Introduction
Hormone-responsive estrogen receptor (ER)+ clinical
breast cancer responds to endocrine therapy involving the use of
selective ER modulators and selective inhibitors of estradiol
biosynthesis (1,2). However, long-term treatment with these
agents is frequently associated with acquired tumor resistance,
resulting in limited efficacy that confers a negative impact on
patient response (1–3). These aspects underscore the importance
of identifying novel therapeutic agents with improved efficacy and
low systemic toxicity.
Chinese nutritional herbs are widely used in the
management of general health issues in women as well as an
alternative treatment option for breast cancer (4–7). Naturally
occurring herbal preparations may exhibit an acceptable toxicity
profile, and thus are likely to favorably interact with
conventional endocrine therapy to reduce toxicity and enhance
efficacy of pharmacological therapeutic agents. However, the impact
of herb-drug interaction on therapeutic efficacy remains unknown
(4).
The estrogen-dependent human breast
carcinoma-derived ER+ MCF-7 cell line represents a well-established
preclinical cell culture system for hormone-responsive clinical
breast cancer. MCF-7 cells depend on 17β-estradiol (E2) for cell
proliferation in vitro and for tumor development in
vivo, and thereby represent an important model for evaluating
the efficacy of novel agents that function via modulation of the
cellular and molecular response to estrogens (8). ER functions as a ligand-activated
nuclear transcription factor, with E2 as the endogenous ligand, to
promote cellular proliferation and tumorigenic growth via a complex
signaling cascade that involves the coordinated functions of
several co-activators and co-repressors, resulting in the
expression of selected estrogen-responsive target genes (2,3,8). Sub-physiological concentrations of
E2 render ER as a transiently non-functional receptor
(3,8).
Our previous studies have demonstrated that MCF-7
cells adapted for growth in chemically defined serum-depleted
culture conditions retain their responsiveness to E2, and respond
to the growth-inhibitory effects of several mechanistically
distinct pharmacological agents and selected herbal extracts
(9–13).
In traditional Chinese medicine, combinations of
several nutritional herbs are commonly used as a palliative
treatment option for breast cancer patients (5,7). The herbs
selected in the present study represent some of these clinically
relevant herbs. In the present study, isogenic MCF-7 phenotypes
with modulated ER function were isolated, and these models were
used to compare the growth-inhibitory efficacy of the selected
Chinese nutritional herbs. The outcome of the present study has
provided data to demonstrate the differential efficacy of herbs
depending upon the functional status of ER, thus identifying
potential leads to prioritize efficacious herbs for secondary
prevention and/or for therapy of ER+ and ER- clinical breast
cancer.
Materials and methods
Cell lines
The parental ER+ MCF-7 cell line was originally
obtained from the Michigan Cancer Foundation (Detroit, MI, USA).
These cells were cultured in Dulbecco's modified Eagle-F12 medium
supplemented with 10% heat inactivated fetal calf serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 10 µg/ml insulin
(Eli Lilly and Company, Indianapolis, IN, USA) and 1 µM
dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), following
published protocols (9,10).
Isolation and characterization of
non-functional ER (ER-NF) and functional ER (ER-F) phenotypes
To isolate and characterize the ER-NF phenotype,
parental MCF-7 cells were adapted for growth in medium supplemented
with decreasing serum concentrations (7.5, 5.0, 2.5, 1.0 and 0.7%),
corresponding to decreased levels of E2 ranging from 15 nM to <1
nM. The cells from the 0.7% serum group (E2 <1 nM) were
continuously maintained in medium supplemented with 0.7% serum for
≥5 passages. These cells were tested for their E2 response by
monitoring their growth in the presence of E2 at 1, 5, 10 and 20 nM
concentrations. The stock cultures of ER-NF cells were routinely
maintained in medium supplemented with 0.7% serum (E2 <1 nM).
The ER-NF phenotype is defined as the cell population that exhibits
progressive growth in the presence of chemically defined
serum-depleted medium (serum, 0.7%; E2 <1 nM).
To isolate the ER-F phenotype, ER-NF cells were
maintained in serum-depleted medium (0.7% serum) + 20 nM E2 for ≥5
passages, and the stock cultures were maintained in E2-supplemented
medium. Routinely, the ER-NF and ER-F phenotypes were maintained in
a humidified atmosphere of 95% air and 5% CO2, and were
sub-cultured at a 1:4 ratio when 80% confluent. The ER-F phenotype
is defined as the cell population that exhibits progressive growth
in the presence of a physiologically relevant concentration of
E2.
To further characterize E2 responsiveness, the
effect of the selective ER modulator tamoxifen (TAM) was evaluated
on the ER-NF and ER-F phenotypes by monitoring population-doubling
time, saturation density and anchorage-independent (AI) colony
formation (12,13). For these experiments, the stock
solutions of E2 and TAM (both from Sigma-Aldrich) were prepared in
100% ethanol (Thermo Fisher Scientific, Inc.) at a 100 mM
concentration, and were serially diluted in the culture medium to
obtain the final concentrations of 20 nM E2 and 20 nM TAM employed
for the treatment of the cell cultures.
Preparation of herbal extracts
The Chinese nutritional herbs used in the present
study were provided by Professor G.Y.C Wong (American Foundation
for Chinese Medicine, New York, NY, USA). The Chinese nutritional
herbs selected for the present study include Angelica
sinensis (AS), Cibotium barometz (CB), Cornus
officinalis (CO), Cuscuta sinensis (CS), Dipsacus
asperoides (DA), Epimedium grandiflorum (EG),
Eucommia ulmoides (EU), Ligusticum chuanxiong (LC),
Ligustium lucidum (LL), Lycium barbarum bark (LBB),
Lycium barbarum fruit (LBF), Morinda officinalis (MO)
and Psoralea corylifolia (PC). The sources of herbal
material for the preparation of aqueous extracts from these
nutritional herbs are presented in Table
I. The selection of the specific parts of the nutritional herbs
and the method to prepare the extracts for the present study were
based on the protocols followed in traditional Chinese medicine
(5,7).
 | Table I.Chinese nutritional herbs. |
Table I.
Chinese nutritional herbs.
| Botanical name | Abbreviation | Source of
extract |
|---|
| Angelica
sinensis | AS | Root |
| Cibotium
barometz | CB | Root |
| Cornus
officinalis | CO | Fruit |
| Cuscuta
sinensis | CS | Seed |
| Dipsacus
asperoides | DA | Root |
| Epimedium
grandiflorum | EG | Leaf, stem |
| Eucommia
ulmoides | EU | Bark |
| Ligusticum
chuanxiong | LC | Root |
| Ligustrum
lucidum | LL | Fruit |
| Lycium barbarum
bark | LBB | Bark |
| Lycium barbarum
fruit | LBF | Fruit |
| Morinda
officinalis | MO | Root |
| Psoralea
corylifolia | PC | Seed |
To prepare non-fractionated aqueous extracts, 20 g
of the herbal materials were boiled in 200 ml of distilled water
until the volume was reduced to 100 ml (extract #1). The residue
from extract #1 was then boiled in 100 ml of distilled water until
the volume was reduced to 50 ml (extract #2). Extracts #1 and #2
were combined (total volume, 150 ml) and boiled until the volume
was reduced to 25 ml. These combined extracts were centrifuged (500
× g at room temperature for 10 min), and the final aqueous
supernatant (20 ml) was collected. This non-fractionated aqueous
supernatant served as the stock solution (100%), which was serially
diluted with the culture medium to obtain the final concentrations
of 2, 1, 0.5, 0.05, 0.02 and 0.01% used for the treatment of the
cell cultures.
Dose-response experiments
For dose-response experiments to identify the
inhibitory concentration (IC)50 values of the different Chinese
herbs, the ER-NF and ER-F phenotypes were treated with the herbal
extracts at concentrations ranging from 2 to 0.01%. The cells were
plated at an initial seeding density of 1.0×105 cells
per T-25 flask. After a 24-h attachment period, the cultures were
treated with the corresponding diluted herbal extracts for 7 days.
At the end of the 7th day of treatment, the cells were trypsinized,
and the cell number and viability were determined by the trypan
blue exclusion test (Sigma-Aldrich).
These dose-response experiments provided data
regarding the IC50 values of the different herbs for the ER-NF and
ER-F phenotypes, while the ER-NF:ER-F ratio determined from the
above IC50 provided the rank order for the growth-inhibitory
efficacy of the herbs tested. Additionally, data obtained from the
dose-response experiments identified the maximum cytostatic
concentrations (IC90) of the individual herbal extracts, and were
used to distinguish the maximum cytostatic effects from the toxic
effects. The IC90 was defined as the highest concentration of the
herbal extract that results in a surviving cell population greater
than, or equal to, the initial seeding density, while a surviving
population of less than that of the initial seeding density was
considered as treatment-associated toxic response.
AI growth assay
The protocol for the AI growth assay has been
optimized and published (12,13). For the assay, ER-NF cells were
suspended in 0.33% agar (Sigma-Aldrich) at a density of 1,000
cells/2 ml/well in 6-well plates. The treatment groups contained
the herbal extracts at their respective IC90 value in the cell
suspension prepared in 0.33% agar. The cell suspension was overlaid
on a basement layer of 0.66% agar, and the cultures were incubated
at 37°C for 21 days in a humidified atmosphere of 95% air and 5%
CO2. The non-adherent AI colonies that developed in the
suspension cultures were counted at the end of the treatment.
Cell cycle progression and cellular
apoptosis
To examine the effects of the herbal extracts on
cell cycle progression and cellular apoptosis, the control and
treated cultures were analyzed by flow cytometry using a previously
published protocol (14). Briefly,
trypsinized cell cultures from the control and treated groups were
stained with propidium iodide (Calbiochem; EMD Millipore,
Billerica, MA, USA), and the percentage of cell population in the
G1, S, G2/M and sub-G0 (apoptotic) phases of the cell cycle were
monitored using the EPICS 752 flow cytometer (Beckman Coulter,
Inc., Brea, CA, USA), which was equipped with 488-nM excitation and
630-nM emission long-pass filters. The cell cycle phase
distribution was analyzed using the MultiCycle MPLUS software
(Phoenix Flow Systems, San Diego, CA, USA). The data were expressed
as percentage of cell population in each phase of the cell cycle,
as well as G1:S+G2/M ratio.
Cellular metabolism of E2
To examine the effects of the herbal extracts on the
cellular metabolism of E2, the medium from the control and treated
cultures after 48-h incubation was analyzed for the presence of
selected E2 metabolites, including estrone (E1), 2-hydroxyestrone
(2-OHE1), 16α-hydroxyestrone (16α-OHE1) and estriol (E3), on a 6980
N gas chromatograph (Agilent Technologies, Inc., Santa Clara, CA,
USA) equipped with a 5973 mass selective detector (MSD) (Agilent
Technologies, Inc.), a 7683 injector (Agilent Technologies, Inc.)
and a HPGI 701CA MSD Chemstation (Agilent Technologies, Inc.),
using a previously published gas chromatography-mass
spectrometry-based assay (10,12,13).
The data were expressed as 2-OHE1:16α-OHE1 and E3:16α-OHE1
ratios.
Statistical analysis
The experiments for dose response,
population-doubling time and saturation density were performed with
n=6 flasks per treatment group. The AI growth assay was performed
with n=18 wells per treatment group, while the experiments for cell
cycle analysis, cellular apoptosis and E2 metabolism were performed
using n=3 flasks per treatment group for each assay.
The statistical significance of the differences in
mean ± standard deviation (SD) values between the data points from
the control and the experimental groups for the individual
experiments was analyzed by a two-sample t-test using
GraphPad Prism software version 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Isolation and characterization of
ER-NF and ER-F phenotypes
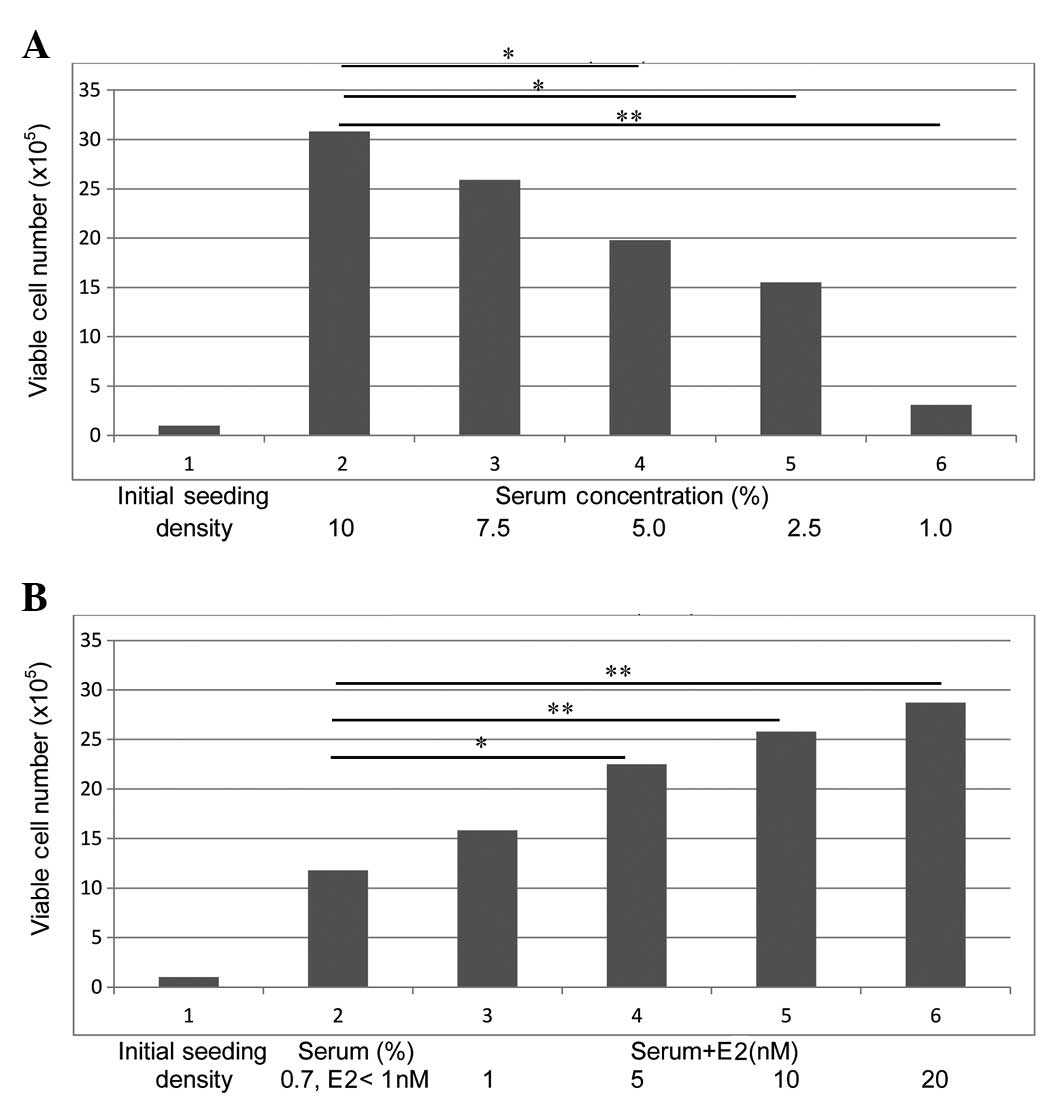
The data presented in Fig.
1A demonstrates that the parental MCF-7 cells exhibit a
progressive decrease in the number of surviving cells as a function
of decreased serum concentration. Thus, relative to the 10% serum
group, the 5.0% and 2.5% serum groups exhibited a 43.7% (P=0.04)
and a 53.1% (P=0.04) decrease, respectively, and the 1.0% serum
group exhibited a 92.2% decrease (P=0.03) in the surviving cell
population. The cells from the 1.0% serum group are also
effectively adapted to progressive growth in the presence of 0.7%
serum. The data presented in Fig. 1B
demonstrates that the cells maintained in the presence of 0.7%
serum exhibit a progressive increase in the number of viable cells
as a function of increasing concentrations of E2. Relative to the
<1 nM E2 group, cells treated with 5 nM E2 exhibited an 83.3%
increase (P=0.03), and those treated with 10 nM E2 and 20 nM E2
exhibited a 1.2-fold (P=0.01) and a 1.4-fold (P=0.01) increase,
respectively, in the surviving population. Thus, the surviving
population from the 0.7% serum group (E2 <1 nM) represents the
ER-NF phenotype, while that from the 0.7% serum + 20 nM E2 group
represents the ER-F phenotype.
The data presented in Table II examines the effect of TAM on
E2-promoted growth in the ER-NF and ER-F phenotypes. The ER-NF
phenotype, in response to treatment with E2 + TAM, exhibits a 29.1%
increase (P=0.05) in population-doubling time, a 59.1% decrease
(P=0.04) in saturation density and a 59.3% decrease (P=0.04) in the
number of AI colonies, relative to the E2-treated control.
Similarly, in the ER-F phenotype, treatment with E2 + TAM induces a
26.5% increase (P=0.05) in the population doubling time, a 65.5%
decrease (P=0.04) in the saturation density and a 62.1% decrease
(P=0.04) in the number of AI colonies, relative to the E2-treated
control. Thus, these data demonstrate that TAM antagonizes the
growth-promoting effect of E2 in the ER-NF and ER-F phenotypes.
 | Table II.Combinatorial effects of E2 and TAM
on ER-NF and ER-F phenotypes. |
Table II.
Combinatorial effects of E2 and TAM
on ER-NF and ER-F phenotypes.
|
| ER-NF | ER-F |
|---|
|
|
|
|
|---|
| Biomarker | E2 | E2+TAM | E2 | E2+TAM |
|---|
| Population-doubling
time (h)a | 27.8±1.7 |
35.9±1.9d | 27.2±1.4 |
34.4.±1.4e |
| Saturation density
(×105 cells)b | 18.1±0.3 |
7.4±0.5f | 26.1±1.8 |
9.0±1.6g |
| AI colonies
(no.)c | 36.9±2.1 |
15.0±1.6h | 37.2±2.1 |
14.1±1.0i |
Effects of herbal extracts on ER-NF
and ER-F phenotypes
The data on growth inhibition of ER-NF and ER-F
phenotypes by herbal extracts are presented as IC50 values in
Tables III and IV, respectively. The rank order of
preferential growth-inhibitory effects of herbal extracts on the
ER-NF and ER-F phenotypes are presented as the IC50 ratio of the
ER-NF vs. ER-F phenotype (Table V).
These data revealed that an IC50 ratio of >1 was exhibited by
LBB, EU, LBF, PC and DA, thus representing preferential efficacy
towards the ER-F phenotype. An IC50 ratio of 1 was exhibited by LL,
CS, EG and LC, thus representing equal efficacy towards the ER-NF
and ER-F phenotypes, while an IC50 ratio of <1 was exhibited by
AS and CO, thus representing preferential efficacy towards the
ER-NF phenotype. The extract prepared from CB exhibited a
non-significantly different 8 and 16% growth inhibition for the
ER-NF and ER-F phenotypes, respectively, at the highest
concentration tested (2.0%). Similarly, the extract prepared from
MO exhibited a non-significantly different 9.7 and 8.5% growth
inhibition for the two phenotypes, respectively, at the equivalent
highest concentration. Thus, the extracts from CB and MO were
demonstrated to be essentially ineffective in the present
experimental system.
 | Table III.Rank order of efficacy by
IC50 of Chinese nutritional herbs on the estrogen
receptor non-functional phenotype. |
Table III.
Rank order of efficacy by
IC50 of Chinese nutritional herbs on the estrogen
receptor non-functional phenotype.
| Herbal extract | IC50
(%)a |
|---|
| LBB | 0.01 |
| CO | 0.03 |
| DA | 0.19 |
| EU | 0.23 |
| LL | 0.30 |
| PC | 0.40 |
| EG | 0.46 |
| CS | 0.60 |
| LC | 0.60 |
| AS | 0.70 |
| LBF | 0.90 |
| CB | Not
reachedb |
| MO | Not
reachedb |
 | Table IV.Rank order of efficacy by
IC50 of Chinese nutritional herbs on the estrogen
receptor functional phenotype. |
Table IV.
Rank order of efficacy by
IC50 of Chinese nutritional herbs on the estrogen
receptor functional phenotype.
| Herbal extract | IC50
(%)a |
|---|
| LBB | 0.001 |
| CO | 0.070 |
| EU | 0.090 |
| DA | 0.130 |
| PC | 0.260 |
| LL | 0.280 |
| LBF | 0.390 |
| EG | 0.440 |
| CS | 0.570 |
| LC | 0.620 |
| AS | 1.170 |
 | Table V.Comparative efficacy by
IC50 of Chinese nutritional herbs on the isogenic ER-NF
and ER-F phenotypes. |
Table V.
Comparative efficacy by
IC50 of Chinese nutritional herbs on the isogenic ER-NF
and ER-F phenotypes.
| Herbal extract | ER-NF:ER-F ratio
(IC50)a |
|---|
| LBB | 10.00 |
| EU | 2.56 |
| LBF | 2.31 |
| PC | 1.54 |
| DA | 1.46 |
| LL | 1.07 |
| CS | 1.05 |
| EG | 1.04 |
| LC | 0.97 |
| AS | 0.59 |
| CO | 0.43 |
Based on the data from the rank order of effective
IC50 values in the ER-NF and ER-F phenotypes, herbal extracts from
LBB (which demonstrated preferential efficacy for ER-F cells), EG
(which demonstrated identical efficacy in ER-NF and ER-F cells) and
CO (which demonstrated preferential efficacy for ER-NF cells), were
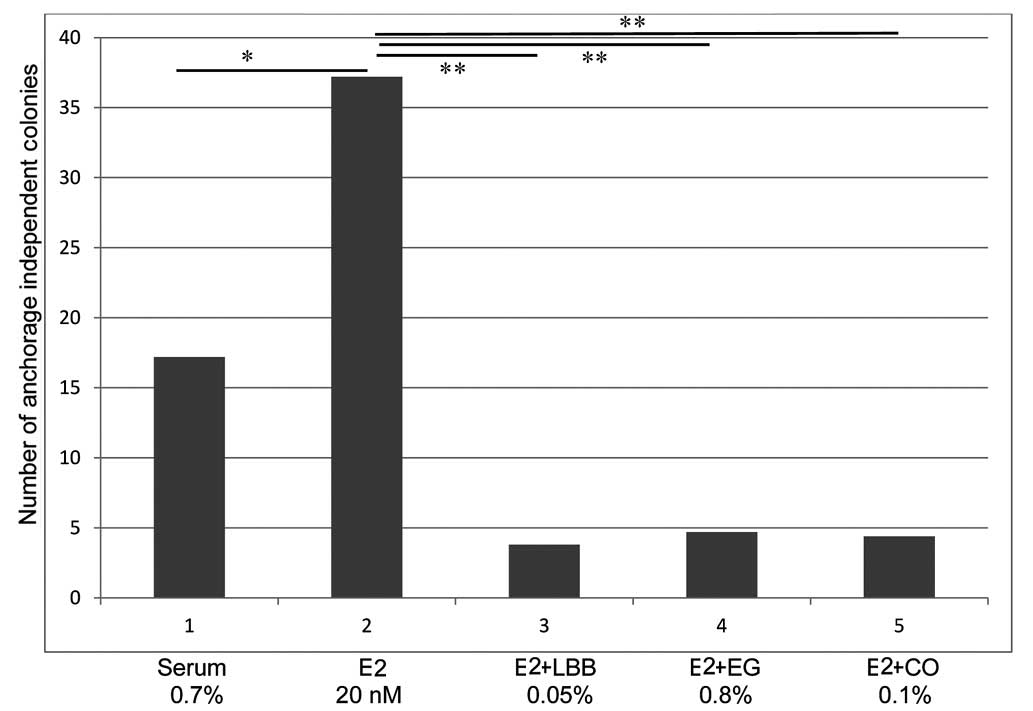
examined for their effect on AI growth in MCF-7 cells. The data
presented in Fig. 2, which are
expressed as the number of colonies [mean ± SD, n=18/treatment
group) demonstrate that MCF-7 cells maintained in the presence of
0.7% serum (E2 <1 nM) plus a physiologically relevant
concentration of 20 nM E2 exhibit a 116.3% increase (P=0.01) in the
number of AI colonies (mean AI colony number, 36.7±2.4), relative
to that observed in the 0.7% serum-treated control group (mean AI
colony number, 17.2±3.4). Furthermore, LBB treatment resulted in a
mean AI colony number of 4.5±1.3, while treatment with EG and CO
led to a mean AI colony number of 4.9±2.5 and 4.7±2.4,
respectively. Thus, extracts from LBB, EG and CO at their
respective IC90 value produced a >80% decrease (P=0.02) in the
number of E2-promoted AI colonies.
Mechanisms for efficacy of selected
herbal extracts
The data on the effect of extracts from LBB, EG and
CO on the phases of the cell cycle are presented in Table VI. The LBB extract induced a 48.2%
reduction (P=0.04) in the S-phase population and a 1.5-fold
increase (P=0.01) in the G2/M-phase population, leading to
G2/M-phase arrest. In contrast, treatment with EG resulted in a
55.1% increase (P=0.04) in the population in G1 phase and a 83.9%
decrease (P=0.02) in the G2/M-phase population. Treatment with CO
resulted in a 33.5% increase (P=0.04) in the number of cells in the
G1 phase and a 45.9% decrease (P=0.04) in that in the S phase.
Thus, these data demonstrate that the above three extracts
counteract the growth-promoting effect of E2 via their specific
effects on distinct phases of the cell cycle, leading to an altered
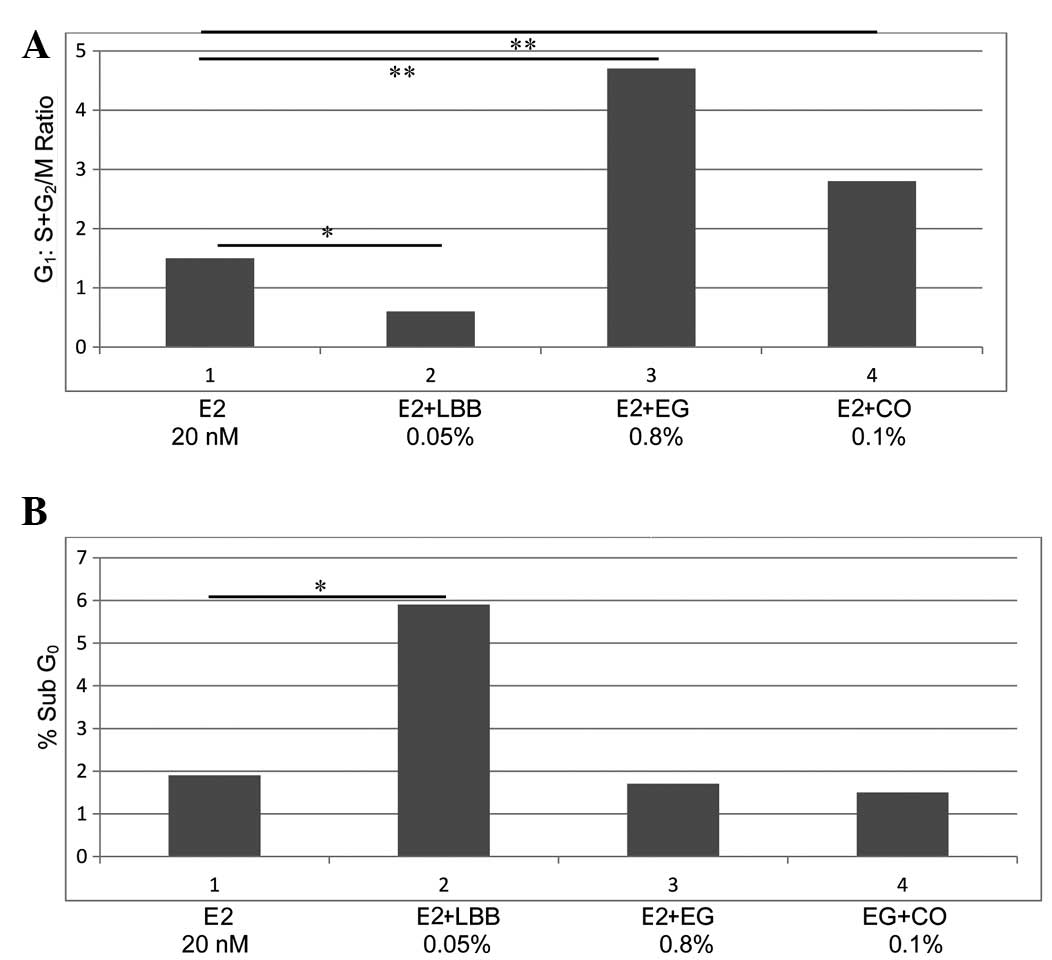
G1:S+G2/M ratio. Thus, the data on the cell cycle progression,
expressed as G1:S+G2/M ratio (mean ± SD, n=3/treatment group),
which was obtained using extracts from the selected herbs LBB, EG
and CO (Fig. 3A), demonstrated that,
compared with the control, which exhibited a G1:S+G2/M ratio of
1.02±0.3, the extract from LBB at its IC90 value led to a ratio of
0.8±0.1, which represents a 25.5% reduction (P=0.04). In contrast,
the extract from EG at its IC90 value induced a 2.4-fold increase
(ratio, 3.5±1.1; P=0.02), while the extract from CO induced a 98.0%
increase (ratio, 2.02±0.6; P=0.02).
 | Table VI.Effects of extracts from LBB, EG and
CO on the cell cycle progression of the estrogen receptor
non-functional phenotype. |
Table VI.
Effects of extracts from LBB, EG and
CO on the cell cycle progression of the estrogen receptor
non-functional phenotype.
|
|
| Cell cycle-phase
population (%)a |
|---|
|
|
|
|
|---|
| Treatment | Concentration | G1 | S | G2/M |
|---|
| E2 | 20 nM | 50.1±5.8 | 34.2±3.9 | 14.9±1.6 |
| LBB | 0.05% | 42.2±4.9 |
17.7±1.9c |
38.1±1.8d |
| EG | 0.80% |
77.7±7.8b |
19.8±1.9c |
2.4±0.2e |
| CO | 0.10% |
66.9±6.7b |
18.5±1.8c | 14.6±1.4 |
The data on the status of cellular apoptosis
presented in Fig. 3B demonstrate
that, compared with the control (sub-G0 population, 1.9±0.5%), the
extract from LBB induced a 2.1-fold increase (sub-G0 population,
5.9±1.3%; P=0.01). Additionally, fluorescence microscopy of these
apoptotic cells stained with the ApopTag Fluorescein In Situ
Apoptosis Detection kit (Merck Millipore, Darmstadt, Germany)
exhibited nuclear fragmentation corresponding to the extent of
apoptosis, as detected by the percentage of sub-G0 population (data
not shown). By contrast, extracts from EG (sub-G0 population,
1.6±0.9%) and CO (sub-G0 population, 1.4±0.9%) were essentially
ineffective in inducing cellular apoptosis. These data were not
significantly different from the control group.
The data from the experiments on the cellular
metabolism of E2 are presented as 2-OHE1:16α-OHE1 ratio (mean ± SD,
n=3/treatment group) in Fig. 4A and
as E3:16α-OHE1 ratio in Fig. 4B.
Relative to the control (ratio, 0.5±0.1), extracts from LBB (ratio,
5.2±1.1), EG (ratio, 4.9±1.0) and CO (ratio, 6.8±1.4), induced a
9.2, 8.8 and 12.6-fold increase, respectively (P=0.01), in the
2-OHE1:16α-OHE1 ratio (Fig. 4A).
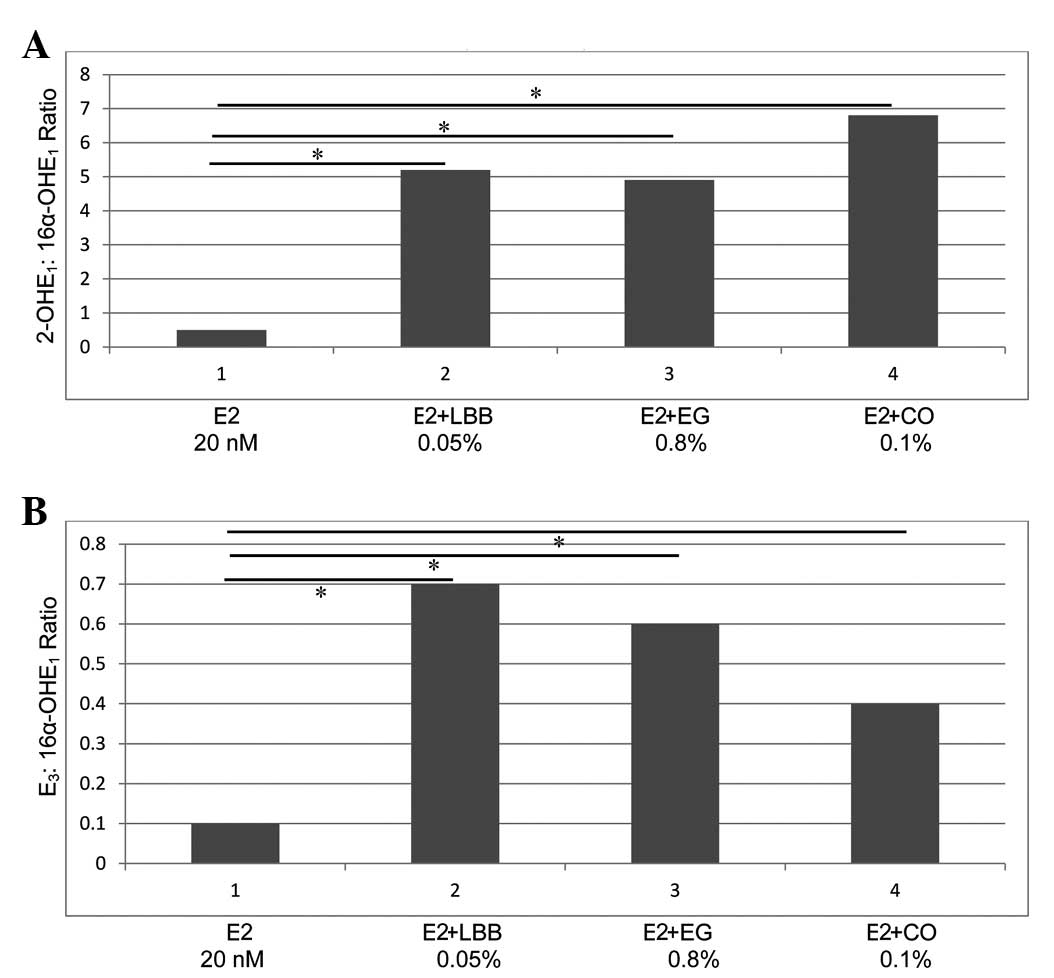 | Figure 4.(A and B) Alteration in the cellular
metabolism of E2. (A) Effect of extracts from LBB, EG and CO on the
2-OHE1:16α-OHE1 ratio. An increase in 2-OHE1:16α-OHE1 ratio was
observed in response to treatment with the three extracts, relative
to the ratio exhibited by the E2-treated control (*P=0.01). (B)
Effect of extracts from LBB, EG and CO on the
E3:16α-OHE1 ratio. An increase in
E3:16α-OHE1 ratio (*P=0.01) in response to
treatment with the three extracts was observed, relative to that
displayed by the E2−treated control. E2, 17β-estradiol;
LBB, Lycium barbarum bark; EG, Epimedium grandiflorum; CO, Cornus
officinalis; 2-OHE1; 2-hydroxyestrone; 16α-OHE1,
16α-hydroxyestrone; E3, estriol. |
Regarding the E3:16α-OHE1 ratio (Fig. 4B), relative to the control (ratio,
0.1±0.04), the LBB extract exhibited a ratio of 0.7±0.3, while the
extract from EG exhibited a ratio of 0.6±0.2 and that from CO
exhibited a ratio of 0.4±0.2, thus inducing a 6, 5 and 3-fold
increase, respectively (P=0.01).
Discussion
Global gene expression profiling of clinical breast
cancer has provided improved molecular/genetic classification of
cancer subtypes, and thereby, has facilitated rational
subtype-targeted therapy (15).
Similar classification of commercially available breast carcinoma
cell lines has refined the applicability of these human
tissue-derived preclinical models for specific molecular subtypes
(16–18). It is well established that long-term
treatment with endocrine therapy is frequently associated with de
novo or acquired resistance via deregulation of multiple
genetic/molecular pathways that are critical for proliferation and
survival signaling, and may lead to compromised therapeutic
efficacy (3). Reliable cell culture
models that facilitate the identification of cellular/molecular
pathways of therapeutic resistance should provide valuable
experimental approaches for screening of potential lead compounds
that are efficacious against therapy-resistant breast cancer.
The cell culture models for ER+/progesterone
receptor (PR)+ /human epidermal growth factor (HER-2)- luminal A
and ER-/PR-/HER-2- triple negative subtypes have been commonly used
as comparative experimental systems for ER+ and ER- clinical breast
cancer, respectively (2,8,16).
Specific genetic differences in these two models other than the
status of ER expression have been documented (16–18). These
intrinsic molecular/genetic differences may limit the utility of
these models for comparative investigations. Availability of
isogenic phenotypes that are genetically identical but differ only
in ER function should facilitate stringently controlled comparative
studies. Additionally, the present comparative study on ER-NF and
ER-F phenotypes provides an experimental approach that simulates a
situation in the progression of clinical breast cancer where
relapse is due to a change in ER status from positive to negative
(3).
The present approach on the human mammary
carcinoma-derived MCF-7 cells, which served as a model for the
breast cancer luminal A subtype (8,16,17), has provided isogenic cell phenotypes
with modulated ER function. Long-term growth adaptation of the
parental MCF-7 cells in chemically defined serum-depleted medium
(0.7% serum, E2 <1 nM) selected the transient ER-NF phenotype
due to limited availability of a physiologically relevant
concentration of E2. The ER-NF phenotype continued to exhibit
progressive growth in the presence of E2 within the physiologically
achievable range. Additionally, the growth-promoting effect of E2
was abrogated in the presence of the selective ER modulator TAM.
Collectively, these observations provided evidence that the ER-NF
and ER-F phenotypes effectively retain E2 responsiveness.
The ER-NF cells maintained in the presence of 0.7%
serum (E2 <1 nM) and the ER-F cells maintained in the presence
of 0.7% serum + 20 nM E2 provided the isogenic models for the
present study to compare the growth-inhibitory efficacy of extracts
from selected Chinese nutritional herbs. Although these ER-NF and
ER-F isogenic phenotypes adequately facilitated preliminary
screening of efficacious herbs, both these phenotypes must be
further characterized at the molecular level to confirm the
functional status of ER.
The comparative dose-response experiments of the
herbal extracts on the ER-NF and ER-F phenotypes identified the
IC50 values of the individual extracts independently for each
phenotype, and the IC50 ratio of the ER-NF vs. the ER-F phenotype
facilitated the determination of preferential efficacy and rank
ordering of the Chinese nutritional herbs on isogenic MCF-7
phenotypes with modulated ER function. The rank order of
growth-inhibitory activity based on the individual ER-NF:ER-F IC50
ratios revealed that the extracts from five herbs (LBB, EU, LBF, PC
and DA) were preferentially effective against ER-F cells, while the
extracts from four herbs (LL, CS, EG and LC) were equally effective
against ER-NF and ER-F cells, and the extracts from two herbs (AS
and CO) were preferentially effective against ER-NF cells. Thus,
the data on differential susceptibility for growth inhibition on
isogenic cells with modulated ER function provide potential leads
for the efficacy of Chinese nutritional herbs on ER+ and ER-
clinical breast cancer.
The IC90 values distinguished the maximum cytostatic
response from the toxic response. Therefore, the IC90 values for
the ER-NF and ER-F phenotypes were used for the mechanistic
biomarker experiments on AI growth, cell cycle progression,
cellular apoptosis and cellular E2 metabolism.
Selected herbal extracts representing specific
phenotype-dependent preference for growth inhibition were also
effective in inhibiting the AI growth of the ER-NF phenotype. Thus,
at their respective IC90 value, these extracts induced a >80%
reduction in the number of AI colonies, relative to those observed
in the E2-treated control group.
To identify the potential mechanisms responsible for
their growth-inhibitory efficacy, the extracts from LBB
(preferential activity against the ER-F phenotype), EG (equally
effective in ER-NF and ER-F cells) and CO (preferential activity
against the ER-NF phenotype) were examined for their effects on
cell cycle progression and cellular apoptosis. These extracts
exhibited distinct effects on cell cycle progression. Thus, the LBB
extract induced G2/M arrest and cellular apoptosis, while the
extracts from EG and CO induced G1 arrest but were ineffective
regarding the induction of cellular apoptosis. Thus, extracts from
effective herbs representative of each of the aforementioned three
groups exhibit distinct effects on cell cycle progression and/or
cellular apoptosis. Taken together, these data suggest that the
anti-proliferative activity of the above three herbal extracts may
be due to their distinct effect on specific phases of the cell
cycle.
Oxidative metabolism of E2 impacts the
carcinogenesis of endocrine-responsive target organs (19–22). E2
metabolites such as 2-OHE1 and 16α-OHE1 exhibit distinct
growth-modulatory effects on mammary epithelial cells at the
initial phases of carcinogenesis induced by certain oncogenes such
as RAS, c-Myc and HER-2, as well as on breast carcinoma-derived
cells (22–25). Thus, 2-OHE1 exhibits
anti-proliferative effects on these cells, while 16α-OHE1 promotes
cellular proliferation (22–25). Experimentally induced alterations in
E2 metabolism, measured as 2-OHE1:16α-OHE1 and E3:16α-OHE1 ratios,
provide an endocrine biomarker for carcinogenic risk and for
effective cancer prevention/therapy (10,12,13). In
the light of these observations, the effects of extracts from LBB,
EG and CO on E2 metabolism were examined in the ER-NF phenotype of
MCF-7 cells. The data from these experiments demonstrated that
these three extracts at their respective IC90 value increased the
2-OHE1:16α-OHE1 and E3:16α-OHE1 ratios due to enhanced production
of the anti-proliferative metabolites 2-OHE1 and E3.
The outcome of the present study demonstrates that
non-fractionated aqueous extracts from Chinese nutritional herbs
effectively down-modulate the growth-promoting effects of E2 via
distinct mechanisms in the isogenic cell culture model with
modulated ER function. It is noteworthy that human breast
carcinoma-derived cell culture models with differing expression of
ER, PR, HER-2, epidermal growth factor receptor, p53 and
retinoblastoma protein have been utilized to examine the
growth-inhibitory effects of aqueous extracts prepared from several
Chinese medicinal herbs (26,27). However, it should be recognized that
intrinsic genetic differences in expression of hormone and growth
factor receptors in individual cell lines preclude justifiable
comparative investigations within the existing models. By contrast,
the data from the present study provide evidence that isogenic
phenotypes with modulated ER function as the only experimental
variable represent a facile comparative approach for a
mechanism-based screening of extracts prepared from
multi-functional herbs. Furthermore, rank ordering of
preferentially efficacious herbal extracts on isogenic ER-NF and/or
ER-F phenotypes may provide valuable mechanistic leads for their
potential efficacy towards ER- and/or ER+ clinical breast
cancer.
It must be emphasized that, in the present study,
the non-fractionated aqueous extracts from the herbs were used
specifically to simulate the administration of herbal tea to
patients in traditional Chinese medicine (4,5,7). It is therefore conceivable that a
mixture of water soluble bio-active agents may be responsible for
the observed growth-inhibitory effects in the present experimental
models, and that these agents, acting individually or in
combination, may be affecting the growth of MCF-7 cells.
It is also noteworthy that the parental MCF-7 cells,
which represent a model for the luminal A molecular subtype of
clinical breast cancer, have been previously tested as a model for
endocrine therapy-resistant cancer stem cells (28). The response of MCF-7-derived stem
cells to nutritional herbs may therefore identify a testable
approach for efficacious cancer stem cell-targeted herbal therapy.
Indeed, efficacy of natural phytochemicals such as quercetin and
sulforaphane towards pancreatic cancer stem cells has been
documented (29).
In conclusion, the present study demonstrates that
the non-fractionated aqueous extracts from Chinese nutritional
herbs effectively down-modulate the growth-promoting effects of E2
via distinct mechanisms in the present isogenic cell culture model.
This aspect is specifically strengthened by the data that extracts
from herbs selectively efficacious for the ER-F or ER-NF phenotype
function via distinct effects on cell cycle progression, cellular
apoptosis and formation of anti-proliferative E2 metabolites.
Furthermore, the models developed and the results obtained in the
present study provide relevant significance to the herbal
management of ER+ or ER- clinical breast cancer, as well to the
relapse of ER+ breast cancer, in which the ER expression has become
negative (4,5,26,27,30).
Clearly, clinical usefulness of the nutritional herbs investigated
in the present study will have to be established in future human
studies.
Acknowledgements
Major funding for the present study was provided by
philanthropic contributions to the American Foundation for Chinese
Medicine (New York, NY, USA) by the family of Mr. Daniel and Ms.
Kathleen Mezzalingua, the family of Mr. Hakan and Ms. Marie Ledin,
the Laura and Isaac Perlmutter Foundation Inc. (Lake Worth, FL,
USA), the Saint Agatha Foundation (Jenkintown, PA, USA) and the
Sophie Stenbeck Family Foundation (New York, NY, USA).
References
|
1
|
Lippman ME: Efforts to combine endocrine
and chemotherapy in the management of breast cancer: Do two and two
equals three? Breast Cancer Res Treat. 3:117–127. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnston SR and Dowsett M: Aromatase
inhibitors for breast cancer: Lessons from the laboratory. Nat Rev
Cancer. 3:821–831. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Musgrove EA and Sutherland RL: Biological
determinants of endocrine resistance in breast cancer. Nat Rev
Cancer. 9:631–643. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rock E and De Michelle A: Nutritional
approaches to late toxicities of adjuvant chemotherapy in breast
cancer survivors. J Nutr. 133(Suppl 1): S3785–S3793. 2003.
|
|
5
|
Heyler LK, Chin S, Chu BK, Fitzgerald B,
Verma S, Rakovitch E, Dranitsaris G and Clemons M: The use of
complementary and alternative medicine among patients with locally
advanced breast cancer: A descriptive study. BMC Cancer. 6:392006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oudin C, Bonnetain F, Boidot R, Vegran F,
Soubeyrand MS, Arnould L, Riedinger JM and Lizard-Nacol S: Patterns
of loss of heterozygosity in breast carcinoma during neoadjuvant
chemotherapy. Int J Oncol. 30:1145–1151. 2007.PubMed/NCBI
|
|
7
|
Mathews AK, Sellergren SA, Huo D, List M
and Fleming G: Complementary and alternative medicine use among
breast cancer survivors. J Alt Comp Med. 13:555–562. 2007.
View Article : Google Scholar
|
|
8
|
Lippman ME, Osborne CK, Knazek R and Young
N: In vitro model systems for the study of hormone-dependent breast
cancer. N Engl J Med. 296:154–159. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suto A, Bradlow HL, Kubota T, Kitajima H,
Wong GY, Osborne MP and Telang NT: Alteration in proliferative and
endocrine responsiveness of human mammary carcinoma cells by
prototype tumor suppressing agents. Steroids. 58:215–219. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li G, Sepkovic DW, Bradlow HL, Telang NT
and Wong GYC: Lycium barbarum inhibits growth of estrogen receptor
positive human breast cancer cells by favorably altering estradiol
metabolism. Nutr Cancer. 61:408–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mukherjee B, Telang N and Wong GY: Growth
inhibition of estrogen receptor positive human breast cancer cells
by Taheebo from the inner bark of Tebebuia avellandae tree. Int J
Mol Med. 24:253–260. 2009.PubMed/NCBI
|
|
12
|
Telang NT, Li G, Sepkovic DW, Bradlow HL
and Wong GY: Anti-proliferative effects of Chinese herb Cornus
officinalis in a cell culture model for estrogen receptor positive
clinical breast cancer. Mol Med Rep. 5:22–28. 2012.PubMed/NCBI
|
|
13
|
Telang N, Li G, Sepkovic D, Bradlow HL and
Wong GY: Comparative efficacy of extracts from Lycium barbarum bark
and fruit on estrogen receptor positive human mammary carcinoma
MCF-7 cells. Nutr Cancer. 66:278–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Katdare M, Osborne M and Telang NT: Soy
isoflavone genestein modulates cell cycle progression and induces
apoptosis in HER-2/neu oncogene expressing human breast epithelial
cells. Int J Oncol. 21:809–815. 2002.PubMed/NCBI
|
|
15
|
Sorlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical interpretations. Proc
Natl Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neve RM, Chin K, Fridyand J, Yeh J,
Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al: A
collection of breast cancer cell lines for the study of
functionally distinct cancer subtypes. Cancer Cell. 10:515–527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Subik K, Lee JF, Baxter L, Strzepak T,
Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG and
Tang P: The expression patterns of ER, PR, HER-2, CK5/6, EGFR, Ki
67 and AR by immunohistochemical analysis in breast cancer cell
lines. Breast Cancer (Auckl). 4:35–41. 2010.PubMed/NCBI
|
|
18
|
Muller PA and Vousden KH: Mutant p53 in
cancer: New functions and therapeutic opportunities. Cancer Cell.
25:304–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schneider J, Kinne D, Frachia A, Pierce V,
Anderson KE, Bradlow HL and Fishman J: Abnormal oxidative
metabolism of estradiol in women with breast cancer. Proc Natl Acad
Sci USA. 79:3047–3051. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fishman J, Schneider J, Herschkopf RJ and
Bradlow HL: Increased estrogen 16alpha-hydroxylase activity in
women with breast and endometrial cancer. J Steroid Biochem.
20:1077–1081. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yager JD and Davidson NE: Estrogen
carcinogenesis in breast cancer. N Engl J Med. 354:270–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Telang NT: Oncogenes, estradiol
biotransformation, and mammary carcinogenesis. Ann NY Acad Sci.
784:277–287. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suto A, Telang NT, Tanino H, Takeshita T,
Ohmiya H, Osborne MP and Kubota T: In vitro and in vivo modulation
of growth regulation in the human breast cancer cell line MCF-7 by
estradiol metabolites. Breast Cancer. 6:87–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lottering ML, Haag M and Segers JC:
Effects of 17beta-estradiol metabolites on cell cycle events in
MCF-7 cells. Cancer Res. 52:5926–5932. 1992.PubMed/NCBI
|
|
25
|
Schneider J, Huh MM, Bradlow HL and
Fishman J: Antiestrogen action of 2-hydroxyestrone on MCF-7 human
breast carcinoma cells. J Biol Chem. 259:4840–4845. 1984.PubMed/NCBI
|
|
26
|
Campbell MJ, Hamilton B, Shoemaker M,
Tagliaferri M, Cohen I and Tripathy D: Anti-proliferative activity
of Chinese medicinal herbs on breast cancer cells in vitro.
Anticancer Res. 22:3843–3852. 2002.PubMed/NCBI
|
|
27
|
Chiu JH, Chang C, Wu JC, Liu HJ, Wen CS,
Hsu CH, Chen JL, Tseng LM, Chen WS and Shyr YM: Screening to
identify commonly used Chinese herbs that affect ERBB2 and ESR1
gene expression using the human breast cancer MCF-7 cell line. Evid
Based Complement Alternat Med. 2014:9654862014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Telang N: Putative cancer-initiating stem
cells in cell culture models for molecular subtypes of clinical
breast cancer. Oncol Lett. 10:3840–3846. 2015.PubMed/NCBI
|
|
29
|
Zhou W, Kallifatidis G, Baumann B, Rausch
V, Mettern J, Gladkich J, Giese N, Moldenhauer G, Wirth T, Büchler
MW, et al: Dietary polyphenol quercetin targets pancreatic cancer
stem cells. Int J Oncol. 37:551–561. 2010.PubMed/NCBI
|
|
30
|
Ye L, Jia Y, Ji KE, Sanders AJ, Xue K, Ji
J, Mason MD and Jiang WG: Traditional Chinese medicine in the
prevention and treatment of cancer and cancer metastasis. Oncol
Lett. 10:1240–1250. 2015.PubMed/NCBI
|