Introduction
Urothelial cell carcinoma (UCC) is one of the most
common types of cancer in the USA, accounting for ~4.5% of all
newly diagnosed cancer cases and 2.8% of all cancer-associated
mortalities in 2013 (1). In the USA,
the most common site of UCC is the bladder, and bladder UCC is the
fourth most common type of cancer and the sixth leading cause of
cancer-associated mortality in males (1). Bladder UCC may be classified into two
subtypes at diagnosis, non-muscle and muscle-invasive, which
exhibit distinct clinical features (2). The non-muscle-invasive subtype commonly
recurs in the bladder cavity and accounts for 70℃80% of cases of
bladder UCC, with muscle invasion present in only 10–20% of cases.
The 5-year survival rate of the non-muscle-invasive subtype of UCC
is >90%. By contrast, muscle-invasive bladder UCC, which
accounts for ~20% of bladder UCC cases, exhibits a poor prognosis
with a 5-year survival rate of <50%. Carcinoma in situ
(CIS), which is classified into the non-muscle invasive aggressive
subtype, is localized to the epithelium and exhibits invasive and
metastatic potential. In patients with aggressive bladder UCC
(muscle invasive UCC and CIS) without lymph nodes or distant
metastasis, the standard and most effective treatment is total
resection of the bladder and urethra, which includes the prostate
in male patients. However, ≤50% of patients who undergo total
cystectomy exhibit recurrence and develop distant metastases
(3–5).
Aggressive UCC with malignant potential may also originate from the
upper urinary tract, renal calyx, renal pelvis and ureter (6). In patients with UCC of the upper urinary
tract without lymph node or distant metastasis, the most effective
treatment is total resection of the diseased section of the upper
urinary tract, including the kidney, ureter and a section of the
bladder (nephroureterectomy); however, ~40% of UCC patients that
undergo total nephroureterectomy have succumbed to the disease 5
years after surgery (7). In general,
patients with muscle invasive bladder UCC, CIS of the lower urinary
tract and UCC of the upper urinary tract are at high risk of
recurrence, metastases and mortality even after radical surgery
(8,9).
Therefore, to improve prognosis of the patients with aggressive
UCC, further treatments in addition to radical surgery are
required. Presurgical neoadjuvant cisplatin chemotherapy with
methotrexate, vinblastine, doxorubicin and cisplatin (10) and gemicitabin plus cisplatin (11) have been demonstrated to improve the
survival of patients with muscle invasive UCC. Immunohistochemical
markers are useful for the prediction of cancer behavior and
appropriate treatment choice and thus may contribute to improving
patient survival. The cell cycle regulators, p53, retinoblastoma
protein, p21, p27 and cyclin E1, have been reported to predict
disease recurrence and mortality following radical cystectomy in
patients with pTa-pT3 node-negative UCC (12). A combination of these markers
exhibited significantly higher predictive accuracy for disease
recurrence and cancer specific mortality compared with isolated use
of the markers; however, the use of individual markers did not
improve the predictive accuracy (12–14).
Therefore, the identification of a novel marker that predicts
survival of patients with aggressive UCC more accurately is
urgently required.
The anaphylatoxin complement component 5 fragment a
(C5a) is an N-terminal 74-amino acid fragment that is released from
the α-chain of C5, which functions as a leukocyte chemoattractant
and inflammatory mediator (15,16).
Increasing evidence has identified that the complement system is
activated in human cancer tissues (17,18) and in
animal cancer models (19,20), indicating that C5a may be present in
the cancer microenvironment. C5a functions by binding to the C5a
receptor (C5aR), which was originally identified in leukocyte cell
lines (21). In our previous study,
aberrant C5aR expression in cancer cells was identified in a number
of different organs and C5a was demonstrated to enhance cancer cell
invasiveness by activating motility and increasing matrix
metalloproteinase release in a C5aR-dependent manner (22). Furthermore, direct C5a release from C5
by cell membrane proteases of cancer cells and promotion of
C5aR-expressing cancer cell invasiveness by C5a was demonstrated,
indicating that a self-activation circuit via C5aR exists in cancer
cells that express C5aR and proteases on the cell surface (23). Notably, a previous study revealed that
a C5aR agonist increased cancer cell proliferation and C5aR
silencing reduced tumor growth (24).
Thus, we hypothesize that C5aR-positive cancer cells are more
aggressive than C5aR-negative cells and thus, C5aR expression in
cancer cells may be associated with poor prognosis of cancer
patients.
To determine whether C5aR expression affects the
prognosis of patients with aggressive UCC undergoing radical
surgery, the association of UCC C5aR expression with
clinicopathological parameters and survival outcomes of patients
was evaluated.
Materials and methods
Patients and UCC samples
Cancerous and non-cancerous adjacent tissues were
obtained from patients during surgery. A total of 52 patients with
aggressive UCC who underwent radical cystectomy for bladder UCC (41
patients; 78%) or radical nephroureterectomy for upper urinary
tract UCC (11 patients; 22%) at Kumamoto University Hospital
(Kumamoto, Japan) between April 1996 and March 2013 were enrolled
in the present study (Table I). The
patient cohort included 39 male patients and 13 female patients
with a median age of 72 years (range, 43–86 years). The median
duration of follow-up was 27.4 months (range, 0.53–150.0 months). A
total of 16 patients (30.8%) succumbed to urothelial cancer and 5
patients (9.6%) succumbed due to other causes (4 pneumonitis; 1
unknown). Written informed consent for the use cancer and adjacent
noncancerous tissues and clinicopathological records was obtained
from all the patients prior to surgery. The Kumamoto University
Hospital Ethics Committee approved the use of the samples and
records for the present study.
 | Table I.Association between C5aR expression
and the clinicopathological parameters of 52 urothelial carcinoma
cancer patients. |
Table I.
Association between C5aR expression
and the clinicopathological parameters of 52 urothelial carcinoma
cancer patients.
| Parameters | Patients,
na | C5aR (+), n | C5aR (−), n | P-value |
|---|
| Age |
| Median,
years (range) | 52 | 73.0 (43–86) | 66.5 (53–83) | 0.3640b |
| Gender |
| Male | 39 | 28 | 11 | 0.5116c |
|
Female | 13 | 10 | 3 |
|
| Tumor location |
| Upper
urinary tract | 11 | 7 | 4 | 0.3302c |
|
Bladder | 41 | 31 | 10 |
|
| WHO grade |
| G1–2 | 13 | 10 | 3 | 0.5047c |
| G3 | 35 | 25 | 10 |
|
| T stage |
|
T1–2 | 28 | 22 | 6 | 0.3659c |
|
T3–4 | 20 | 14 | 6 |
|
| Blood vessel
invasion, +/− | 19/26 | 13/21 | 6/5 | 0.2726c |
| Lymph node
invasion, +/− | 19/28 | 13/22 | 6/6 | 0.3265c |
| Stage of
disease |
|
I–II | 24 | 20 | 4 | 0.1590c |
|
III–IV | 24 | 16 | 8 |
|
Immunohistochemistry and retrospective
analysis
Deparaffinized tissue sections (2-µm) were
pretreated with 0.3% H2O2 in methanol for 20
min, followed by treatment with Protein Block Serum-Free (Dako
Cytomation, Carpinteria, CA, USA) for 20 min. Sections were
incubated with primary mouse anti-human C5aR antibody (2 µg/ml;
dilution, 1:50; catalog no., HM2094; Hycult Biotech, Uden, The
Netherlands) or nonspecific mouse IgG (2 µg/ml; dilution, 1:50;
catalog no., ×0944; Dako Cytomation) at room temperature for 1 h
followed by staining with EnVision+ solution (Dako Cytomation) and
3,3′-diaminobenzidine tetrahydrochloride solution (Wako Pure
Chemical Industries, Ltd., Osaka, Japan) containing 0.006%
H2O2, according to the manufacturer's
instructions. Nuclei were counterstained with hematoxylin (Kanto
Chemical Co., Inc., Tokyo, Japan) and visualized with an optical
microscope. Histopathological analyses were performed by senior
pathologists and reviewed by the chief of clinical pathology. All
tumors used in this study were classified as UCC according to the
World Health Organization (WHO) grading criteria (25,26). The
association between UCC C5aR expression and patient survival, tumor
stage and grade was analyzed. All the analyses were conducted by
investigators blinded to the outcome of the patients.
Statistical analysis
In the analysis of clinicopathological parameters in
association with UCC C5aR expression, Fisher's exact test was used,
with the exception of age, which was analyzed with the Mann-Whitney
U test. Kaplan-Meier analysis was performed to calculate 5-year
survival rates and the log-rank test was conducted to analyze
overall survival (OS). The Cox proportional hazards model was used
to calculate the hazard ratio (HR) and 95% confidence interval (CI)
for univariate and multivariate analysis. P<0.05 was considered
to indicate a statistically significant difference. All statistical
analyses were performed using SPSS version 20.0 statistical
software (IBM Corporation, Armonk, NY, USA).
Results
C5aR expression in UCC
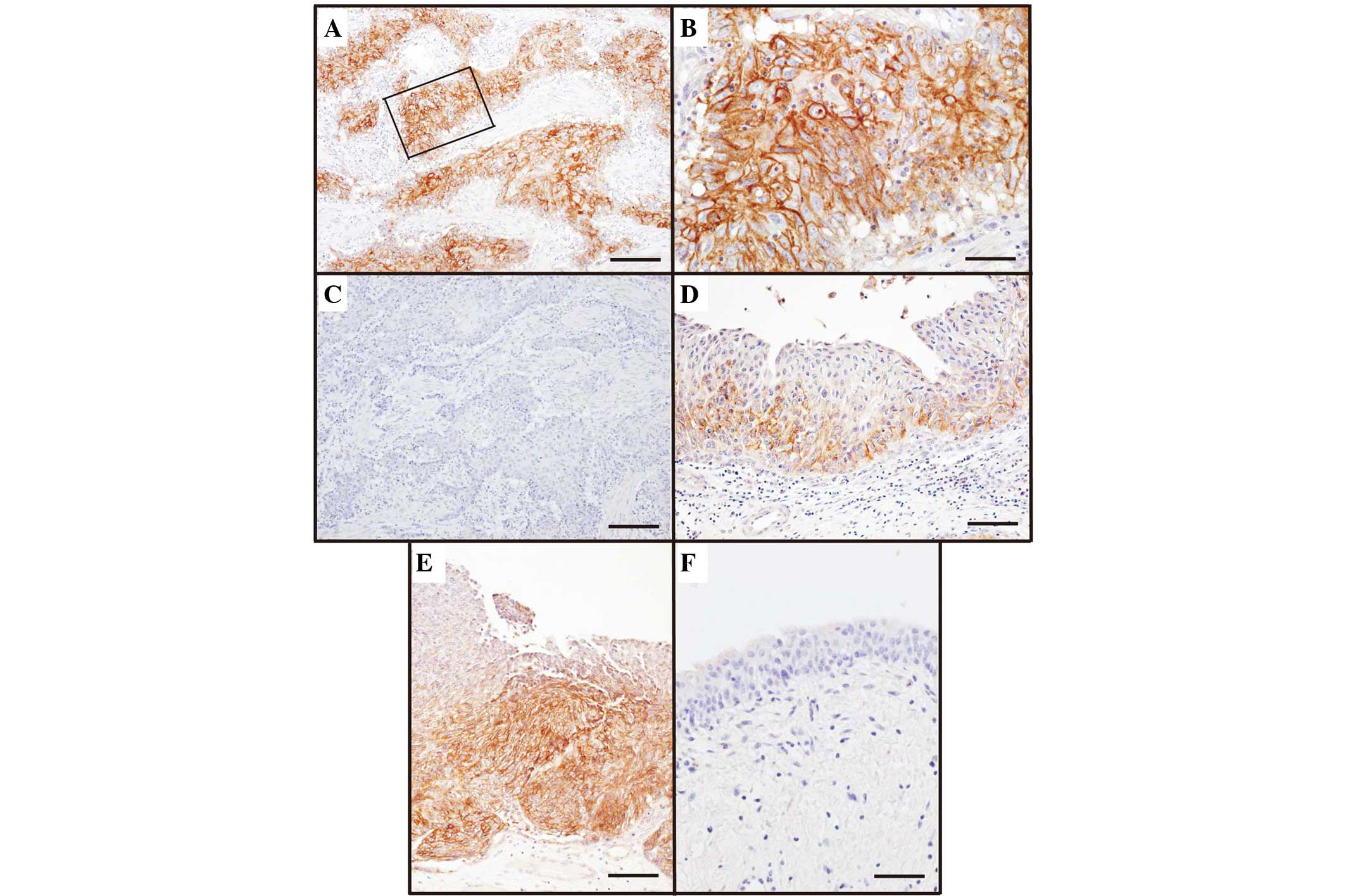
C5aR expression was identified in 38 UCC patients
(73%) (Table I). C5aR staining
(Fig. 1) was localized to the UCC
cell membrane (Fig. 1A-C), however,
no C5aR expression was identified in noncancerous urothelial cells
or adjacent cells (Fig. 1F). Notably,
UCC cells at the invasive front rather than the surface layer
expressed C5aR, and almost all UCC cells in the deeper invasion
site were C5aR-positive (Fig. 1D and
E).
Association between UCC C5aR
expression and clinicopathological parameters
The associations between clinicopathological
parameters and C5aR expression were investigated (Table I). Previously, age was demonstrated as
a risk factor for developing UCC of the bladder and advanced
bladder UCC is more common in females who exhibit worse survival
rates than males (27). Since there
was no significant association of UCC C5aR expression with age and
gender in the present study, the two parameters are unlikely to
show bias towards the low overall survival rate of patients with
C5aR-positive UCC. No significant differences in C5aR expression
were identified between different UCC locations, including the
bladder and upper urinary tract. Furthermore, UCC C5aR expression
was not associated with tumor grade, pathological tumor stage,
vessel invasion or clinical stage; however, it is notable that UCC
C5aR-positive rate was markedly high in tumors at T1–2 and of
patients at stage I–II.
Association between UCC C5aR
expression and patient survival
To determine whether C5aR expression correlated with
UCC patient prognosis, patient survival times were investigated. No
significant differences in 5-year survival rate and OS rate were
identified between UCC of the upper (pelvic/ureter) and lower
(bladder) urinary tract (Table II),
which enabled survival rate analysis to be conducted for all
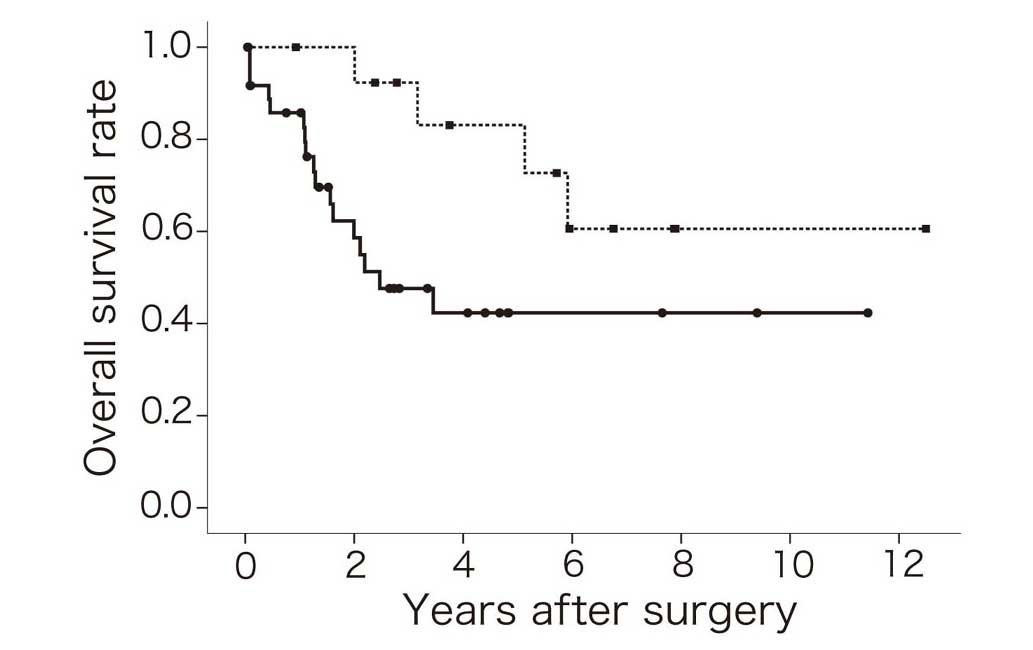
patients in this study. The OS rate of C5aR-positive UCC patients
was lower than that of C5aR-negative patients (Fig. 2). Furthermore, the 5-year survival
rate of C5aR-positive UCC patients was ~50% lower compared with
C5aR-negative UCC patients (Table
II). Univariate (HR, 3.14; P=0.035) and multivariate (HR, 3.92;
P=0.029) analyses revealed that the OS rate of patients with
C5aR-positive UCC was significantly lower than that of patients
with C5aR-negative UCC. However, no significant differences in OS
rate were identified between UCC WHO grade or stage of disease
(Table II). Furthermore, UCC C5aR
expression was not associated with the disease-specific survival
rate of patients (P=0.092); however, the disease-specific 5-year
survival rate was significantly lower in patients with
C5aR-positive UCC (0.51; 95% CI, 0.30–0.71) than patients with
C5aR-negative UCC (0.83; 95% CI, 0.62–1.00) (P=0.032).
 | Table II.Univariate and multivariate analysis
of overall survival in 52 urothelial carcinoma patients. |
Table II.
Univariate and multivariate analysis
of overall survival in 52 urothelial carcinoma patients.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Parameter | 5-year survival
rate | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Location
(PU/B) | 0.62/0.53 | 1.89 | 0.56–6.45 | 0.299 | 2.02 | 0.51–7.94 | 0.313 |
| C5aR expression
(−/+) | 0.83/0.42 | 3.14 | 1.03–9.60 | 0.035 | 3.92 | 1.15–13.4 | 0.029 |
| WHO grade
(3/1–2) | 0.54/0.50 | 1.04 | 0.38–2.88 | 0.938 | 1.46 | 0.48–4.41 | 0.508 |
| Stage of disease
(I–II/III–IV) | 0.64/0.43 | 1.19 | 0.77–4.82 | 0.156 | 2.30 | 0.86–6.16 | 0.099 |
Discussion
The present study identified that C5aR is expressed
in aggressive type UCC cells and revealed that patients with
C5aR-positive UCC exhibit lower survival rates when compared with
C5aR-negative UCC patients. Aggressive type UCC cells expressed
C5aR at the greatest rate (73%) (Fig.
1; Table I) compared with the
C5aR-positive rates of other types of cancer, as previously
reported (22). This finding may
indicate an association between C5aR expression and UCC
aggressiveness. Invasiveness of C5aR-expressing cancer cells is
augmented by C5a (22), which is
present in the cancer microenvironment (17–20). UCC
cells at the invasive front are in direct contact with interstitial
fluid that contains C5, which facilitates C5a release by cancer
cell proteases (23). C5aR expression
in UCC cells at the invasive front (Fig.
1D and E) appears to be correlated with the invasiveness of
aggressive UCC (3). Thus, the poor
outcomes of patients with C5aR-positive UCC (Fig. 2; Table
II) may be associated with the invasive potential of
C5a-activated C5aR-positive cancer cells (22).
Pathological factors have been widely used to
predict the outcomes of patients with cancer, including UCC. In
UCC, advanced tumor stage and grade are associated with distant
metastasis and increased invasive potential (28). In addition, tumor size (diameter ≥3
cm) and lymph node invasion indicate a worse prognosis for UCC
patients (29,30). However, ~20% of aggressive UCC
patients that undergo total cystectomy experience recurrence
(4) and the survival rate of UCC
patients that undergo total nephroureterectomy is relatively low
(~60%) (7). These studies indicate
the limited efficacy of radical surgeries and highlight the urgent
requirement for other reliable prognostic/predictive markers to
identify patients that require additional treatments to decrease
the risk of cancer recurrence and mortality. In the present study,
C5aR expression was identified as a possible negative prognostic
factor for aggressive UCC (Table
II). In this study, C5aR-expressing UCC patients exhibited the
shortest OS times and the highest HRs, which may indicate that
disease-associated mortality is more closely correlated with C5aR
expression than high cancer grade or advanced clinical stage. In
addition, no significant associations were identified between UCC
C5aR expression and negative prognostic factors, including tumor
grade and stage, vessel invasion or clinical stage (Table I). Multivariate analysis revealed that
C5aR-positive UCC patients exhibited a significantly lower OS rate
than C5aR-negative UCC patients (Table
II). Therefore, these results indicate that C5aR expression is
an independent prognostic factor of poor outcomes for UCC
patients.
A previous study demonstrated a synergistic effect
of the apoptosis markers, Bcl-2, caspase-3, p53 and survivin, on
the progression of bladder cancer and revealed that changes in the
expression of the four markers in patients that underwent radical
cystectomy was independently predictive of high risk for disease
recurrence and mortality (31).
Although it has not yet been demonstrated in human UCC, C5aR
signaling suppresses apoptosis, induces T-cell expansion via the
enhanced expression of Bcl-2 (32)
and enhances neuronal cell survival via inhibition of caspase-3
activation (33). C5a is present in
the cancer microenvironment (17,20,23),
where, by stimulation with C5a, the apoptosis of C5aR-expressing
UCC may be suppressed; this supression facilitates the growth and
development of cancer in concert with the C5a-elicited enhancement
of proliferation (24) and
invasiveness (22). Thus, C5aR
expression in UCC may correlate with the low survival rate
exhibited by patients (Table II). It
has been reported that high C5aR mRNA expression in cancer cells is
associated with short survival time in patients with ovarian or
lung cancer (24), in accordance with
the results of the present study, which revealed that C5aR protein
expression of UCC cells correlated with low survival rates in UCC
patients (Fig. 2; Table II). These results indicate that
cancer cell C5aR expression is a marker of poor prognosis.
In bladder cancer patients that underwent radical
cystectomy, survivin overexpression was demonstrated to be
associated with increased overall mortality, cancer specific
mortality and tumor recurrence (34).
However, increased survivin expression was also associated with
advanced pathological stage, lymphovascular invasion and lymph node
metastasis, which indicates that survivin expression is lower at
the early stages of UCC than the advanced stages. By contrast,
higher C5aR expression at organ-confined cancer stages, T1 and T2,
and higher HR of C5aR expression as an independent prognostic
factor of poor outcomes (Tables I and
II), indicates that UCC C5aR
expression may lead to the use of additional treatments at
relatively early stages of disease for patients undergoing surgery.
Since C5aR-positive UCC patients exhibit a lower disease-specific
5-year survival rate than C5aR-negative UCC patients, additional
treatments at the earlier stages of disease may improve prognosis.
Further study is required to establish an association between UCC
C5aR expression and clinical outcomes, including cancer recurrence,
progression and mortality, for early stage UCC patients with
C5aR-positive cells in the urine or tissues obtained from
transurethral resection or bladder tumor biopsy.
In conclusion, UCC C5aR expression was identified as
an independent marker of poor prognosis in aggressive UCC in the
present study. Notably, C5aR expression is higher at earlier stages
of UCC and thus, immunohistochemical staining of C5aR in UCC may be
applicable for clinical examination (cytological analysis of urine
and/or biopsied tissues), to provide information regarding tumor
aggressiveness. This information may also be used to predict the
prognosis of UCC patients. Future studies that evaluate the
efficacy of additional treatments, such as adjuvant chemotherapy,
radiotherapy and/or molecular-targeted therapy, for the prevention
of recurrence and improvement of survival outcomes in C5aR-positive
UCC patients are required.
Acknowledgements
This study was partially supported by The Japanese
Science Progress Society (grant no's. 22590363 and 25460498).
References
|
1
|
Siegel R, Naishadham MA and Jemal A:
Cancer statistics, 2013. CA Cancer J Clin. 63:11–30. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knowles MA: Molecular subtypes of bladder
cancer: Jekyll and Hyde or chalk and cheese? Carcinogenesis.
27:361–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of invasive bladder
cancer: Long-term results in 1,054 patients. J Clin Oncol.
19:666–675. 2001.PubMed/NCBI
|
|
4
|
Hautmann RE, Gschwend JE, de Petriconi RC,
Kron M and Volkmer BG: Cystectomy for transitional cell carcinoma
of the bladder: Results of a surgery only series in the neobaldder
era. J Urol. 176:486–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shariat SF, Karakiewicz PI, Palapattu GS,
Lotan Y, Rogers CG, Amiel GE, Vazina A, Gupta A, Bastian PJ,
Sagalowsky AI, et al: Outcomes of radical cystectomy for
transitional cell carcinoma of the bladder: A comtemporary series
from the Bladder Cancer Research Consortium. J Urol. 176:2414–2422.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dinney CP, McConkey DJ, Millikan RE, Wu X,
Bar-Eli M, Adam L, Kamat AM, Siefker-Radtke AO, Tuziak T, Sabichi
AL, et al: Focus on bladder cancer. Cancer Cell. 6:111–116. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi Y, Saika T, Manabe D, Nasu Y and
Kumon H: Prognostic factors influencing survival after
nephroureterectomy for transitional cell carcinoma of the upper
urinary tract. Acta Med Okayama. 65:143–149. 2011.PubMed/NCBI
|
|
8
|
Hall MC, Womack S, Sagalowsky AI, Carmody
T, Erickstad MD and Roehrborn CG: Prognostic factors, recurrence,
and survival in transitional cell cartinoma of the upper tract: A
30-year experience in 252 patients. Urology. 52:594–601. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Casey RG, Catto JWF, Cheng L, Cookson MS,
Herr H, Shariat S, Witjes JA and Black PC: Diagnosis and management
of urothelial carcinoma in situ of the lower urinary tract: A
systematic review. Eur Urol. 67:876–888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grossman HB, Natale RB, Tangen CM,
Speights VO, Vogelzang NJ, Trump DL, White RW deVere, Sarosdy MF,
Wood DP Jr, Raghavan D and Crawford ED: Neoadjuvant chemotherapy
plus cystectomy compared with cystectomy alone for locally advanced
bladder cancer. N Engl J Med. 349:859–866. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077.
2000.PubMed/NCBI
|
|
12
|
Shariat SF, Karakiewicz PI, Ashfaq R,
Lerner SP, Palapattu GS, Cote RJ, Sagalowsky AI and Lotan Y:
Multiple biomarkers improve prediction of bladder cancer recurrence
and mortality in patients undergoing cystectomy. Cancer.
112:315–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shariat SF, Zlotta AR, Ashfaq R,
Sagalowsky AI and Lotan Y: Cooperative effect of cell-cycle
regulators expression on bladder cancer development and biological
aggressiveness. Mod Pathol. 20:445–459. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shariat SF, Chade DC, Karakiewicz PI,
Ashfaq R, Isbarn H, Fradet Y, Bastian PJ, Nielsen ME, Capitanio U,
Jeldres C, et al: Combination of multiple markers can improve
prognostication in patients with locally advanced and lymph node
positive bladder cancer. J Urol. 183:68–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo RF and Ward PA: Role of C5a in
inflammatory responses. Annu Rev Immunol. 23:821–852. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Markiewski MM and Lambris JD: The role of
complement in inflammatory diseases from behind the scenes into the
spotlight. Am J Pathol. 171:715–727. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niculescu F, Rus HG, Retegan M and Vlaicu
R: Persistent complement activation on tumor cells in breast
cancer. Am J Pathol. 140:1039–1043. 1992.PubMed/NCBI
|
|
18
|
Bjørge L, Hakulinen J, Vintermyr OK, Jarva
H, Jensen TS, Iversen OE and Meri S: Ascitic complement system in
ovarian cancer. Br J Cancer. 92:895–905. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Markiewski MM, DeAngelis RA, Benencia F,
Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G and
Lambris JD: Modulation of the antitumor immune response by
complement. Nat Immunol. 9:1225–1235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Corrales L, Ajona D, Rafail S, Lasarte JJ,
Riezu-Boj JI, Lambris JD, Rouzaut A, Pajares MJ, Montuenga LM and
Pio R: Anaphylatoxin C5a creates a favorable microenvironment for
lung cancer progression. J Immunol. 189:4674–4683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gerard NP and Gerard C: The chemotactic
receptor for human C5a anaphylatoxin. Nature. 349:614–617. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nitta H, Wada Y, Kawano Y, Murakami Y,
Irie A, Taniguchi K, Kikuchi K, Yamada G, Suzuki K, Honda J, et al:
Enhancement of human cancer cell motility and invasiveness by
anaphylatoxin C5a via aberrantly expressed C5a receptor (CD88).
Clin Cancer Res. 19:2004–2013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nitta H, Murakami Y, Wada Y, Eto M, Baba H
and Imamura T: Cancer cells release anaphylatoxin C5a from C5 by
serine protease to enhance invasiveness. Oncol Rep. 32:1715–1719.
2014.PubMed/NCBI
|
|
24
|
Cho MS, Vasquez HG, Rupaimoole R, Pradeep
S, Wu S, Zand B, Han HD, Rodriguez-Aguayo C, Bottsford-Miller J,
Huang J, et al: Autocrine effects of tumor-derived complement. Cell
Rep. 6:1085–1095. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eble JN, Sauter G, Epstein JI and
Sesterhenn IA: World Health Organization Classification of
TumoursPathology and Genetics of Tumours of the Urinary System and
Male Genital Organs. IARC Press; Lyon: 2004
|
|
26
|
Mostofi FK, Sobin LH and Torloni H:
Histological typing of urinary bladder tumoursInternational
Classification of Tumours. 10. World Health Organization; Geneva:
1973
|
|
27
|
Shariat SF, Sfakianos JP, Droller MJ,
Karakiewicz PI, Meryn S and Bochner BH: The effect of age and
gender on bladder cancer: A critical review of the literature. BJU
Int. 105:300–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lughezzani G, Burger M, Margulis V, Matin
SF, Novara G, Roupret M, Shariat SF, Wood CG and Zigeuner R:
Prognostic factors in upper urinary tract urothelial carcinomas: A
comprehensive review of the current literature. Eur Urol.
62:100–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Simone G, Papalia R, Loreto A, Leonardo C,
Sentinelli S and Gallucci M: Independent prognostic value of tumour
diameter and tumournecrosis in upper urinary tract urothelial
carcinoma. BJU Int. 103:1052–1057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kikuchi E, Margulis V, Karakiewicz PI,
Roscigno M, Mikami S, Lotan Y, Remzi M, Bolenz C, Langner C, Weizer
A, et al: Lymphovascular invasion predicts clinical outcomes in
patients with node-negative upper tract urothelial carcinoma. J
Clin Oncol. 27:612–618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karam JA, Lotan Y, Karakiewicz PI, Ashfaq
R, Sagalowsky AI, Roehrborn CG and Shariat SF: Use of combined
apoptosis biomarkers for predicition of bladder cancer recurrence
and mortality after radical cystectomy. Lancet Oncol. 8:128–136.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lalli PN, Strainic MG, Yang M, Lin F,
Medof ME and Heeger PS: Locally produced C5a binds to T
cell-expressed C5aR to enhance effector T-cell expansion by
limiting antigen-induced apoptosis. Blood. 112:1759–1766. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bénard M, Gonzalez BJ, Schouft MT,
Falluel-Morel A, Vaudry D, Chan P, Vaudry H and Fontaine M:
Characterization of C3a and C5a receptors in rat cellebellar
granule neurons during maturation. Neuroprotective effect of C5a
against apoptotic cell death. J Biol Chem. 279:43487–43496. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shariat SF, Ashfaq R, Karakiewicz PI,
Saeedi O, Sagalowsky AI and Lotan Y: Survivin expression is
associated with bladder cancer presence, stage, progression and
mortality. Cancer. 109:1106–1113. 2007. View Article : Google Scholar : PubMed/NCBI
|