes lung cancer cell invasion through
ERK-MMP9 pathway. Mol Carcinog. 54(Suppl 1): E35–E44. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Dong QZ, Fu L, Stoecker M, Wang E
and Wang EH: Overexpression of CRKL correlates with poor prognosis
and cell proliferation in non-small cell lung cancer. Mol Carcinog.
52:890–899. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Senechal K, Heaney C, Druker B and Sawyers
CL: Structural requirements for function of the Crkl adapter
protein in fibroblasts and hematopoietic cells. Mol Cell Biol.
18:5082–5090. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schönherr C, Yang HL, Vigny M, Palmer RH
and Hallberg B: Anaplastic lymphoma kinase activates the small
GTPase Rap1 via the Rap1-specific GEF C3G in both neuroblastoma and
PC12 cells. Oncogene. 29:2817–2830. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao T, Miao Z, Wang Z, Xu Y, Wu J, Liu X,
You Y and Li J: Overexpression of CRKL correlates with malignant
cell proliferation in breast cancer. Tumour Biol. 34:2891–2897.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao S, Li H, Wang Q, Su C, Wang G, Song
H, Zhao L, Luan Z and Su R: The role of c-Src in the invasion and
metastasis of hepatocellular carcinoma cells induced by association
of cell surface GRP78 with activated α2M. BMC Cancer. 15:3892015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tamura M, Sasaki Y, Kobashi K, Takeda K,
Nakagaki T, Idogawa M and Tokino T: CRKL oncogene is downregulated
by p53 through miR-200s. Cancer Sci. 106:1033–1040. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ladanyi M: CRKL as a lung cancer oncogene
and mediator of acquired resistance to EGFR inhibitors: Is it all
that it is cracked up to be? Cancer Discov. 1:560–561. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koval AP, Karas M, Zick Y and LeRoith D:
Interplay of the proto-oncogene proteins CrkL and CrkII in
insulin-like growth factor-I receptor-mediated signal transduction.
J Biol Chem. 273:14780–14787. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu L, Dong Q, Xie C, Wang Y and Li Q: CRKL
protein overexpression enhances cell proliferation and invasion in
pancreatic cancer. Tumour Biol. 36:1015–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singer CF, Hudelist G, Lamm W, Mueller R,
Handl C, Kubista E and Czerwenka K: Active (p)CrkL is overexpressed
in human malignancies: Potential role as a surrogate parameter for
therapeutic tyrosine kinase inhibition. Oncol Rep. 15:353–359.
2006.PubMed/NCBI
|
|
29
|
Kim YH, Kwei KA, Girard L, Salari K, Kao
J, Pacyna-Gengelbach M, Wang P, Hernandez-Boussard T, Gazdar AF,
Petersen I, et al: Genomic and functional analysis identifies CRKL
as an oncogene amplified in lung cancer. Oncogene. 29:1421–1430.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fathers KE, Bell ES, Rajadurai CV, Cory S,
Zhao H, Mourskaia A, Zuo D, Madore J, Monast A, Mes-Masson AM, et
al: Crk adaptor proteins act as key signaling integrators for
breast tumorigenesis. Breast Cancer Res. 14:R742012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Graf R, Barbero S, Keller N, Chen L, Uryu
S, Schlaepfer D and Stupack D: Src-inducible association of CrkL
with procaspase-8 promotes cell migration. Cell Adh Migr.
7:362–369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen Q, Rahn JJ, Zhang J, Gunasekera N,
Sun X, Shaw AR, Hendzel MJ, Hoffman P, Bernier A and Hugh JC: MUC1
initiates Src-CrkL-Rac1/Cdc42-mediated actin cytoskeletal
protrusive motility after ligating intercellular adhesion
molecule-1. Mol Cancer Res. 6:555–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guarino M: Src signaling in cancer
invasion. J Cell Physiol. 223:14–26. 2010.PubMed/NCBI
|
|
34
|
Cui A, Hua H, Shao T, Song P, Kong Q, Luo
T and Jiang Y: Aflatoxin B1 induces Src phosphorylation and
stimulates lung cancer cell migration. Tumour Biol. 36:6507–6513.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun B, Yang N, Jiang Y, Zhang H, Hou C, Ji
C, Liu Y and Zuo P: Antagomir-1290 suppresses CD133+
cells in non-small cell lung cancer by targeting fyn-related Src
family tyrosine kinase. Tumour Biol. 36:6223–6230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang JH, Nao JF, Zhang M and He P: 20
(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer
HO-8910 cells through PI3K/Akt and XIAP pathways. Tumour Biol.
35:11985–11994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ye G, Lu Q, Zhao W, Du D, Jin L and Liu Y:
Fucoxanthin induces apoptosis in human cervical cancer cell line
HeLa via PI3K/Akt pathway. Tumour Biol. 35:11261–11267. 2014.
View Article : Google Scholar : PubMed/NCBI
|