Introduction
Breast cancer is the most prevalent malignancy in
women, accounting for ~29% of all female cancer cases (1). Despite progress in early diagnosis and
treatment, breast cancer remains the second most frequent cause of
cancer-associated mortality in women in United States (1). Traditionally, histologic tumor grade,
tumor size, estrogen receptor (ER) and progesterone receptor (PgR)
expression, axillary lymph node involvement, Ki-67 expression and
human epidermal growth factor receptor-2 (HER-2) status have been
employed as prognostic factors for breast cancer patients.
The association between tumorigenesis, hypoxia and
tumor development is well-documented (2). Tumor hypoxia is facilitated by various
factors, including tumor localization, tumor size and blood flow,
all of which affect oxygen accessibility (3). The most recognized hypoxia signaling
factor is hypoxia-inducible factor (HIF)-1 (3). HIF-1 is made up of two subunits: HIF-1α
and HIF-1β (4,5). While HIF-1β is expressed constitutively,
HIF-1α is only expressed during hypoxic conditions and is a
measurement of overall activity (4–7). Increased
levels of HIF-1α have been documented in multiple tumors, in
particular breast cancer (8).
Notably, cells under low oxygen stress often switch to aerobic
glycolysis (lactate production), a low energy-generating state,
from oxidative phosphorylation, which is termed the Warburg effect
(9). Previous studies have reported
that aerobic glycolysis increases ~2-fold under acute hypoxia,
which results in augmented cytoplasmic hexokinase and
membrane-localized glucose transporters (GLUT), including GLUT-1
and GLUT-3 (10). Although hypoxia
has been generally recognized for its impact on glucose metabolism,
certain hypoxic tumors demonstrate marginal increases in glucose
uptake, glycolytic shift and lactate production (9).
Augmented and differential glucose metabolism was
established in 1931 by Otto Warburg (11). A measure of glucose uptake is an
important feature of the Warburg effect phenomenon and may be
measured in tumors with 18F-fluorodeoxyglucose (FDG)
tracers and positron emission tomography (PET). FDG-PET provides
semiquantitative measurement of glucose standardized uptake value
(SUV), which may aid in predicting tumor activity, treatment
monitoring, staging and detection of disease recurrence (12,13). The
level of FDG uptake in breast tumors has been documented for its
heterogeneity compared with other forms of cancer (14). Notably, breast cancer FDG uptake is
reported to be associated with several tumorigenic factors,
including histologic grade, tumor size and hormone receptor
expression levels, among others (15–17). Based
on this, breast cancer with augmented glucose uptake is more
aggressive compared to breast cancer with low glucose uptake
(15–17).
The present study aimed to investigate the
association between FDG uptake and HIF-1α expression in breast
cancer. The link between known breast cancer parameters (tumor
size, axillary lymph node involvement, ER and PgR expression,
HER-2, Ki-67 expression, grade and histology) and FDG uptake was
also evaluated.
Materials and methods
Patient characteristics and tissue
samples
A total of 92 patients, who were diagnosed with no
particular type of breast carcinoma between August 2013 and April
2015 in the Department of Surgical Oncology, Süleyman Demirel
University (Isparta, Turkey), were included in the study. Diagnosis
of primary breast cancer was defined by core-needle biopsy at least
15 days prior to PET/computed tomography (CT). Initial diagnosis
and re-analysis of biopsies were performed by a pathologist
(Department of Pathology, Süleyman Demirel University) for
diagnostic confirmation. The tumors were subjected to the modified
Scarff-Bloom-Richardson grading system (18) and categorized based on the World
Health Organization classification system (19). FDG-PET analysis was performed on all
92 patients between August 2013 and April 2015 to determine the
stage of the disease prior to therapy. Patients were eligible for
surgery if no indication of distant metastatic spread was
determined. Patients diagnosed with excisional biopsy and/or
lesions <1 cm (based on CT images) were not included in the
study. Each individual was subjected to surgery three weeks after
FDG-PET examination. No patients received neoadjuvant therapy. The
study was approved by the Ethics Committee of the Süleyman Demirel
Universty and informed consent was obtained from all patients prior
to examination.
FDG-PET image acquisition
Whole-body FDG-PET scans were perfomed as described
using the Philips Gemini TF PET/CT scanner (Philips Medical Systems
B.V., Eindhoven, The Netherlands). Patients were prepared by a 6-h
fast, as serum glucose levels had to be <150 mg/dl prior to
glucose tracer administration. At 60 min after the intravenous
injection of 3.7 MBq/kg (0.1 mCi/kg) 18F-FDG (Monrol,
Eczacıbaşı, İstanbul, Turkey), PET/CT was performed. Subsequently,
an emission scan was recorded in three-dimensional mode following
CT for 2 min per position. PET and CT images were examined in the
cross-sectional planes view and in the rotating maximum-intensity
projection. FDG uptake in the tumor and lymph nodes were
semiquantified using maximum SUV (SUVmax).
Immunohistochemistry
HIF-1α expression was evaluated by
immunohistochemistry using monoclonal rabbit anti-human HIF-1α
antibodies (clone, EP1215Y; dilution, 1:100; Abcam, Cambridge, MA,
USA). A biotynilated goat anti-polyvalent secondary antibody
(TP-125-BN; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
experiment was run in parallel as a negative control and human
ovarian carcinoma was used as positive control. The
avidin-biotin-peroxidase complex method was used while performing
immunostaining. Immunohistochemistry was performed on
formalin-fixed, paraffin-embedded resection specimens. Tissues were
kept in 10% formalin at room temperature for overnight fixation and
processed in a fully automated tissue processor. Sections from
paraffin-embedded tissues (4 µm thick) were obtained for
immunohistochemical staining. The level of staining was determined
using a light microscope. In the evaluation of immunostaining,
nuclear immunoreactivity in neoplastic cells was considered as
positive. A value ≥10% was set as the cut-off to distinguish
positive and negative immunoreactivity (20).
Statistical analysis
Median and interquartile ranges (IQR) for the SUVmax
for each prognostic indicator, including hormone receptor
expression, Allred score above or below 4, grade 1, 2 vs. grade 3,
HIF-1α, HER-2, Ki67 index, histology, and tumor (T1, T2 and T3) and
nodal (N0, N1, N2 and N3) status, were calculated (21). SUVmax and prognostic indicators were
analyzed by the Mann-Whitney U test or Kruskal Wallis test.
Categorical data were studied using the χ2 test. Mean
IQR (IQRM) was calculated and used instead of mean ±
standard deviation. Multiple regression analysis was employed using
the forward enter method by recruiting predictors with P<0.05
and excluding if P>0.10. Outliers were identified and excluded
from analysis. Multiple regression analysis yielded the best
predictors of SUVmax. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using by MedCalc v12.5 (MedCalc Software bvba, Ostend,
Belgium).
Results
Patient characteristics
A total of 92 patients were enrolled in the present
study. The mean age was 58.9±11.5 years (IQRM, 58.3
years). Tumors were determined in the right breast of 35 (38.0%)
patients and in the left breast of 57 (62.0%) patients (P=0.02).
The IQRM tumor size, number of metastatic lymph nodes,
tumor SUVmax and axillary tumor SUVmax were 2.5 cm, 2.2, 7 and 2.6,
respectively.
Tumor characteristics
The characteristics of all tumors are presented in
Table I. The tumors were grade 1, 2
and 3 in 16.3, 53.3 and 30.4% of patients, respectively. In
addition, 32.6, 30.4, 21.7 and 15.2% of patients were N0, N1, N2
and N3, respectively, and tumor size was T1, T2 and T3 in 20.7,
69.6 and 9.8% of patients, respectively. ER was positive in 82.6%
of patients and negative in 17.4%, while PgR was positive in 81.5%
of patients and negative in 18.5%. The IQRM estrogen and
progesterone Allred scores were 6.2 and 5, respectively. HER-2
expression was classified as 0, 1, 2 and 3 in 44.6, 16.3, 14.1 and
25.0% of patients, respectively. Silver in situ
hybridization (SISH) was applied to 13 patients and 7 (53.8%) were
positive. A total of 84 (91.3%) patients were triple-negative.
Ki-67 expression was ≤10% in 34 (37.0%) patients and >10% in 58
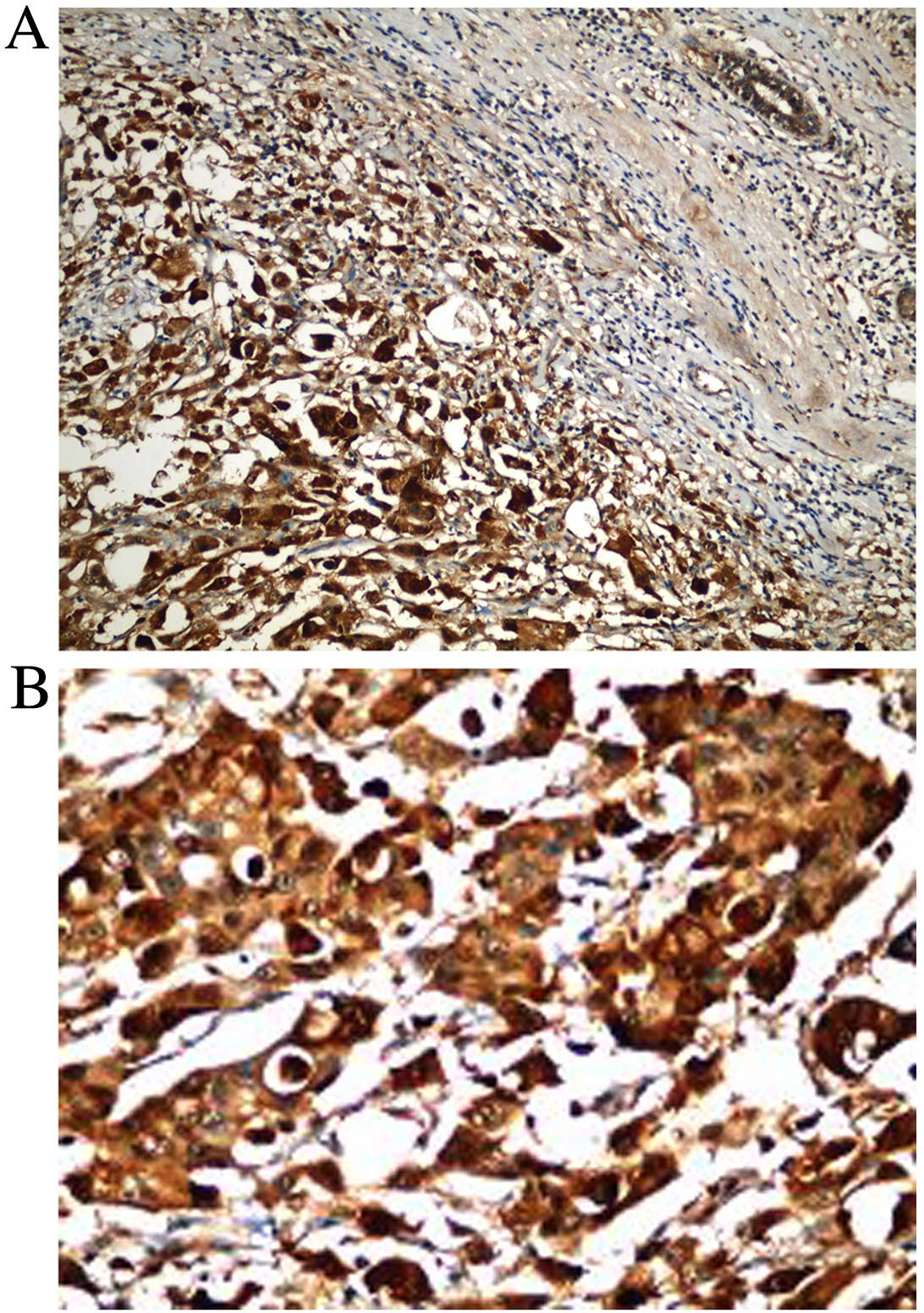
(63.0%) patients. HIF-1α was positive in 83.7% of patients
(Table I; Fig. 1A and B).
 | Table I.Breast cancer tumor
characteristics. |
Table I.
Breast cancer tumor
characteristics.
| Characteristic | Number | % |
|---|
| Grade |
|
|
| 1 | 5 | 16.3 |
| 2 | 49 | 53.3 |
| 3 | 28 | 30.4 |
| Nodal status |
|
|
| N0 | 30 | 32.6 |
| N1 | 28 | 30.4 |
| N2 | 20 | 21.7 |
| N3 | 14 | 15.2 |
| Tumor size |
|
|
| T1 | 19 | 20.7 |
| T2 | 64 | 69.6 |
| T3 | 9 | 9.8 |
| ER |
|
|
|
Negative | 16 | 17.4 |
|
Positive | 76 | 82.6 |
| PgR |
|
|
|
Negative | 17 | 18.5 |
|
Positive | 75 | 81.5 |
| ER Allred
score |
|
|
|
<4 | 20 | 21.7 |
| ≥4 | 72 | 78.3 |
| PgR Allred
score |
|
|
|
<4 | 29 | 31.5 |
| ≥4 | 63 | 68.5 |
| HER-2 |
|
|
| 0 | 41 | 44.6 |
| 1 | 15 | 16.3 |
| 2 | 13 | 14.1 |
| 3 | 23 | 25 |
| Ki-67 |
|
|
|
≤10% | 34 | 37 |
|
>10% | 58 | 63 |
| HIF-1α |
|
|
|
Negative | 15 | 16.3 |
|
Positive | 77 | 83.7 |
Clinicopathological parameters, SUVmax
and HIF-1α
Comparisons between SUVmax, HIF-1α and
clinicopathological parameters of the primary tumors are presented
in Table II. The median SUVmax
values of ER- and PgR-negative tumors were significantly increased
(P=0.004 and P=0.008). This difference in SUVmax was also evident
in the Allred score of ER and PgR. The SUVmax values of the T2 and
T3 tumors were significantly different from those of the T1 tumors
(P=0.02), and the SUVmax values between the Ki-67 >10% group and
the Ki-67 <10% group were also significantly different (P=0.01).
Although median SUVmax values were not different in HER-2-positive
and -negative tumors, it was higher in triple-negative tumors
(P=0.04). With regard to tumor grade, median SUVmax was
significantly higher in high-grade tumors (Fig. 2A and B). SUVmax did not exhibit a
significant correlation with HIF-1α expression (P=0.28); however,
HIF-1α was associated with tumor size and PgR levels. HIF-1α
expression increased with a larger tumor size (r=0.27; P=0.008) and
decreased PgR expression (r=−0.26; P=0.0002).
 | Table II.Univariate analysis of median SUVmax
for different tumor characteristics. |
Table II.
Univariate analysis of median SUVmax
for different tumor characteristics.
| Characteristic | Median SUVmax
(IQR) | P-value |
|---|
| ER |
|
|
|
Negative | 9.6 (4.0) | 0.004a,c |
|
Positive | 6 (6.2) |
|
| PgR |
|
|
|
Negative | 9.8
(3.3) | 0.008a,c |
|
Positive | 6
(6.1) |
|
| ER Allred
score |
|
|
|
<4 | 9.2
(3.6) | 0.01a,c |
| ≥4 | 6
(6.3) |
|
| PgR Allred
score |
|
|
|
<4 | 9.1
(4.2) | 0.03a,c |
| ≥4 | 5.9
(6.1) |
|
| HER-2 |
|
|
|
Negative | 5.7
(6.6) | 0.07a |
|
Positive | 7.9
(3.8) |
|
|
Triple-negative |
|
|
|
Non-TN | 6.8
(5.9) | 0.04a,c |
| TN | 10.1 (3.6) |
|
| Ki-67 |
|
|
|
≤10% | 4.7
(6.8) | 0.01a,c |
|
>10% | 7.8
(5.5) |
|
| HIF-1α |
|
|
|
Negative | 6.0
(6.0) | 0.28a |
|
Positive | 7.8
(5.5) |
|
| Grade |
|
|
|
1–2 | 5.7
(5.8) | 0.001a,c |
| 3 | 9.6
(4.1) |
|
| Tumor size |
|
|
| T1 | 4.8
(3.7) | 0.02b,c,d |
| T2 | 7.8
(6.2) |
|
| T3 | 7.9
(3.8) |
|
Axillary nodal status and SUVmax
The IQRM of axillary nodal SUVmax was
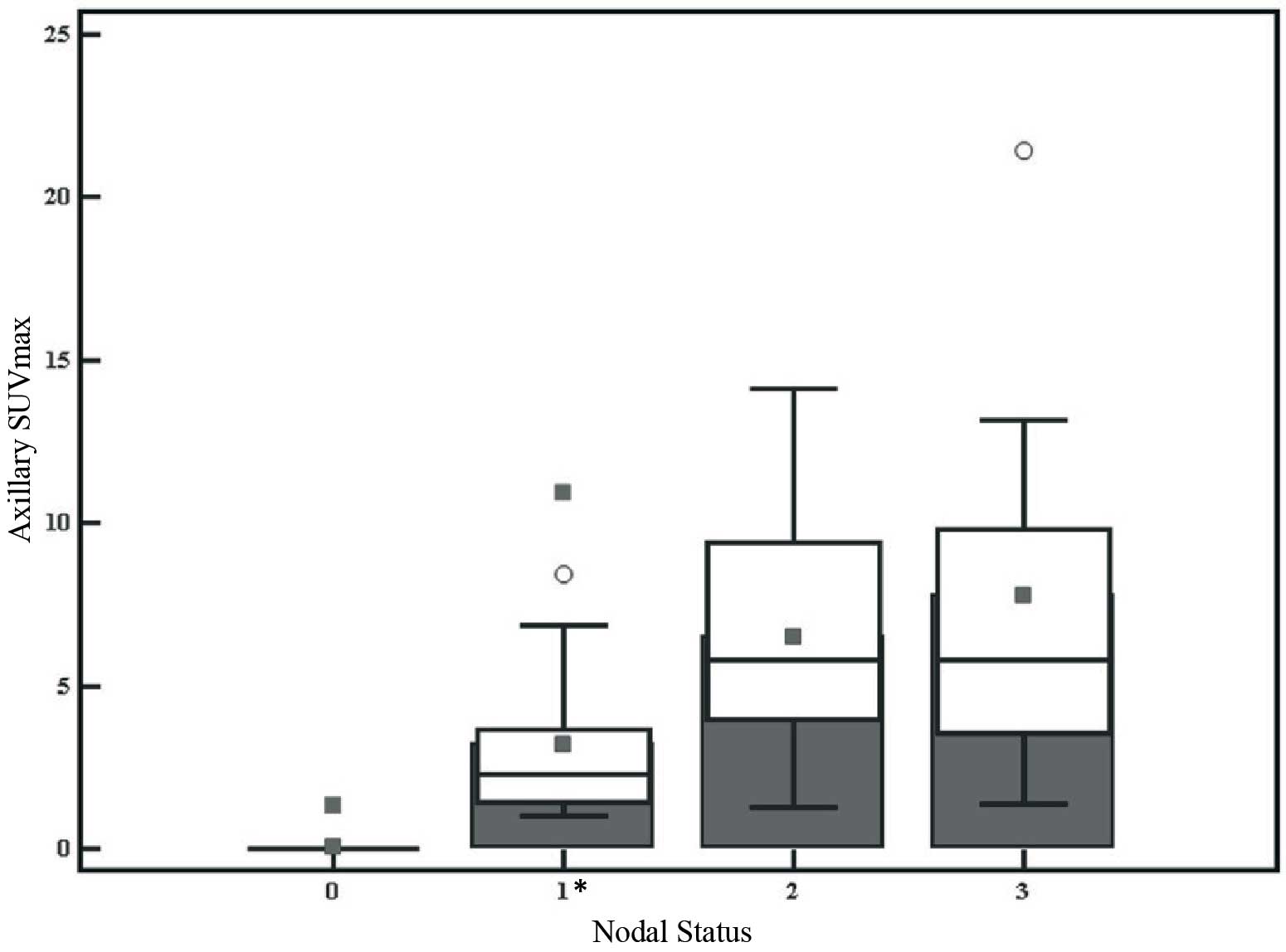
2.7. SUVmax according to nodal status is presented in Table III. Axillary SUVmax of N1 was
statistically lower than N2 and N3 (P<0.0001) (Fig. 3).
 | Table III.Axillary SUVmax according to nodal
status. |
Table III.
Axillary SUVmax according to nodal
status.
| Nodal status | Median SUVmax
(IQR) | Statistical
significance |
|---|
| N0 | 0 (0) | Statistically
different from N1, N2 and N3 |
| N1 | 2.2 (2.4) | Statistically
different from N0, N2 and N3 |
| N2 | 5.7 (5.4) | Statistically
different from N0 and N1 |
| N3 | 5.8 (6.3) | Statistically
different from N0 and N1 |
Multiple regression analysis
Multiple regression analysis was performed to
determine the association between SUVmax and independent factors
affecting SUVmax (Table IV). The
SUVmax of the axillary lymph nodes was only predicted by nodal
status in multiple regression analysis (coefficient, 2.7;
rpartial=0.70; t=9.1; r2=0.49;
P<0.0001).
 | Table IV.Multivariate model demonstrating the
effects of different factors on SUVmax. |
Table IV.
Multivariate model demonstrating the
effects of different factors on SUVmax.
|
| Coefficient |
rpartial | T | P-value |
|---|
| ER Allred
score | −0.3322 | −0.2436 | −2.302 | 0.0238 |
| Ki-67 | 0.04052 | 0.2306 | 2.172 | 0.0327 |
| Size | 0.6996 | 0.2557 | 2.424 | 0.0175 |
Discussion
Various studies have demonstrated an association
between FDG uptake in tumors and multiple prognostic indicators
(22–24). Additional studies have evaluated the
association among hypoxia, namely HIF-1α, and FDG uptake in breast
cancer (25). To the best of our
knowledge, no study has yet investigated the combined association
between hypoxia, FDG uptake and other clinicopathological
prognostic factors.
In the present study, univariate analysis identified
that augmented SUVmax values were associated with a higher nuclear
grade, larger tumor size, negative hormone receptor status,
triple-negativity and high Ki-67 expression. The median SUVmax
value was higher in HER-2-positive breast cancer, but this was not
significant statistically (P=0.07). Conversely, there was no
association observed between HIF-1α expression and FDG uptake.
Factors for tumor proliferation and metastasis were significantly
associated with augmented SUVmax. Oshida et al (26) reported that high FDG uptake in breast
tumors is associated with poor prognosis. Conversely, results
regarding the link between tumor proliferation and FDG uptake in
multiple tumors remains controversial (27,28). On
the other hand, in a number of previous studies positive
associations were observed between proliferation rates and FDG
uptake in breast cancer (15,16,22,25,29,30).
The present study is consistent with this, as Ki-67 expression and
tumor size were considered independent factors for SUVmax in breast
cancer according to multivariate regression analysis. Several
studies have produced contradictory results, with some
demonstrating a correlation between FDG uptake and tumor size
(16,17,30,31), while
others have not had consistent results (14,15,22,23,32).
Tumor and axillary lymph node grade are major
predictive factors for breast cancer. In the present study, the
most significant correlation was observed between SUVmax and tumor
grade (P=0.001), which is consistent with previous studies
(16,17,26,30,33,34).
Additionally, multiple regression analysis performed in current
study demonstrated that the SUVmax of axillary lymph nodes was only
predicted by nodal status (coefficient, 2.7;
rpartial=0.70; t=9.1; r2=0.49; P<0.0001).
Song et al (12) reported that
axillary nodal SUVmax on pretreatment FDG-PET may be an independent
factor for disease relapse and prognosis in patients with invasive
ductal carcinoma and axillary lymph node involvement.
Several studies have observed FDG uptake in invasive
lobular cancer, which was reduced compared with other types of
aggressive tumors (14,15,23,30). This
finding is explained by diffuse infiltration, lower tumor cell
density, low levels of GLUT-1 and reduced proliferation (23,35,36).
Although studies regarding hormone receptor
expression and FDG uptake are inconsistent, the present study
detected a significantly higher FDG uptake in ER- and PgR-negative
tumors than positive ones (P=0.004 and P=0.008, respectively).
Meanwhile, ER Allred score was identified to be an independent
factor affecting SUVmax. A number of previous studies reported that
there was correlation between hormone receptor status and SUVmax
(14,15,32,34,35,37).
However, recent studies have demonstrated a positive association
between SUVmax values and ER negativity in tumors (30,32,37,38).
Triple-negative tumors have been documented as more invasive and
are known for their poor prognosis compared with non-triple
negative tumors (37). The present
study also observed that the triple-negative tumors had augmented
SUVmax compared with the non-triple-negative disease (P=0.04),
which is consistent with previous reports (23,37).
Previously, several studies reported that there was
a marked improvement in the survival of patients with HER-2
receptor-positive breast cancer due to the use of antibody
treatment with trastuzumab (39–41).
Although a few previous studies have observed an association
between SUVmax and c-Erb-B2 positivity (16,17), the
current study did not identify a significant correlation, which is
supported by a number of other studies (15,32,37,42).
This may suggest that HER-2 does not have any major effect on
glycolytic pathways (23).
Intratumoral hypoxia has been demonstrated to lead
to poor prognosis (43–45). Hypoxic tumors have been documented for
their association with aggressiveness, augmented metastasis, and
resistance to chemo and radiation therapy (43–45).
Increased expression of HIF-1α has been reported in a various forms
of common cancer. Bos et al (8) observed that HIF-1α was upregulated
during breast pathogenesis following the identification of enhanced
HIF-1α levels in ductal carcinoma in situ and invasive
cancer specimens. However, augmented levels were not observed in
normal breast and ductal hyperplasia (8). Subsequently, Bos et al (25) investigated the association between FDG
uptake and HIF-1α, GLUT-1, hexokinase I–II-III, vascular
endothelial growth factor (VEGF), intratumoral microvessel density
and mitotic index. Despite reporting a significant positive
association with FDG uptake and GLUT-1 and hexokinase I, no
correlation was observed between FDG uptake and HIF-1α or other
biomarkers (25). This was consistent
with the results of the present study. Following investigation of
Ki-67 expression through immunohistochemistry, Evans et al
(46) suggested that hypoxic
localized cell proliferation of tumors was decreased (46). In the current study, HIF-1α levels
were associated with tumor size and PgR negativity.
Clavo et al (47) examined FDG uptake in multiple types of
carcinoma cells after varying the oxygen levels in vitro. An
enhancement in FDG level was observed following moderate hypoxic
treatment. Based on these results, it was suggested that hypoxia
regulates FDG uptake (28,47). Toba et al (48) investigated the association between
HIF-1α, GLUT-1, VEGF and FDG uptake in thymic epithelial tumors.
The results demonstrated a moderate association between FDG uptake
and the expression of HIF-1α (48).
Furthermore, Rajendran et al (9) examined the association between hypoxia
and glycolysis with 18F-fluoromisonidazole (FMISO) and
FDG uptake on PET images in four diverse tumors, including breast
cancer. A moderate association was identified between FDG and FMISO
uptake in head and neck cancer, but no association was observed in
other cancer forms, including breast cancer and glioblastoma
(9).
In conclusion, the results of the current study
suggested that increased FDG uptake was associated with various
poor prognostic indicators, such as large tumor size, high tumor
grade and Ki-67 expression, reduction in hormonal receptors, and
triple-negative primary breast tumors. However, no assoication was
identified between FDG uptake and HIF-1α level. Although hypoxia
impacts glycolysis, tumor hypoxia is a heterogeneous phenomenon.
Therefore, there may be inconsistent patterns observed between
glucose utilization and hypoxia. A major limitation of the present
study may be that that only patients with invasive breast carcinoma
were included. Thus, future studies may benefit from including a
range of histological types, subsequently allowing a comparison
between different types of breast cancer.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knowles HJ and Harris AL: Hypoxia and
oxidative stress in breast cancer. Hypoxia and tumourigenesis.
Breast Cancer Res. 3:318–322. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Serganova I, Humm J, Ling C and Blasberg
R: Tumor hypoxia imaging. Clin Cancer Res. 12:5260–5264. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bos R, van der Groep P, Greijer AE,
Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ and van
der Wall E: Levels of hypoxia-inducible Factor-1alpha independently
predict prognosis in patients with lymph node negative breast
carcinoma. Cancer. 97:1573–1581. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu AY, Frid MG, Shimoda LA, Wiener CM,
Stenmark K and Semenza GL: Temporal, spatial, and oxygen-regulated
expression of hypoxia-inducible factor-1 in the lung. Am J Physiol.
275:L818–L826. 1998.PubMed/NCBI
|
|
7
|
Huang LE, Arany Z, Livingston DM and Bunn
HF: Activation of hypoxia-inducible transcription factor depends
primarily upon redox-sensitive stabilization of its apha subunit. J
Biol Chem. 271:32253–32259. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bos R, Zhong H, Hanrahan CF, Mommers EC,
Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Diest PJ and van
der Wall E: Levels of hypoxia-inducible factor-1 alpha during
breast carcinogenesis. J Natl Cancer Inst. 93:309–314. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rajendran JG, Mankoff DA, O'Sullivan F,
Peterson LM, Schwartz DL, Conrad EU, Spence AM, Muzi M, Farwell DG
and Krohn KA: Hypoxia and glucose metabolism in malignant tumors:
Evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose
positron emission tomography imaging. Clin Cancer Res.
10:2245–2252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burgman P, Odonoghue JA, Humm JL and Ling
CC: Hypoxia-induced increase in FDG uptake in MCF7 cells. J Nucl
Med. 42:170–175. 2001.PubMed/NCBI
|
|
11
|
Warburg O: The Metabolism of Tumors.
Richard R., Inc.; New York, NY: pp. 129–169. 1931
|
|
12
|
Song BI, Lee SW, Jeong SY, Chae YS, Lee
WK, Ahn BC and Lee J: 18F-FDG uptake by metastatic axillary lymph
nodes on pretreatment PET/CT as a prognostic factor for recurrence
in patients with invasive ductal breast cancer. J Nucl Med.
53:1337–1344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Czernin J and Phelps ME: Positron emission
tomography scanning: Current and future applications. Annu Rev Med.
53:89–112. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Avril N, Rosé CA, Schelling M, Dose J,
Kuhn W, Bense S, Weber W, Ziegler S, Graeff H and Schwaiger M:
Breast imaging with positron emission tomography and fluorine-18
fluorodeoxyglucose: Use and limitations. J Clin Oncol.
18:3495–3502. 2000.PubMed/NCBI
|
|
15
|
Buck A, Schirrmeister H, Kühn T, Shen C,
Kalker T, Kotzerke J, Dankerl A, Glatting G, Reske S and Mattfeldt
T: FDG uptake in breast cancer: Correlation with biological and
clinical prognostic parameters. Eur J Nucl Med Mol Imaging.
29:1317–1323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ueda S, Tsuda H, Asakawa H, Shigekawa T,
Fukatsu K, Kondo N, Yamamoto M, Hama Y, Tamura K, Ishida J, et al:
Clinicopathological and prognosti relevance of uptake level using
18F-fluorodeoxyglucose positron emission tomography/computed
tomography fusion imaging (18F-FDG PET/CT) in primary breast
cancer. Jpn J Clin Oncol. 38:250–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanli Y, Kuyumcu S, Ozkan ZG, Işİk G,
Karanlik H, Guzelbey B, Turkmen C, Ozel S, Yavuz E and Mudun A:
Increased FDG uptake in breast cancer is associated with prognostic
factors. Ann Nucl Med. 26:345–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fitzgibbons PL, Page DL, Weaver D, Thor
AD, Allred DC, Clark GM, Ruby SG, O'Malley F, Simpson JF, Connolly
JL, et al: Prognostic factors in breast cancer. Collage of american
pathologists consensus statement 1999. Arch Pathol Lab Med.
124:966–978. 2000.PubMed/NCBI
|
|
19
|
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH
and van de Vijver MJ: WHO Classification of tumours of the breast.
4th. IARC; Lyon, France: 2012
|
|
20
|
Dales JP, Garcia S, Meunier-Carpentier S,
Andrac-Meyer L, Haddad O, Lavaut MN, Allasia C, Bonnier P and
Charpin C: Overexpression of hypoxia-inducible factor HIF-1 alpha
predicts early relapse in breast cancer: Retrospective study in a
series of 745 patients. Int J Cancer. 116:734–739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cohen DA, Dabbs DJ, Cooper KL, Amin M,
Jones TE, Jones MW, Chivukula M, Trucco GA and Bhargava R:
Interobserver agreement among pathologists for semiquantitative
hormone receptorscoring in breast carcinoma. Am J Clin Pathol.
138:796–802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koolen BB, Peeters MJ Vrancken, Wesseling
J, Lips EH, Vogel WV, Aukema TS, van Werkhoven E, Gilhuijs KG,
Rodenhuis S, Rutgers EJ and Valdés Olmora RA: Association of
primary tumour FDG uptake with clinical, histopathological and
molecular characteristics in breast cancer patients scheduled for
neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 39:1830–1838.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Groheux D, Giacchetti S, Moretti JL,
Porcher R, Espié M, Lehmann-Che J, de Roquancourt A, Hamy AS,
Cuvier C, Vercellino L and Hindié E: Correlation of high 18F-FDG
uptake to clinical, pathological and biological prognostic factors
in breast cancer. Eur J Nucl Med Mol Imaging. 38:426–435. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heudel P, Cimarelli S, Montella A,
Bouteille C and Mognetti T: Value of PET-FDG in primary breast
cancer based on histopathological and immunohistochemical
prognostic factors. Int J Clin Oncol. 15:588–593. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bos R, van Der Hoeven JJ, van Der Wall E,
van Der Groep P, van Diest PJ, Comans EF, Joshi U, Semenza GL,
Hoekstra OS, Lammertsma AA and Molthoff CF: Bioglogic correlates of
(18)fluorodeoxyglucose uptake in human breast cancer measured by
positron emission tomography. J Clin Oncol. 20:379–387. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oshida M, Uno K, Suzuki M, Nagashima T,
Hashimoto H, Yagata H, Shishikura T, Imazeki K and Nakajima N:
Predicting the prognoses of breast carcinoma patients with positron
emission tomography using 2-deoxy-2-fluoro[18F]-D-glucose. Cancer.
82:2227–2234. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buck AC, Schirrmeister HH, Guhlmann CA,
Diederichs CG, Shen C, Buchmann I, Kotzerke J, Birk D, Mattfeldt T
and Reske SN: Ki-67 immunostaining in pancreatic cancer and chronic
active pancreatitis: Does in vivo FDG uptake correlate with
proliferative activity? J Nucl Med. 42:721–725. 2001.PubMed/NCBI
|
|
28
|
Higashi K, Clavo AC and Wahl RL: Does FDG
uptake measure proliferative activity of human cancer cells? In
vitro comparison with DNA flow cytometry and tritiated thymidine
uptake. J Nucl Med. 34:414–419. 1993.PubMed/NCBI
|
|
29
|
Simpson JF, Gray R, Dressler LG, Cobau CD,
Falkson CI, Gilchrist KW, Pandya KJ, Page DL and Robert NJ:
Prognostic value of histologic grade and proliferative activity in
axillary node-positive breast cancer: Results from the Eastern
Cooperative Oncology Group Companion Study, EST 4189. J Clin Oncol.
18:2059–2069. 2000.PubMed/NCBI
|
|
30
|
Gil-Rendo A, Martínez-Regueira F, Zornoza
G, Garcia-Velloso MJ, Beorlegui C and Rodriguez-Spiteri N:
Association between [18F]fluorodeoxyglucose uptake and prognostic
parameters in breast cancer. Br J Surg. 96:166–170. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakajo M, Kajiya Y, Kaneko T, Kaneko Y,
Takasaki T, Tani A, Ueno M, Koriyama C and Nakajo M: FDG PET/CT and
diffusion-weighted imaging for breast cancer: Prognostic value of
maximum standardized uptake values and apparent diffusion
coefficient values of the primary lesion. Eur J Nucl Med Mol
Imaging. 37:2011–2020. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Osborne JR, Port E, Gonen M, Doane A,
Yeung H, Gerald W, Cook JB and Larson S: 18F-FDG PET of locally
invasive breast cancer and association of estrogen receptor status
with standardized uptake value: Microarray and immunohistochemical
analysis. J Nucl Med. 51:543–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Berriolo-Riedinger A, Touzery C, Riedinger
JM, Toubeau M, Coudert B, Arnould L, Boichot C, Cochet A, Fumoleau
P and Brunotte F: [18F]FDG-PET predicts complete pathological
response of breast cancer to neoadjuvant chemotherapy. Eur J Nucl
Med Mol Imaging. 34:1915–1924. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumar R, Chauhan A, Zhuang H, Chandra P,
Schnall M and Alavi A: Clinicopathologic factors associated with
false negative FDG-PET in primary breast cancer. Breast Cancer Res
Treat. 98:267–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Crippa F, Seregni E, Agresti R, Chiesa C,
Pascali C, Bogni A, Decise D, De Sanctis V, Greco M, Daidone MG and
Bombardieri E: Association between [18F]fluorodeoxyglucose uptake
and postoperative histopathology, hormone receptor status,
thymidine labelling index and p53 in primary breast cancer: A
preliminary observation. Eur J Nucl Med. 25:1429–1434. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Avril N, Rosé CA, Schelling M, Dose J,
Kuhn W, Bense S, Weber W, Ziegler S, Graeff H and Schwaiger M:
Breast imaging with positron emission tomography and fluorine-18
fluorodeoxyglucose: Use and limitations. J Clin Oncol.
18:3495–3502. 2000.PubMed/NCBI
|
|
37
|
Kim BS and Sung SH: Usefulness of 18F-FDG
uptake with clinicopathologic and immunohistochemical prognostic
factors in breast cancer. Ann Nucl Med. 26:175–183. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mavi A, Cermik TF, Urhan M, Puskulcu H,
Basu S, Yu JQ, Zhuang H, Czerniecki B and Alavi A: The effects of
estrogen, progesterone, and C-erbB-2 receptor states on 18F-FDG
uptake of primary breast cancer lesions. J Nucl Med. 48:1266–1272.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Romond EH, Perez EA, Bryant J, Suman VJ,
Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman
PA, et al: Trastuzumab plus adjuvant chemotherapy for operable
HER2-positive breast cancer. N Engl J Med. 353:1673–1684. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Piccart M, Lohrish C, Di Leo A and
Larsimont D: The predictive value of HER2 in breast cancer.
Oncology. 61(Suppl 2): 73–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gonzalez-Angulo AM, Hortobagyi GN and
Esteva FJ: Adjuvant therapy with trastuzumab for HER-2/neu-positive
breast cancer. Oncologist. 11:857–867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ikenaga N, Otomo N, Toyofuku A, Ueda Y,
Toyoda K, Hayashi T, Nishikawa K and Tanaka M: Standardized uptake
values for breast carcinomas assessed by
fluorodeoxyglucose-positron emission tomography correlate with
prognostic factors. Am Surg. 73:1151–1157. 2007.PubMed/NCBI
|
|
43
|
Spence AM, Muzi M, Graham MM, O'Sullivan
F, Krohn KA, Link JM, Lewellen TK, Lewellen B, Freeman SD, Berger
MS and Ojemann GA: Glucose metebolism in human malignant gliomas
measured quantitatively with PET, 1-[C-11]glucose and FDG: Analysis
of the FDG lumped constant. J Nucl Med. 39:440–448. 1998.PubMed/NCBI
|
|
44
|
Kallinowski F, Schlenger KH, Kloes M,
Stohrer M and Vaupel P: Tumor blood flow: The principal modulator
of oxidative and glycolytic metablism and of the metabolic
micromilieu of human tumor xenografts in vivo. Int J Cancer.
44:266–272. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fleming IN, Manavaki R, Blower PJ, West C,
William KJ, Harris AL, Domarkas J, Lord S, Baldry C and Gilbert FJ:
Imaging tumour hypoxia with positron emission tomography. Br J
Cancer. 112:238–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Evans SM, Hahn SM, Magarelli DP and Koch
CJ: Hypoxic heterogeneity in human tumors: EF5 binding,
vasculature, necrosis and proliferation. Am J Clin Oncol.
24:467–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Clavo AC, Brown RS and Wahl RL:
Fluorodeoxyglucose uptake in human cancer cell lines is increased
by hypoxia. J Nucl Med. 36:1625–1632. 1995.PubMed/NCBI
|
|
48
|
Toba H, Kondo K, Sadohara Y, Otsuka H,
Morimoto M, Kajiura K, Nakagawa Y, Yoshida M, Kawakami Y, Takizawa
H, et al: 18F-fluorodeoxyglucose positron emission
tomography/computed tomography and the relationship between
fluorodeoxyglucose uptake and the expression of hypoxia-inducible
factor-1α, glucose transporter-1 and vascular endothelial growth
factor in thymic epithelial tumours. Eur J Cardiothorac Surg.
44:105–112. 2013. View Article : Google Scholar
|