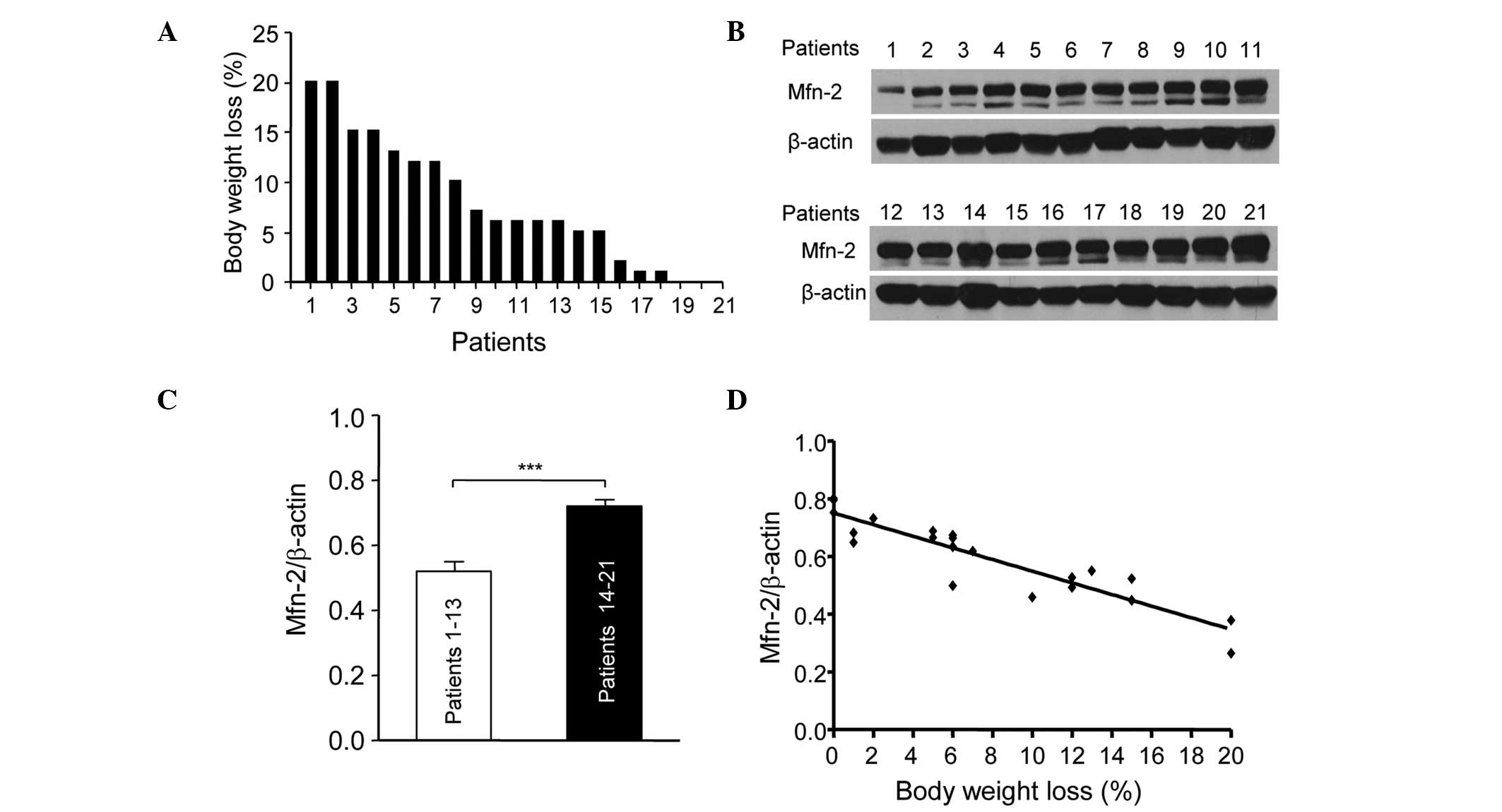
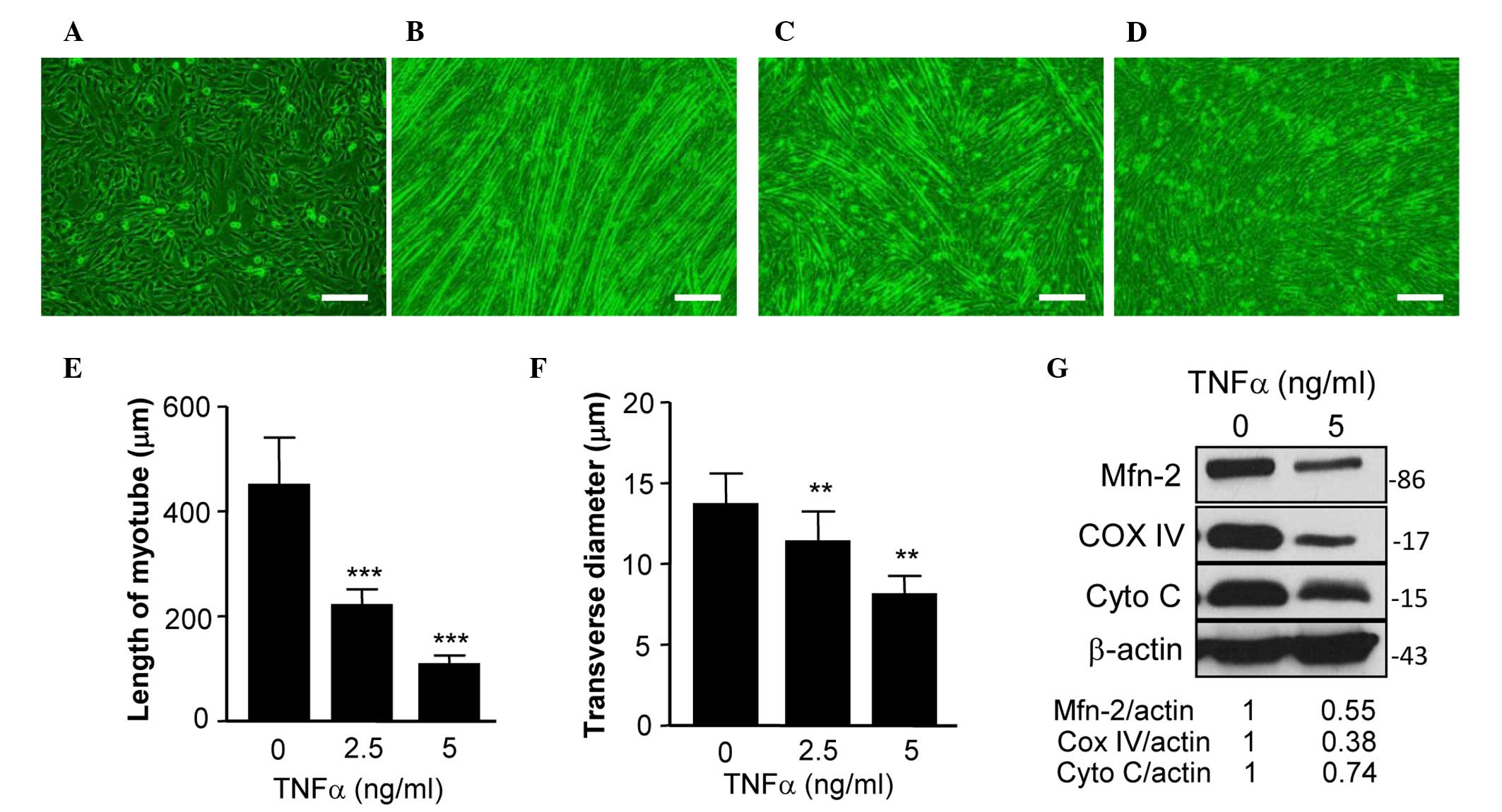
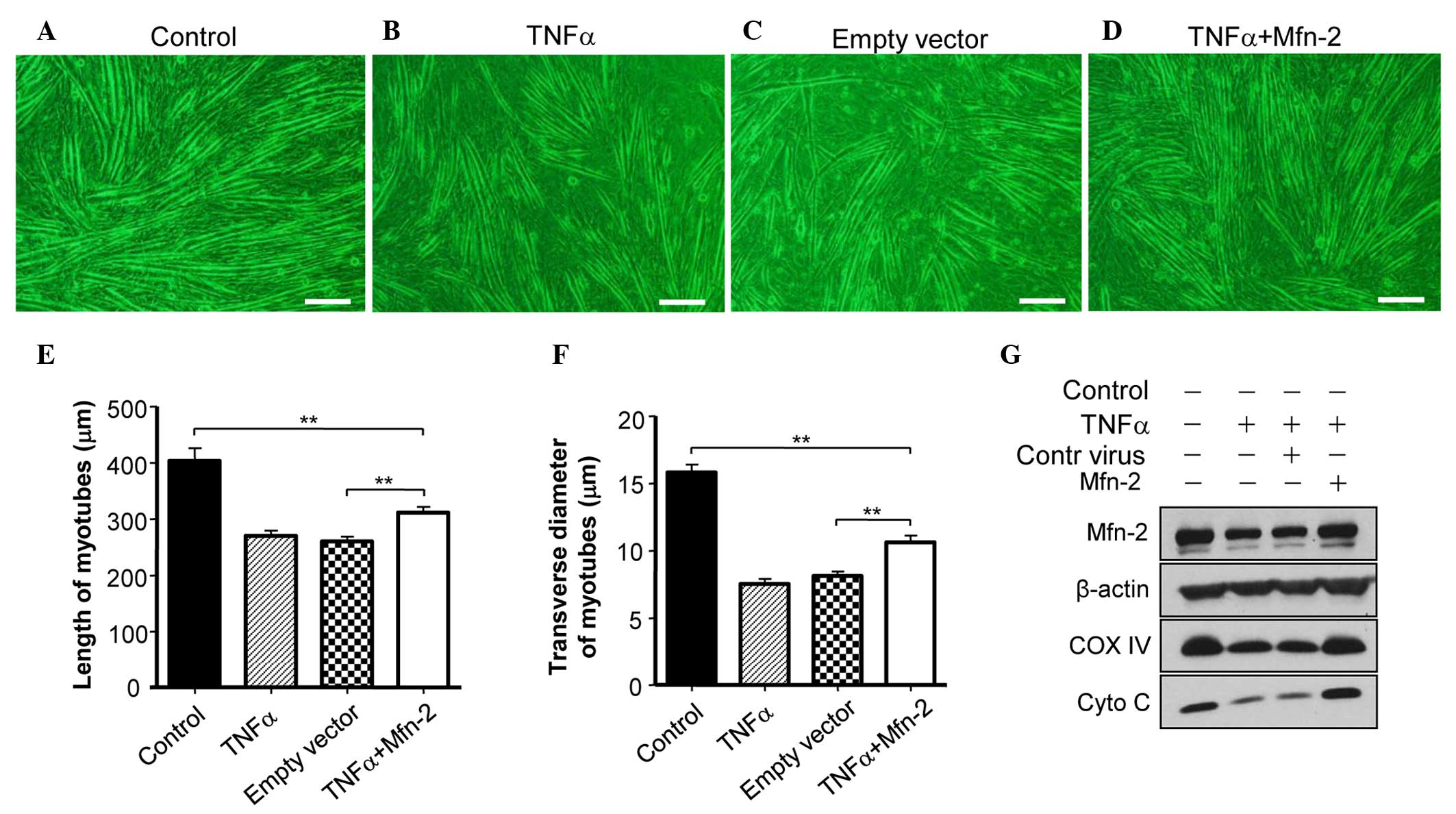
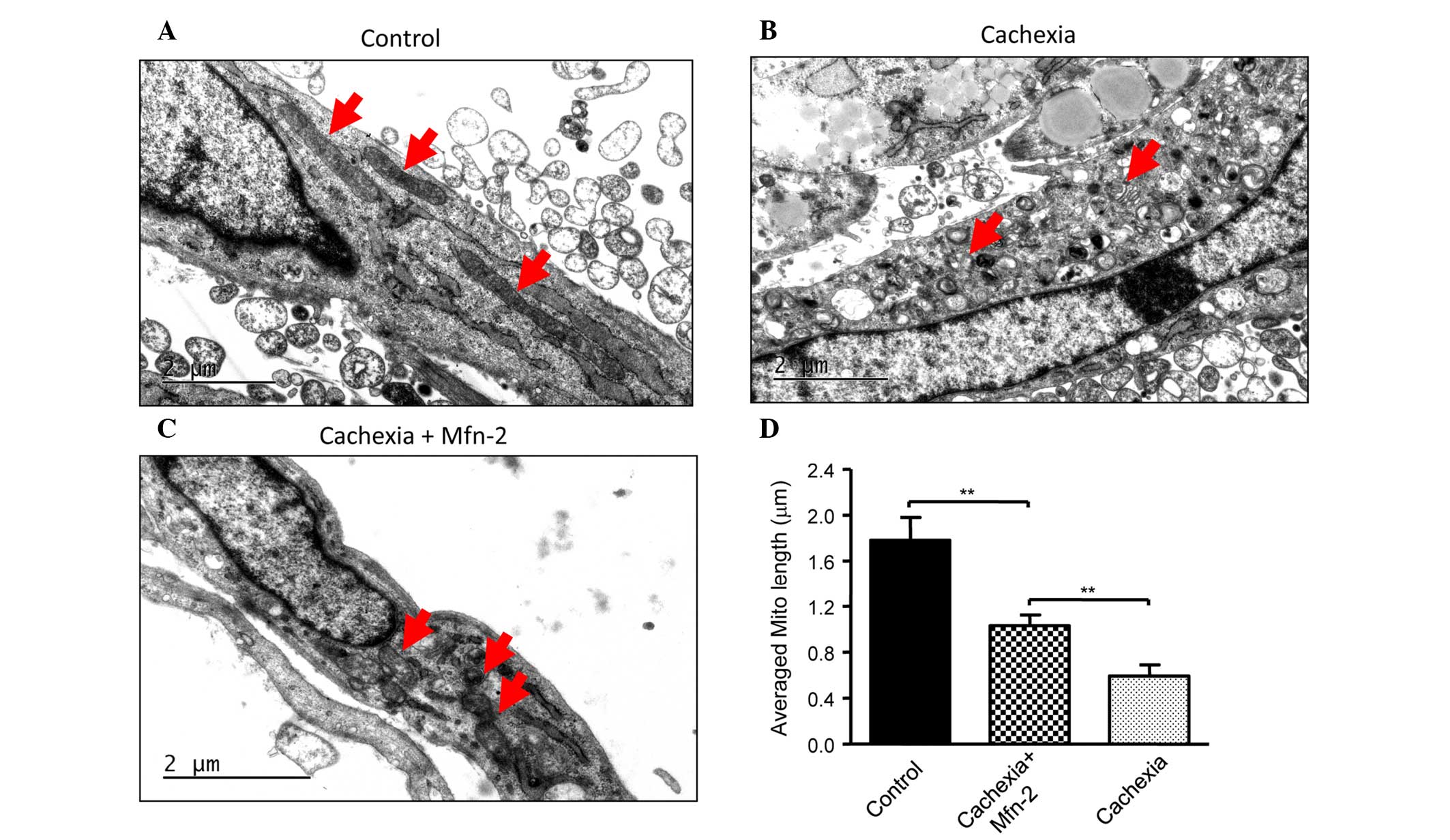
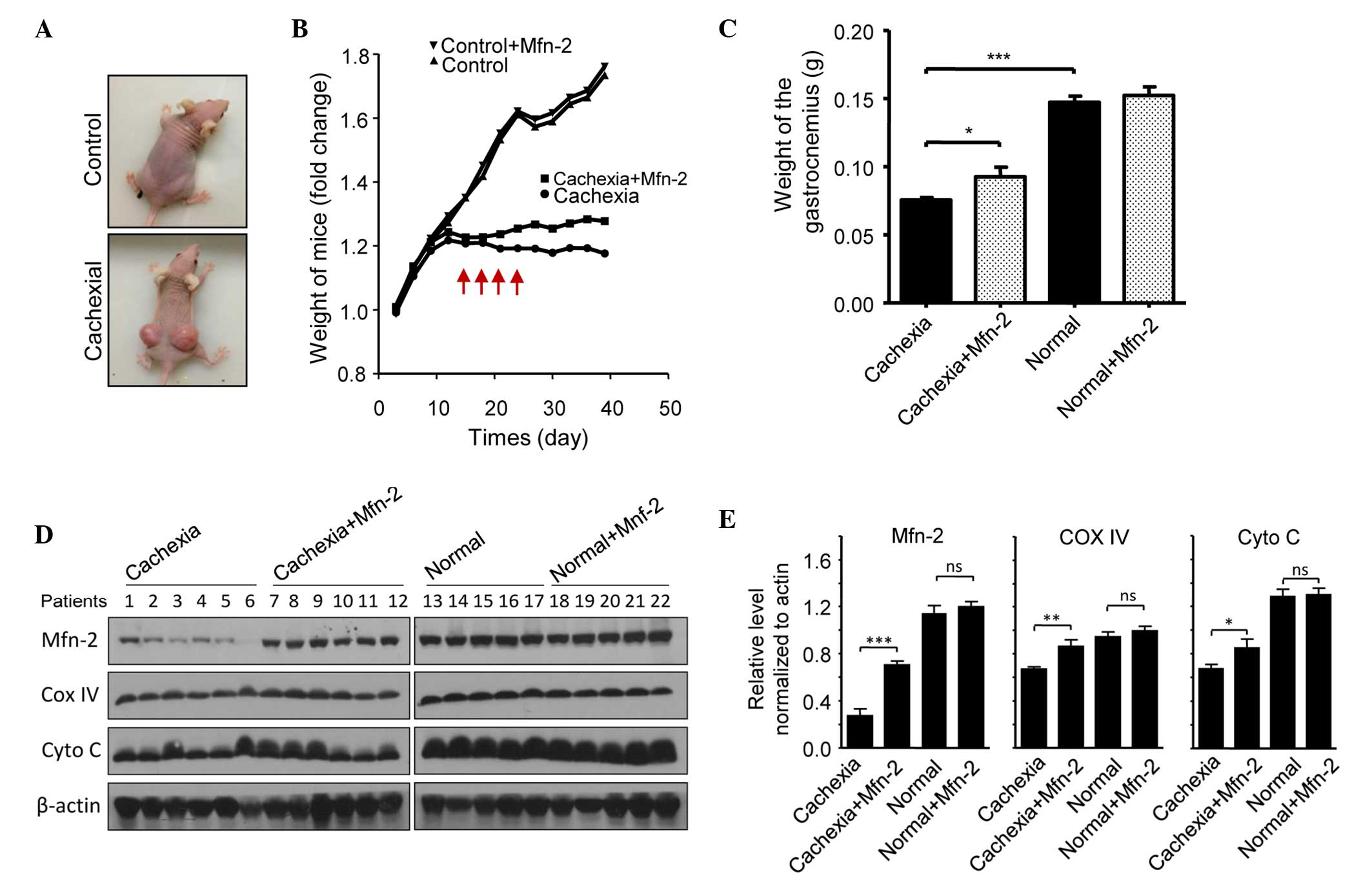
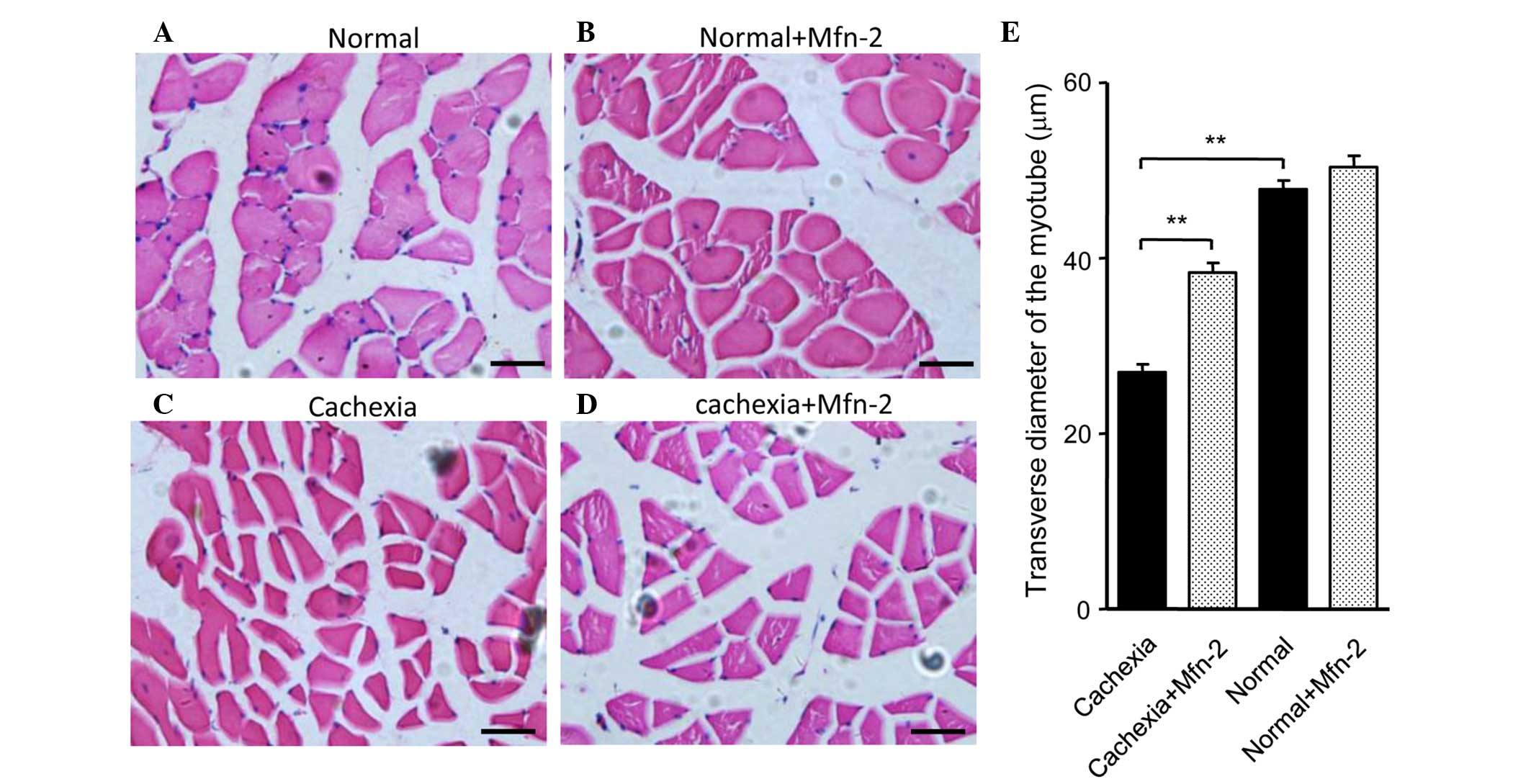
|
1
|
Fearon KC, Voss AC and Hustead DS: Cancer
Cachexia Study Group: Definition of cancer cachexia: Effect of
weight loss, reduced food intake, and systemic inflammation on
functional status and prognosis. Am J Clin Nutr. 83:1345–1350.
2006.PubMed/NCBI
|
|
2
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bachmann J, Heiligensetzer M,
Krakowski-Roosen H, Büchler MW, Friess H and Martignoni ME:
Cachexia worsens prognosis in patients with resectable pancreatic
cancer. J Gastrointest Surg. 12:1193–1201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dewys WD, Begg C, Lavin PT, Band PR,
Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr, Engstrom PF,
Ezdinli EZ, et al: Prognostic effect of weight loss prior to
chemotherapy in cancer patients. Eastern Cooperative Oncology
Group. Am J Med. 69:491–497. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Donohoe CL, Ryan AM and Reynolds JV:
Cancer cachexia: Mechanisms and clinical implications.
Gastroenterol Res Pract. 2011:6014342011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bozzetti F, Arends J, Lundholm K,
Micklewright A, Zurcher G and Muscaritoli M: ESPEN: ESPEN
guidelines on parenteral nutrition: Non-surgical oncology. Clin
Nutr. 28:445–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deans DA, Tan BH, Wigmore SJ, Ross JA, de
Beaux AC, Paterson-Brown S and Fearon KC: The influence of systemic
inflammation, dietary intake and stage of disease on rate of weight
loss in patients with gastro-oesophageal cancer. Br J Cancer.
100:63–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Romanello V, Guadagnin E, Gomes L, Roder
I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz
M, et al: Mitochondrial fission and remodelling contributes to
muscle atrophy. EMBO J. 29:1774–1785. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soriano FX, Liesa M, Bach D, Chan DC,
Palacín M and Zorzano A: Evidence for a mitochondrial regulatory
pathway defined by peroxisome proliferator-activated receptor-gamma
coactivator-1alpha, estrogen-related receptor-alpha, and mitofusin
2. Diabetes. 55:1783–1791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang P, Galloway CA and Yoon Y: Control
of mitochondrial morphology through differential interactions of
mitochondrial fusion and fission proteins. PLoS One. 6:e206552011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
White JP, Baltgalvis KA, Puppa MJ, Sato S,
Baynes JW and Carson JA: Muscle oxidative capacity during
IL-6-dependent cancer cachexia. Am J Physiol Regul Integr Comp
Physiol. 300:R201–R211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen T, Zheng M, Cao C, Chen C, Tang J,
Zhang W, Cheng H, Chen KH and Xiao RP: Mitofusin-2 is a major
determinant of oxidative stress-mediated heart muscle cell
apoptosis. J Biol Chem. 282:23354–23361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song Z, Ghochani M, McCaffery JM, Frey TG
and Chan DC: Mitofusins and OPA1 mediate sequential steps in
mitochondrial membrane fusion. Mol Biol Cell. 20:3525–3532. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ranieri M, Brajkovic S, Riboldi G, Ronchi
D, Rizzo F, Bresolin N, Corti S and Comi GP: Mitochondrial fusion
proteins and human diseases. Neurol Res Int. 2013:2938932013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kluge MA, Fetterman JL and Vita JA:
Mitochondria and endothelial function. Circ Res. 112:1171–1188.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bach D, Naon D, Pich S, Soriano FX, Vega
N, Rieusset J, Laville M, Guillet C, Boirie Y, Wallberg-Henriksson
H, et al: Expression of Mfn2, the Charcot-Marie-Tooth neuropathy
type 2A gene, in human skeletal muscle: Effects of type 2 diabetes,
obesity, weight loss, and the regulatory role of tumor necrosis
factor alpha and interleukin-6. Diabetes. 54:2685–2693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hernández-Alvarez MI, Thabit H, Burns N,
Shah S, Brema I, Hatunic M, Finucane F, Liesa M, Chiellini C, Naon
D, et al: Subjects with early-onset type 2 diabetes show defective
activation of the skeletal muscle PGC-1{alpha}/Mitofusin-2
regulatory pathway in response to physical activity. Diabetes Care.
33:645–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang HS, Chen Y, Fan L, Xi QL, Wu GH, Li
XX, Yuan TL, He SQ, Yu Y, Shao ML, Liu Y, et al: The endoplasmic
reticulum stress sensor IRE1α in intestinal epithelial cells is
essential for protecting against colitis. J Biol Chem.
290:15327–15336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue J, Li X, Jiao S, Wei Y, Wu G and Fang
J: Prolyl hydroxylase-3 is down-regulated in colorectal cancer
cells and inhibits IKKbeta independent of hydroxylase activity.
Gastroenterology. 138:606–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eura Y, Ishihara N, Yokota S and Mihara K:
Two mitofusin proteins, mammalian homologues of FZO, with distinct
functions are both required for mitochondrial fusion. J Biochem.
134:333–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yaffe D and Saxel O: Serial passaging and
differentiation of myogenic cells isolated from dystrophic mouse
muscle. Nature. 270:725–727. 1977. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reid MB and Li YP: Tumor necrosis
factor-alpha and muscle wasting: A cellular perspective. Respir
Res. 2:269–272. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Larichaudy J, Zufferli A, Serra F,
Isidori AM, Naro F, Dessalle K, Desgeorges M, Piraud M, Cheillan D,
Vidal H, et al: TNF-α- and tumor-induced skeletal muscle atrophy
involves sphingolipid metabolism. Skelet Muscle. 2:22012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Z, Puigserver P, Andersson U, Zhang C,
Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC and
Spiegelman BM: Mechanisms controlling mitochondrial biogenesis and
respiration through the thermogenic coactivator PGC-1. Cell.
98:115–124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tisdale MJ: Wasting in cancer. J Nutr.
129:(1S Suppl). S243–S246. 1999.
|
|
26
|
Argilés JM, Busquets S, Stemmler B and
López-Soriano FJ: Cancer cachexia: Understanding the molecular
basis. Nat Rev Cancer. 14:754–762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sugioka R, Shimizu S and Tsujimoto Y:
Fzo1, A protein involved in mitochondrial fusion, inhibits
apoptosis. J Biol Chem. 279:52726–52734. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deans C and Wigmore SJ: Systemic
inflammation, cachexia and prognosis in patients with cancer. Curr
Opin Clin Nutr Metab Care. 8:265–269. 2005. View Article : Google Scholar : PubMed/NCBI
|