Introduction
Breast cancer is considered to be one of the most
common malignances in women (1). Its
occurrence and development is a multi-step process, which results
from progressive accumulation of genetic and epigenetic
alternations (2). Chromosomal loss,
which leads to the inactivation of tumor suppressor genes, is one
of the most common genetic alterations detected in human breast
cancer (3). Previous publications
have reported that human chromosome 1p36 is a region that is
consistently deleted in human cancer (4–6), and
chromodomain helicase DNA binding protein 5 (CHD5) is located on
the short arm of this human chromosome (1p) (7). As a tumor suppressor, CHD5 is involved
in proliferation, apoptosis and senescence via the
p19ARF/p53 signaling pathway (8). CHD5 belongs to the chromodomain helicase
DNA binding domain family, which includes nine members (CHD1-9) and
is a subclass of the SWItch/sucrose non-fermentable proteins
(9). Although CHD5 is involved in key
cellular processes, the disrupted regulation of its expression has
not been fully elucidated. To date, homozygous deletion or mutation
cannot totally explain the loss of CHD5 expression, and additional
mechanisms require investigation.
Impairment in chromatin remodeling activity,
mediated by aberrant promoter methylation of candidate genes,
including the CHD family, may be important in cancer pathology
(10). During carcinogenesis, DNA
methylation increases at promoters in selected CpG islands, but is
lost at the majority of other genomic regions, resulting in
silencing of tumor suppressor genes (11–13). These
changes in DNA methylation are not due to any alteration in the DNA
sequence (14). CHD5, as an
ATP-dependent chromatin-remodeling enzyme, has been observed to
exhibit aberrant methylation of CpG islands in human cancer cell
lines and primary tumors, particularly gliomas and colon and breast
carcinomas (10,15). The present study focused on DNA
methylation analysis of CHD5 protein, in order to elucidate an
epigenetic mechanism of aberrant gene silencing. CHD5 expression
was investigated using semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR) in 137 fresh
breast cancer specimens, as well as in corresponding normal
tissues. In addition, CHD5 methylation was detected by
nested-methylation specific PCR (MSP) in 389 sporadic breast cancer
tissues. The association between CHD5 expression, CHD5 methylation
status and several clinicopathological features of breast cancer
tissues was also analyzed. The present study performed DNA
methylation analysis of CHD5 protein, in order to elucidate an
epigenetic mechanism of aberrant gene silencing in breast
carcinoma.
Materials and methods
Tissue samples
All tissue samples were collected from surgical
specimens of patients who underwent a mastectomy at the Affiliated
Hospital of Qingdao University, (Qingdao, China) between January
2011 and January 2012. All patients provided informed consent and
all procedures were approved by the hospital's ethics board. The
patients were unrelated Chinese women, aged 26–86 years (mean,
52.3±10.6 years), with sporadic breast cancer. The present study
analyzed a total of 389 tumor samples (252 paraffin-embedded
tissues and 137 fresh-frozen tissues), which constituted >50% of
a tumor area. In addition, the present study analyzed fresh-frozen
normal tissues from the same patients, located at least 5 cm away
from the tumor sites. These tissues were collected following
reconfirmation by a senior pathologist from the Affiliated Hospital
of Qingdao University. The histological grade of each tumor was
determined according to the modified Bloom-Richardson criteria
(16), and Tumor-Node-Metastasis
stages were determined using the official classification method
(17).
Cell culture
Human breast cancer cell lines MDA-MB-231
(CHD5-negative) and MCF-7 (CHD5-positive) were obtained from Peking
Union Medical College (Beijing, China). The cell lines were
maintained in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and supplemented with 10% fetal bovine serum (GE
Healthcare Life Sciences, Logan, UT, USA) at 37°C in a humidified
atmosphere with 5% CO2.
RNA isolation and RT-PCR analysis
Total RNA was isolated using an RNAfast kit
(Fastagen, Shanghai, China). RT-PCr was performed using the Access
RT-PCR System (Promega Corporation, Madison, WI, USA). The PCR
cycling conditions were as follows: 35 cycles of 95°C for 30 sec,
60°C for 40 sec and 72°C for 40 sec, followed by a final extension
at 72°C for 5 min The primer sequences were as follows: CHD5
forward, 5′-TCAAGACAAACGTGTTCAAGTC-3′ and reverse,
5′-ATTCAAGTGTTCTTCCACACAGC-3′; and GAPDH forward,
5′-CAAGGTCATCCATGACAACTTTG-3′ and reverse,
5′-GTCCACCACCCTGTTGCTGTAG-3′. The PCR products were resolved by gel
electrophoresis on 2% agarose gels, visualized under UV light and
quantified using the JS-380 Gel Imaging Analysis System (Shanghai
Peiqing Science and Technology, Co., Ltd., Shanghai, China). The
CHD5 expression levels were normalized to GAPDH.
Bisulfite genomic sequencing analysis
and nested-MSP
Genomic tumor DNA was isolated from the MDA-MB-231
and MCF-7 breast cancer cell lines or tissue samples using
phenol-chloroform. Unmethylated cytosines in the DNA were to
uracils using bisulfite in the EZ DNA Methylation-Gold™ kit (Zymo
Research, Irvine, CA, USA). Previous studies have revealed that the
region surrounding the transcription start sites (+1) of genes may
regulate their expression (18). The
present study selected the region from −651 to −232 as the target
fragment. The primers were designed by Methyl Primer Express
version 1.0 software (Thermo Fisher Scientific, Inc.) using GenBank
NM_015,557 (CHD5) as the reference sequence (outside primer: Sense,
5′-AGAAATTTTGAGGTAGAGATGGG-3′, antisense,
5′-ACTTCAACACCAACTAAAAACCA-3′, 418 bp; methylated primer: Sense,
5′-GGTTTCGGCGTTTGTGAATC-3′, antisense, 5′-AACTTAACGAACCCGAACGC-3′,
180 bp; unmethylated primer: Sense, 5′-TGGGTTTTGGTGTTTGTGAATT-3′;
antisense, 5′-CAAAACTTAACAAACCCAAACAC-3′, 187 bp). First-round
amplifications were performed in 25 µl reactions using PerfectShot
Ex Taq (Takara Bio, Inc., Otsu, Japan), including 10 pmol outside
primer and 30 ng modified DNA, and the following cycle parameters:
95°C for 5 min, 35 cycles at 95°C for 30 sec, 52°C for 40 sec and
72°C for 40 sec, followed by a final extension at 72°C for 5 min.
PCR products (1 µl) were subjected to second-round amplifications
using methylated and unmethylated primers, with the above cycle
parameters. The PCR products were resolved by electrophoresis on
2.5% agarose gels, visualized under UV light and quantified using
the JS-380 Gel Imaging Analysis System.
Statistical analysis
RT-PCR results are presented as the mean ± standard
deviation, and a Student's t–test was performed. Promoter
methylation data were analyzed using the χ2 test in SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA), and potential
correlations with clinicopathological data were studied using
unconditional logistic regression to estimate odds ratios (OR) and
95% confidence intervals (CI). The model was adjusted for age
during diagnosis. P<0.05 was considered to indicate a
statistically significant difference.
Results
CHD5 mRNA levels in breast tumors and
normal tissues
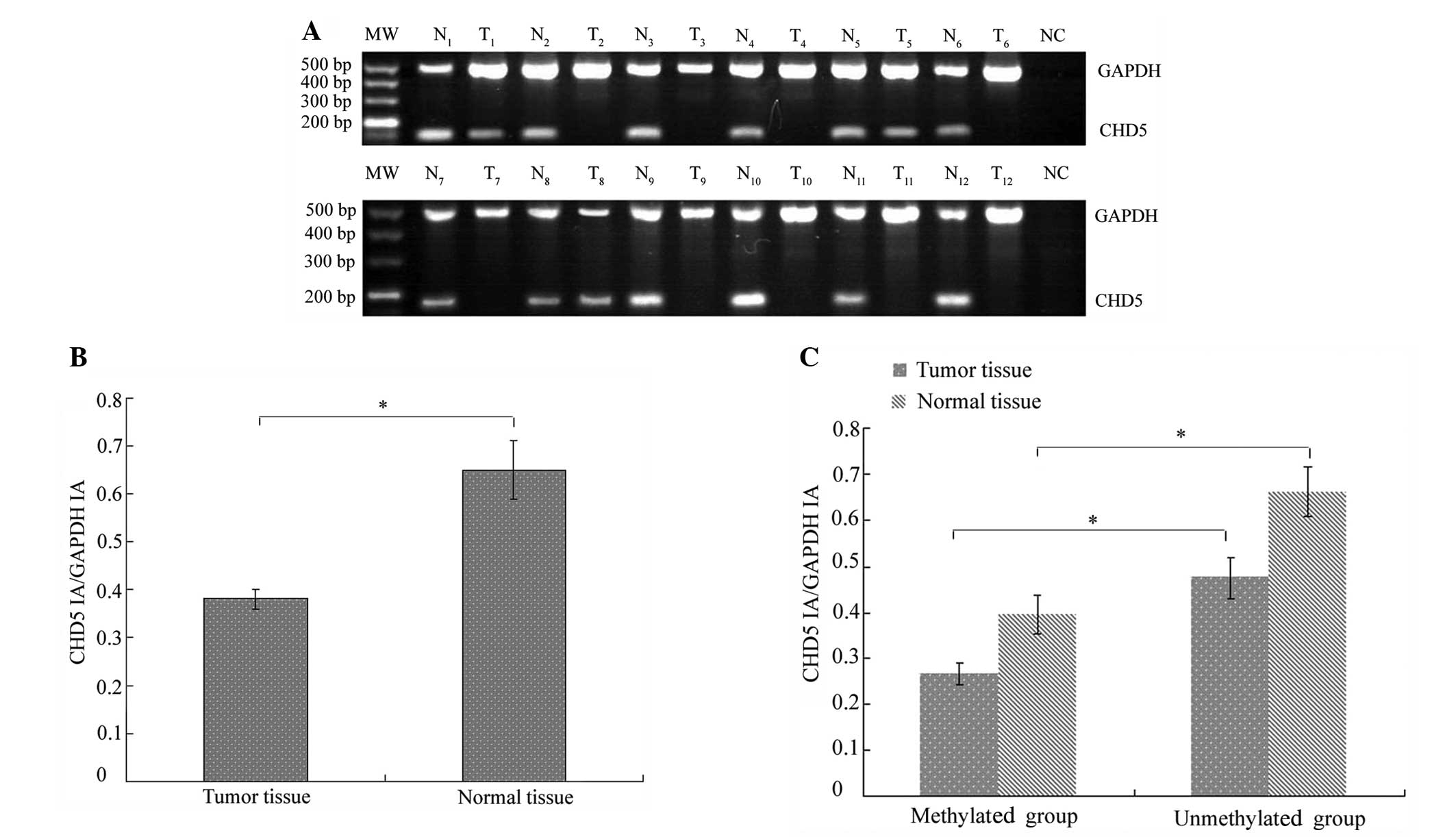
CHD5 mRNA was not detected or decreased in 92/137
fresh-frozen tumor samples and in 31/137 normal tissues (Fig. 1A). Therefore, downregulation of CHD5
expression was significantly increased in tumor tissue compared
with corresponding normal tissue (χ2=54.894;
P<0.001). In addition, CHD5 mRNA levels were significantly
reduced in cancer specimens (0.38±0.02) compared with normal tissue
(0.65±0.06; P<0.001; Fig. 1B).
Correlation between CHD5 promoter
methylation and CHD5 expression
A correlation analysis revealed that loss of CHD5
expression was correlated with CHD5 methylation. Fig. 1C shows that the difference in CHD5
expression levels between tissues in which CHD5 was methylated and
tissues in which CHD5 was unmethylated was statistically
significant for cancerous and normal tissue samples. However, a
small number of normal tissue samples exhibited CHD5 expression and
promoter methylation (Table I).
 | Table I.Association between CHD5 methylation
and expression. |
Table I.
Association between CHD5 methylation
and expression.
|
| CHD5 expression |
|
|
|---|
|
|
|
|
|
|---|
| CHD5 methylation
status | + | – | χ2 | P-value |
|---|
| Tumor sample |
|
| 4.513 | 0.034 |
| + | 9 | 35 |
|
|
| – | 36 | 57 |
|
|
| Normal sample |
|
| 6.673 | 0.010 |
| + | 3 | 17 |
|
|
| – | 103 | 14 |
|
|
Silencing of CHD5 is associated with
promoter hypermethylation
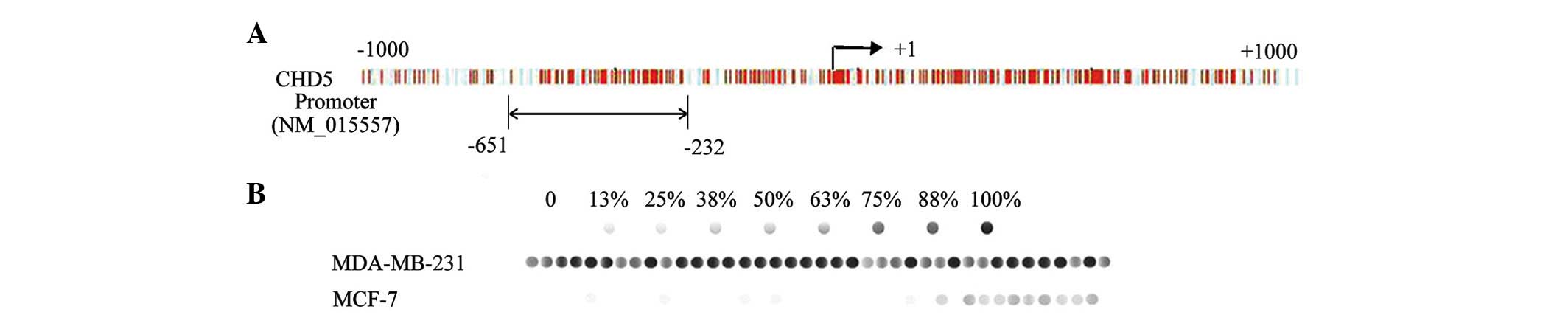
Sodium bisulfite sequencing was performed on a 418
bp fragment with 39 CpG dinucleotides located within the −651 to
−232 island (Fig. 2A). The
CHD5-negative cell line, MDA-MB-231, demonstrated hypermethylation
of the CpG dinucleotides (Fig. 2B).
By contrast, the CHD5-positive cell line MCF-7, exhibited lower
levels of CpG dinucleotide methylation.
Nested-MSP analysis in primary breast
tissues
The present study used nested-MSP analysis to study
the methylation status of CpG islands. CHD5 promoter methylation
was detected in 105/389 (27.1%) primary breast tumor samples (data
not shown), 61/252 paraffin-embedded tissue samples (data not
shown), 44/137 fresh-frozen tumor samples and 20/137 normal tissue
samples (Table I). Therefore, CHD5
promoter methylation was observed more frequently in breast cancer
tissue samples compared with corresponding normal tissue samples
(χ2 =8.590; P=0.003), as confirmed by nested
methylation-specific PCR (Fig.
3).
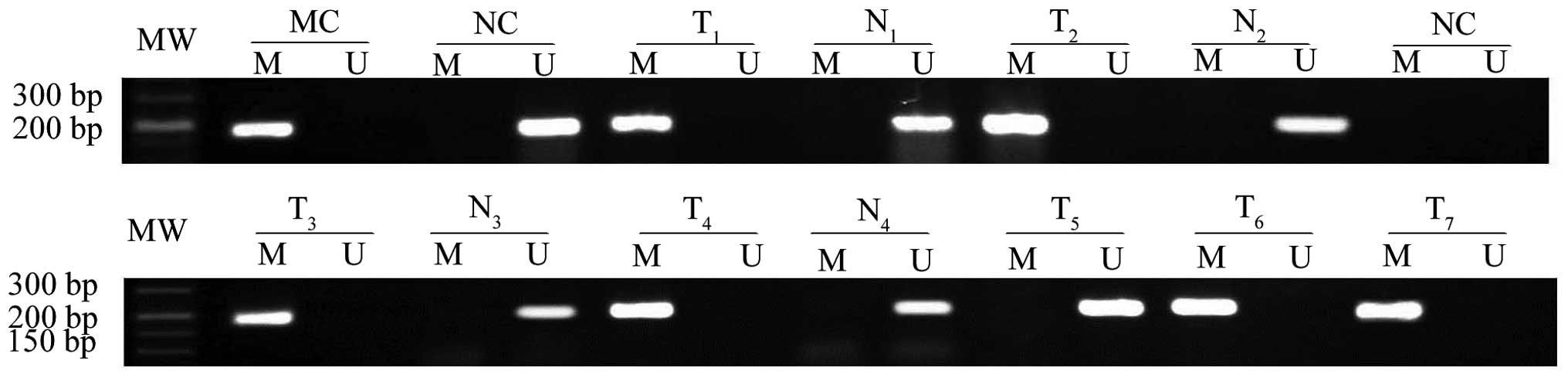 | Figure 3.Nested methylation-specific PCR was
used to analyze CHD5 methylation in breast cancer tissues and
corresponding normal breast tissues. Genomic DNA from breast
tissues treated with sodium bisulfite was amplified using
methylated and unmethylated primers. MC and UC human genomic DNA
were used as the positive control for methylated and unmethylated
reactions, respectively. A blank control (NC) containing all PCR
components without template DNA was also included in all PCR
reactions. PCR, polymerase chain reaction; CHD5, chromodomain
helicase DNA binding protein 5; T, breast cancer tissue; N,
corresponding normal breast tissues; M, methylated; U,
unmethylated; MC, universal methylated; UC, universal unmethylated;
MW, molecular weight. |
Correlation between promoter
methylation and clinicopathological characteristics
The associations between individual gene methylation
status and clinicopathological features of breast cancer are
evaluated in Table II. Table II shows that the highest levels of
CHD5 methylation were observed in breast tumor samples with either
(or both) estrogen receptor (ER)/progesterone receptor (PR)
negative status [OR, 0.47; 95% CI, 0.24–0.92; P=0.023; OR, 0.50;
95%CI, 0.29–0.87; P=0.028).
 | Table II.Clinicopathological features of the
389 patients with primary breast tumors according to the
methylation status of CHD5. |
Table II.
Clinicopathological features of the
389 patients with primary breast tumors according to the
methylation status of CHD5.
|
|
| CHD5 |
|
|---|
|
|
|
|
|
|---|
| Clinical data | Samples, n | M | OR (95% CI) | P-value |
|---|
| Menopausal
status |
|
|
|
|
| Pre– | 227 | 54 | 1.00 |
|
|
Post– | 162 | 33 | 0.58 (0.24–1.43) | 0.4251 |
| Tumor size (cm) |
|
|
|
|
|
<1.0 | 7 | 1 | 1.00 |
|
|
1.0–1.9 | 136 | 27 | 1.65
(0.15–17.98) | 0.7170 |
|
2–3 | 200 | 46 | 2.34
(0.22–25.42) | 0.5890 |
|
≥3.1 | 46 | 13 | 3.20
(0.27–37.46) | 0.4350 |
|
Differentiation |
|
|
|
|
|
Well | 32 | 6 | 1.00 |
|
|
Moderate | 207 | 50 | 1.33
(0.45–3.90) |
|
|
Poor | 150 | 31 | 1.25
(0.42–3.79) | 0.6467 |
| Lymph node
status |
|
|
|
|
|
Negative | 238 | 55 | 1.00 |
|
|
Positive | 151 | 32 | 0.87
(0.50–1.53) | 0.6583 |
| Metastatic disease
at presentation |
|
|
|
|
|
Negative | 303 | 67 | 1.00 |
|
|
Positive | 86 | 20 | 1.07
(0.56–2.04) | 0.8223 |
| TNM stage |
|
|
|
|
|
0/I | 87 | 15 | 1.00 |
|
| II | 216 | 52 | 1.51
(0.77–2.95) | 0.1950 |
|
III/IV | 86 | 20 | 1.35
(0.63–2.91) | 0.3250 |
| ER status |
|
|
|
|
|
Negative | 242 | 45 | 1.00 |
|
|
Positive | 147 | 42 | 0.47
(0.27–0.82) | 0.0230 |
| PR status |
|
|
|
|
|
Negative | 205 | 43 | 1.00 |
|
|
Positive | 184 | 44 | 0.67
(0.38–1.19) | 0.4876 |
| ER/PR status |
|
|
|
|
| Both
negative | 187 | 33 | 1.00 |
|
| Either
positive | 73 | 22 | 1.50
(0.24–0.92) | 0.0280 |
| Both
positive | 129 | 32 | 0.65
(0.38–1.12) | 0.1220 |
| p53 status |
|
|
|
|
|
Wild-type | 160 | 36 | 1.00 |
|
|
Mutant | 229 | 51 | 1.09
(0.62–1.91) | 0.6337 |
| C-erbB-2
status |
|
|
|
|
|
Negative | 270 | 58 | 1.00 |
|
|
Positive | 119 | 29 | 1.60
(0.89–2.90) | 0.5287 |
| Ki67 proliferation
index, % |
|
|
|
|
|
<10 | 28 | 9 | 1.00 |
|
|
10–32 | 217 | 44 | 0.85
(0.31–2.33) | 0.1510 |
|
≥33 | 144 | 34 | 0.85
(0.30–2.41) | 0.3400 |
Discussion
Evidence that CHD5 functions as a tumor suppressor
in human cancer has been observed in studies of neuroblastoma, in
which CHD5 mRNA expression was downregulated potentially via
promoter methylation in tumors (19).
Furthermore, it also has been reported that aberrant CHD5 promoter
methylation was detected in gastric, colorectal, ovarian and lung
cancer (20–23). However, to the best of our knowledge,
the role of CHD5 promoter methylation status in breast cancer has
not been evaluated. In the present study, the expression of CHD5
was detected at a transcriptional level. The results revealed that
CHD5 mRNA was downregulated in 92/137 breast tumors and 31/137
normal tissues. Therefore, downregulation of CHD5 expression was
significantly more frequent in tumors compared with corresponding
normal tissues (P<0.001). To the best of our knowledge, aberrant
CHD5 promoter methylation could additionally explain low expression
levels or silencing that are not caused by chromatin deletion or
other mutations. Mulero-Navarro and Esteller (10) reported that CHD5 was silenced by
aberrant promoter CpG island methylation in human cancer. However,
this study mainly focused on cancer cell lines and limited cases of
primary tumors (10). Based on the
above research, the present study selected 389 cases of breast
primary tumors (including 252 paraffin embedded specimens and 137
fresh-frozen cases), determined the methylation status and
investigated the correlation between CHD5 methylation and
expression levels and clinicopathological characteristics.
There is increasing evidence that promoter
methylation of tumor suppressor genes has a significant role in the
pathogenesis of tumors, including breast tumors (10,24). In
the present study, MSP revealed aberrant CHD5 promoter methylation
in 105/389 breast tumor samples, 44/137 fresh-frozen tumor samples
and 20 normal tissue samples. CHD5 methylation was more frequent in
breast tumors compared with normal tissues (P=0.003). Furthermore,
the difference in CHD5 expression levels between tissues in which
CHD5 was methylated and tissues in which CHD5 was unmethylated was
statistically significant for the tumor samples (P=0.034) and for
the corresponding normal tissues (P=0.010). However, a small number
of normal samples exhibited CHD5 expression and aberrant promoter
methylation. A potential explanation for this result is that
samples may have already undergone premalignant mutations affecting
CHD5, as this process has been previously observed in tumor
suppressor genes in certain breast tumors (25,26). The
results of the present study suggest that aberrant DNA methylation
may affect CHD5 expression. Furthermore, it is possible that the
observed aberrant methylation of the CHD5 promoter in breast tumors
is critical for tumorigenesis.
To additionally characterize the aberrant CHD5
promoter methylation in tumor samples, the present study evaluated
potential associations between CHD5 methylation status and various
clinicopathological parameters. CHD5 was more frequently methylated
in breast tumor samples with ER/PR (or both) negative status than
in samples with ER/PR (or both) positive status. The ER/PR status
has been recognized as a prognostic factor in patients with breast
carcinoma, and has been noted to be a predictive marker for the
response to treatment with endocrine therapy (27). The presence of ER and PR is predictive
of the response to treatment with the anti-estrogen drug tamoxifen
(28). Previous studies have provided
evidence that ER and PR expression patterns are influenced by
changes in the chromatin structure during transcription (29,30). The
present study demonstrated that CHD5 exhibited aberrant CpG island
methylation in primary tumors of breast carcinoma. CHD5 is located
at human chromosome 1p36, which is recurrently deleted in human
breast cancer (4–6). Furthermore, the results provided
evidence that there is a correlation between aberrant CHD5 promoter
methylation and ER and PR status. Although a previous study
reported that CHD5 protein expression significantly correlated with
ER/PR status in breast tumors (31),
further research is required, as this has been reported
infrequently. Therefore, it is possible that ER/PR status may be
involved in the association between CHD5 methylation and breast
cancer risk. Further studies are required to investigate whether
the CHD5 methylation status may have a role in predicting the
response of breast cancer with ER/PR negative status to
therapy.
In conclusion, the present study indicated that
promoter methylation and downregulation of CHD5 at the RNA level
were common in breast cancer, and CHD5 downregulation occurred in
part as a result of promoter methylation. In addition, the results
of the present study provide evidence that CHD5 methylation is
correlated with ER/PR status. Such knowledge may assist in
understanding the mechanism underlying the pathophysiology of
breast cancer with ER/PR negative status.
References
|
1
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fucito A, Lucchetti C, Giordano A and
Romano G: Genetic and epigenetic alterations in breast cancer: What
are the perspectives for clinical practice? Int J Biochem Cell
Biol. 40:565–575. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Wezel T, Lombaerts M, van Roon EH,
Philippo K, Baelde HJ, Szuhai K, Cornelisse CJ and Cleton-Jansen
AM: Expression analysis of candidate breast tumour suppressor genes
on chromosome 16q. Breast Cancer Res. 7:R998–R1004. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bagchi A and Mills AA: The quest for the
1p36 tumor suppressor. Cancer Res. 68:2551–2556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bièche I, Champème MH, Matifas F, Cropp
CS, Callahan R and Lidereau R: Two distinct regions involved in 1p
deletion in human primary breast cancer. Cancer Res. 53:1990–1994.
1993.PubMed/NCBI
|
|
6
|
Praml C, Finke LH, Herfarth C, Schlag P,
Schwab M and Amler L: Deletion mapping defines different regions in
1p34.2-pter that may harbor genetic information related to human
colorectal cancer. Oncogene. 11:1357–1362. 1995.PubMed/NCBI
|
|
7
|
Costello JF, Frühwald MC, Smiraglia DJ,
Rush LJ, Robertson GP, Gao X, Wright FA, Feramisco JD, Peltomäki P,
Lang JC, et al: Aberrant CpG-island methylation has non-random and
tumor-type-specific patterns. Nat Genet. 24:132–138. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bagchi A, Papazoglu C, Wu Y, Capurso D,
Brodt M, Francis D, Bredel M, Vogel H and Mills AA: CHD5 is a tumor
suppressor at human 1p36. Cell. 128:459–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kolla V, Zhuang T, Higashi M, Naraparaju K
and Brodeur GM: Role of CHD5 in human cancers: 10 years later.
Cancer Res. 74:652–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mulero-Navarro S and Esteller M: Chromatin
remodeling factor CHD5 is silenced by promoter CpG island
hypermethylation in human cancer. Epigenetics. 3:210–215. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clark SJ and Melki J: DNA methylation and
gene silencing in cancer: Which is the guilty party? Oncogene.
21:5380–5387. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Das PM and Singal R: DNA methylation and
cancer. J Clin Oncol. 22:4632–4642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones PA and Baylin SB: The epigenomics of
cancer. Cell. 128:683–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holliday R: The inheritance of epigenetic
defects. Science. 238:163–170. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mokarram P, Kumar K, Brim H,
Naghibalhossaini F, Saberi-firoozi M, Nouraie M, Green R, Lee E,
Smoot DT and Ashktorab H: Distinct high-profile methylated genes in
colorectal cancer. PLoS One. 4:e70122009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veronesi U, Viale G, Rotmensz N and
Goldhirsch A: Rethinking TNM: Breast cancer TNM classification for
treatment decision-making and research. Breast. 15:3–8. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou B, Chim CS, Zeng H, Leung SY, Yang Y,
Tu SP, Lin MC, Wang J, He H, Jiang SH, et al: Correlation between
the single-site CpG methylation and expression silencing of the
XAF1 gene in human gastric and colon cancers. Gastroenterology.
131:1835–1843. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujita T, Igarashi J, Okawa ER, Gotoh T,
Manne J, Kolla V, Kim J, Zhao H, Pawel BR, London WB, et al: CHD5,
a tumor suppressor gene deleted from 1p36.31 in neuroblastomas. J
Natl Cancer Inst. 100:940–949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Lau KK, So LK and Lam YW: CHD5 is
down-regulated through promoter hypermethylation in gastric cancer.
J Biomed Sci. 16:952009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fatemi M, Paul TA, Brodeur GM, Shokrani B,
Brim H and Ashktorab H: Epigenetic silencing of CHD5, a novel
tumor-suppressor gene, occurs in early colorectal cancer stages.
Cancer. 120:172–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gorringe KL, Choong DY, Williams LH,
Ramakrishna M, Sridhar A, Qiu W, Bearfoot JL and Campbell IG:
Mutation and methylation analysis of the chromodomain-helicase-DNA
binding 5 gene in ovarian cancer. Neoplasia. 10:1253–1258. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao R, Yan Q, Lv J, Huang H, Zheng W,
Zhang B and Ma W: CHD5, a tumor suppressor that is epigenetically
silenced in lung cancer. Lung Cancer. 76:324–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koyama H, Zhuang T, Light JE, Kolla V,
Higashi M, McGrady PW, London WB and Brodeur GM: Mechanisms of CHD5
Inactivation in neuroblastomas. Clin Cancer Res. 18:1588–1597.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rinner B, Gallè B, Trajanoski S, Fischer
C, Hatz M, Maierhofer T, Michelitsch G, Moinfar F, Stelzer I,
Pfragner R and Guelly C: Molecular evidence for the bi-clonal
origin of neuroendocrine tumor derived metastases. BMC Genomics.
13:5942012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Futscher BW: Epigenetic changes during
cell transformation. Adv Exp Med Biol. 754:179–194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferguson NL, Bell J, Heidel R, Lee S,
Vanmeter S, Duncan L, Munsey B, Panella T and Orucevic A:
Prognostic value of breast cancer subtypes, Ki-67 proliferation
index, age, and pathologic tumor characteristics on breast cancer
survival in Caucasian women. Breast J. 19:22–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bardou VJ, Arpino G, Elledge RM, Osborne
CK and Clark GM: Progesterone receptor status significantly
improves outcome prediction over estrogen receptor status alone for
adjuvant endocrine therapy in two large breast cancer databases. J
Clin Oncol. 21:1973–1979. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan L, Yang X and Davidson NE: Role of DNA
methylation and histone acetylation in steroid receptor expression
in breast cancer. J Mammary Gland Biol Neoplasia. 6:183–192. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang X, Ferguson AT, Nass SJ, Phillips DL,
Butash KA, Wang SM, Herman JG and Davidson NE: Transcriptional
activation of estrogen receptor alpha in human breast cancer cells
by histone deacetylase inhibition. Cancer Res. 60:6890–6894.
2000.PubMed/NCBI
|
|
31
|
Wu X, Zhu Z, Li W, Fu X, Su D, Fu L, Zhang
Z, Luo A, Sun X, Fu L and Dong JT: Chromodomain helicase DNA
binding protein 5 plays a tumor suppressor role in human breast
cancer. Breast Cancer Res. 14:R732012. View
Article : Google Scholar : PubMed/NCBI
|