Introduction
Colorectal cancer remains the third leading cause of
cancer-associated mortality in the USA, even though tremendous
efforts have been made to improve the effectiveness of treatment
(1). Morbidity primarily results from
the growth of metastatic tumors in distant organs, and metastasis
is one of the major causes of mortality in cancer patients.
Multiple steps are involved in the metastatic process. One of the
first steps is the invasion of cancer cells through the basement
membrane via the adhesion of cancer cells to the extracellular
matrix (ECM), followed by degradation of the ECM by proteolytic
enzymes (2). Key proteases that
degrade the ECM are the serine proteases (plasmins), urokinase
plasminogen activator (uPA), cathepsins and matrix
metalloproteinases (MMPs). It is well demonstrated that uPA and
MMPs are significant in tumor growth, invasion and metastasis
(3–5).
uPA, a member of the serine proteases, interacts with the uPA
receptor, which modulates various biological functions, including
cell migration, differentiation and wound healing (6). Expression of uPA is critical in cancer
cell metastasis and is involved in cancer cell adhesion and
invasion (4,7). MMP9 is also crucial in tumor invasion
and angiogenesis, since it mediates the degradation of the ECM
(8). Overexpression of MMP9 may be
one step in the multi-step process that results in neoplastic cell
proliferation and metastasis, and it has been demonstrated that
MMP9 is associated with colorectal carcinoma (9). Therefore, inhibition of ECM degradation
enzymes and cell adhesion to the ECM should be considered as
preventative treatment for cancer metastasis (5,10,11).
Numerous studies have suggested that nuclear
factor-κB (NF-κB) is key in regulating a number of cellular
processes, including inflammation, cellular proliferation,
transformation and tumorigenesis (12,13). NF-κB
is a ubiquitous eukaryotic transcription factor, which is
identified in the cytoplasm as an inactive heterotrimer consisting
of p50, p65 and IκBα subunits. Activation of NF-κB is initiated by
the signal-induced degradation of IκB proteins, the most studied of
which is IκBα. IκBα undergoes phosphorylation and
ubiquitination-dependent degradation via activation of IκB kinase
(IKK), which is composed of IKKα, IKKβ and IKKγ (also referred to
as NEMO) (14,15). IκBα phosphorylation/degradation leads
to NF-κB release, which translocates to the nucleus where it acts
as a transcription factor and binds to a specific consensus
sequence in DNA, leading to gene transcription (16).
Nontraditional medicine is becoming an increasingly
attractive approach for the treatment of various inflammatory
disorders among patients unresponsive or unwilling to receive
standard medications. Curcumin is the major constituent of turmeric
powder extracted from the roots of the East Indian plant Curcuma
longa. It has been widely used in therapeutic preparations for
centuries owing to its anti-inflammatory and chemotherapeutic
properties (17,18). Curcumin presents itself as a
pharmacologically safe and effective potential candidate for
anti-metastatic therapy, due to the following reasons: Curcumin has
been demonstrated to suppress NF-κB activation induced by various
inflammatory stimuli (19,20); increasing evidence indicates that
curcumin has anticancer effects against various types of human
tumor cells, including ovarian, breast and colon cancer and
astroglioma (21–25); curcumin has been revealed to
downregulate the expression of various NF-κB-regulated genes,
including B-cell lymphoma 2, cyclooxygenase 2, tumor necrosis
factor and adhesion molecules (19,20,26–29);
administration of curcumin in humans is safe (17,18,30).
However, whether curcumin is involved in the
regulation of colon cancer cell invasion via the NF-κB signaling
pathway is not well elucidated. The present study investigated the
human colon cancer LoVo and SW480 cell lines to identify the
effects and mechanisms of curcumin on colon cancer cell adhesion
and invasion during the metastasis process via the NF-κB signaling
pathway. The present results demonstrated that curcumin inhibits
the adhesion and invasion of colon cancer cells by suppressing the
activation of NF-κB and IKK activity. This led to the
downregulation of the expression of uPA and MMP9, which are
regulated by NF-κB, and thus suppressed the proliferation of colon
cancer cells.
Materials and methods
Materials
Curcumin (diferuloylmethane) was obtained from
Sigma-Aldrich (St. Louis, MO, USA), and was dissolved in dimethyl
sulfoxide (DMSO) as a 10 mM stock solution and stored at −20°C for
further study. Primary antibodies consisted of mouse anti-human
monoclonal uPA (catalog no. sc-59727; dilution, 1:1,000) purchased
from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA) and rat
anti-human polyclonal MMP9 (catalog no. 3852; dilution, 1:1,000),
mouse anti-human monoclonal NF-κB p65 (catalog no. 6956; dilution,
1:1,000), rabbit anti-human polyclonal NF-κB p50 (catalog no. 3035;
dilution, 1:1,000), rabbit anti-human polyclonal p-p65 (catalog no.
3031; dilution, 1:1,000), mouse anti-human monoclonal IKK (catalog
no. 11930; dilution, 1:1,000), rabbit anti-human polyclonal AMPK
(catalog no. 2532; dilution, 1:1,000), rabbit anti-human monoclonal
p-AMPK (catalog no. 2535; dilution, 1:1,000) and mouse anti-human
monoclonal β-actin (catalog no. 3700; dilution, 1:1,000) purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Compound C
was purchased from EMD Millipore (Billerica, MA, USA).
Cell culture
Two human colon cancer cell lines SW480 and LoVo
were purchased from the American Type Culture Collection (Manassas,
VA, USA). The SW480 cell line was cultured in Dulbecco's modified
Eagle's medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing glucose (4.5 g/l) supplemented with 10% fetal calf serum
(FCS; Thermo Fisher Scientific, Inc.), 100 µg/ml streptomycin and
100 U/ml penicillin. The LoVo cell line was grown in F-12 medium
(Thermo Fisher Scientific, Inc.) containing L-glutamine,
supplemented with 10% FCS, 100 µg/ml streptomycin and 100 U/ml
penicillin. All cells were cultured in a humidified atmosphere of
5% CO2 at 37°C.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay
MTT proliferation assay was performed to assess the
cytotoxicity of curcumin in SW480 and LoVo cells. Briefly, cells
were seeded on a 96-well plate at a density of 5,000 cells/well.
Following attachment, various concentrations of curcumin (0, 0.01,
0.1, 1, 10, 20 and 50 µM) were added for 24 h. The cells were
washed with phosphate-buffered saline (PBS) and incubated with 200
µl MTT (0.5 mg/ml) until formazan had formed. Subsequently, the
medium was removed and formazan was dissolved with DMSO. Absorbance
was measured at 570 nm using a microplate reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA). The experiment was repeated
three times.
Invasion assay
A Boyden chamber (BD Biosciences, San Jose, CA, USA)
was used to determine the cell invasion ability of SW480 and LoVo
cells. Cells were pre-cultured in serum-free medium with or without
curcumin (0 and 10 µM) for 24 h. Cells (8×104) suspended
in 0.5 ml serum-free medium were loaded into the upper compartment
of the invasion chamber, which was coated with Matrigel (30
mg/filter). The lower compartment was loaded with complete medium
with or without curcumin. The chamber was incubated at 37°C for 24
h and the filters were removed. Invaded cells were fixed, stained
and counted under a microscope. Each experiment was performed three
times.
Western blotting
Western blot analysis was performed on 100 µg
protein extracts. SW480 and LoVo cells were lysed in lysis buffer
[0.5% sodium deoxycholate, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1%
Nonidet P-40, 0.1% sodium dodecyl sulfate (SDS)] containing 50 mM
NaF, 5 mM EDTA, 1 mM DTT and 10 µg/ml aprotinin. Cell lysates were
resolved on SDS-polyacrylamide gel electrophoresis, according to
standard protocols (31). The samples
were immunoblotted with primary antibodies agaisnt uPA, MMP-9,
NF-κB, IKK, p-Thr172 5′ AMP-activated protein kinase (AMPK), AMPK
and β-actin, followed by incubation with secondary antibodies: Goat
anti-rabbit immunoglobulin G (IgG), horseradish peroxidase
(HRP)-linked antibody (catalog no. 7074; dilution, 1:5,000) and
horse anti-mouse IgG, HRP-linked antibody (catalog no. 7076;
dilution, 1:5,000), purchased from Cell Signaling Technology Inc.
Bands were visualized using an Amersham ECL Western Blotting
Detection kit (GE Healthcare, Chalfont, UK), according to the
manufacturer's protocol.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated from SW480 and LoVo cells
using RNA Bee RNA Isolation Reagent (Amsbio LLC, Cambridge, MA,
USA). cDNA (1 µg) is used as a template for subsequent PCR
amplification using primers specific for MMP-9, uPA and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes. PCR
conditions were: 30 sec at 94°C for denaturation, 30 sec at 54°C
for annealing and 30 sec at 65°C for extension, for a total of 30
cycles. qPCR was performed using the SYBR green real-time PCR kit
(catalog no. 204141; Qiagen, Valencia, CA, USA) and the
QuantStudio® 3 Real-Time PCR System (Applied
Biosystems™; Thermo Fisher Scientific, Inc.). PCR products were run
on 2% agarose gels to confirm that correct molecular sizes were
present. Each sample was tested in triplicate using qPCR. The
following primers for qPCR were used: MMP9, forward
5′-AATCTCTTCTAGAGACTGGGAAGGAG-3′ and reverse
5′-AGCTGATTGACTAAAGTAGCTGGA-3′; uPA, forward
5′-CACGCAAGGGGAGATGAA-3′ and reverse 5′-ACAGCATTTTGGTGGTGACTT-3′;
GAPDH, forward 5′-AGAGAGAGGCCCTCAGTTGCT-3′ and reverse
5′-TTGTGAGGGAGATGCTCAGTGT-3′, synthesized by BGI Tech (Shenzhen
Co., Ltd., Shenzhen, China). GAPDH was used as a loading control.
Quantification was performed using the 2−∆∆Cq method, as
previously described (32)
Enzyme-linked immunosorbent assay
(ELISA)
To investigate whether curcumin affects NF-κB
activation, which is critical for transcriptional activity, the DNA
binding activity of NF-κB was analyzed by ELISA. The NF-κB p65
Transcription Factor Assay kit (catalog no. ab133112) was purchased
from Abcam (Cambridge, MA, USA). NF-κB activation was measured by
ELISA according to the manufacturer's protocol.
Luciferase assay
SW480 cells were seeded in 24-well plates at a
density of 1×105 cells/ml/well, and the following day
they were co-transfected with 100 ng luciferase reporter construct
(synthesized by BGI Tech; Shenzhen Co., Ltd.), 20 ng Renilla
luciferase pRL-TK reporter (Promega, Madison, WI, USA), and 400 ng
HA-tagged p65 NF-κB expression plasmid (Addgene, Cambridge, MA,
USA). Subsequent to 24 h, the cells were harvested and the
luciferase activity was determined using the
Dual-Luciferase® Reporter Assay System Protocol (Promega
Corporation, Madison, WI, USA). The relative light units were
measured using a GloMax® 20/20 Luminometer (Promega
Corporation). Data were normalized by Renilla luciferase. Each
experiment was performed at least three times in triplicate
wells.
Statistical analysis
Data were expressed as the mean ± standard error of
the mean. Differences were analyzed by one-way analysis of variance
followed by Fisher's protected least significant difference test
using SPSS (version 17.0) software (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
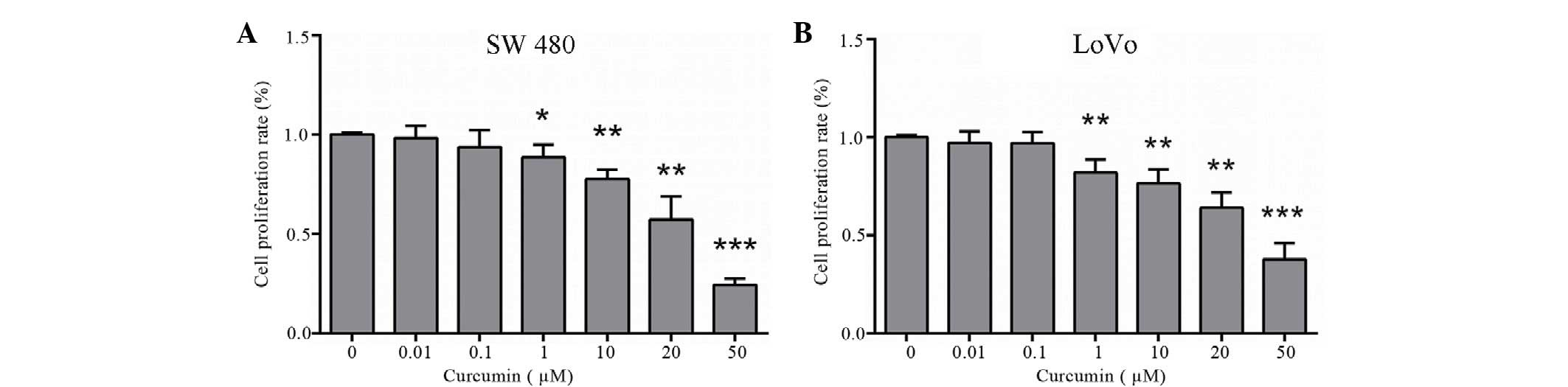
Curcumin inhibits the proliferation of
colon cancer LoVo and SW480 cells
An MTT assay was performed to examine the effect of
curcumin on the proliferation of colon cancer LoVo and SW480 cells.
LoVo and SW480 cells were treated with various concentrations of
curcumin (0–50 µM) for 24 h. Subsequently, curcumin markedly
inhibited cell proliferation of colon cancer LoVo and SW480 cells
in a dose-dependent manner (P=0.014 and P=0.003 vs. control cells,
respectively; Fig. 1A and B). These
inhibitory effects were observed following incubation with 1, 10,
20 and 50 µM curcumin. A dose of up to 20 µM curcumin was used in
further experiments.
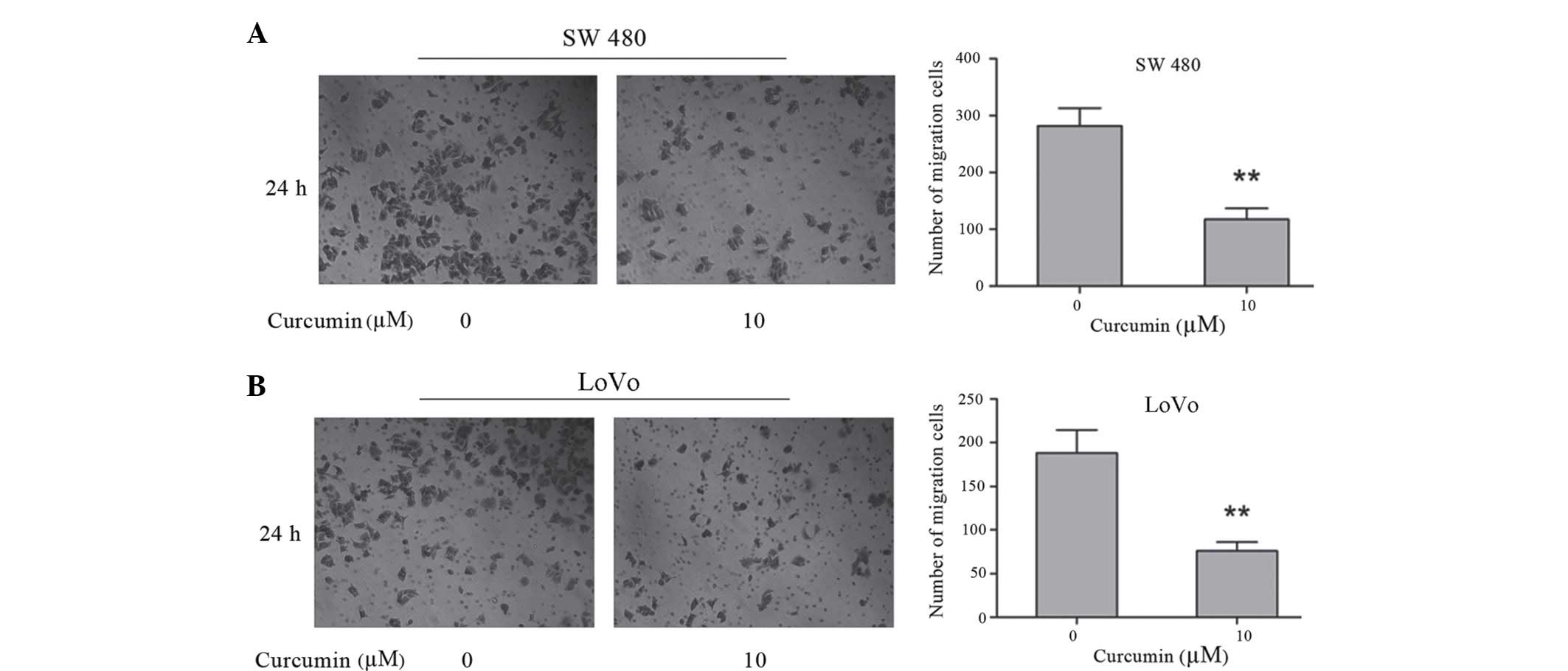
Curcumin inhibits the invasion of
colon cancer SW480 and LoVo cells
To investigate the effects of curcumin on the
invasion of colon cancer SW480 and LoVo cells, a Matrigel-coated
polycarbonate filter in a Boyden chamber was used. Following a 24 h
incubation, curcumin was demonstrated to significantly decrease
cell invasiveness. The number of SW480 cells that penetrated the
membrane in 10 µM curcumin-treated cells (117.00±11.36) was
significantly lower compared with control cells (281.33±11.22)
(P=0.002; Fig. 2A). The number of
LoVo cells that penetrated the membrane in 10 µM curcumin-treated
cells (188.30±15.03) was significantly lower compared with control
cells (76.03±5.77) (P=0.002; Fig.
2B). These results demonstrate that curcumin has a significant
inhibitory effect on the invasive activity of human colon cancer
SW480 and LoVo cells.
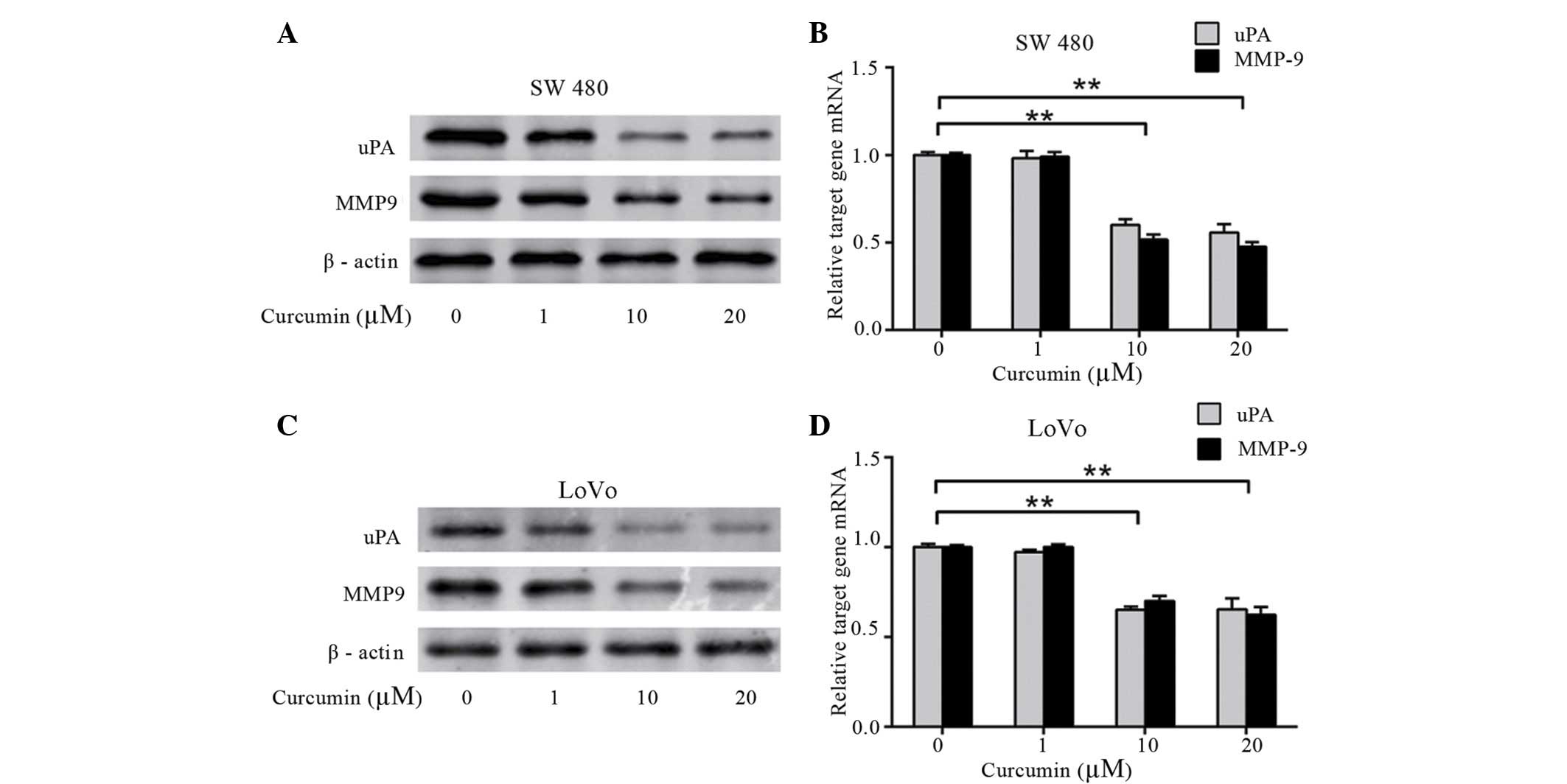
Curcumin inhibits the expression of
MMP9 and uPA in colon cancer LoVo and SW480 cells
To investigate whether curcumin affects the
expression of uPA and MMP9, which are involved in tumor metastasis
and invasion, western blotting and qPCR were performed. The protein
and mRNA levels of uPA and MMP9 was dose-dependently decreased by
curcumin in SW480 (Fig. 3A and B) and
LoVo cells (P=0.003; Fig. 3C and D).
This data demonstrates that curcumin significantly decreases the
expression of uPA and MMP9 in a dose-dependent manner, which
results in the inhibitory effect of colon cancer cell adhesion and
invasion.
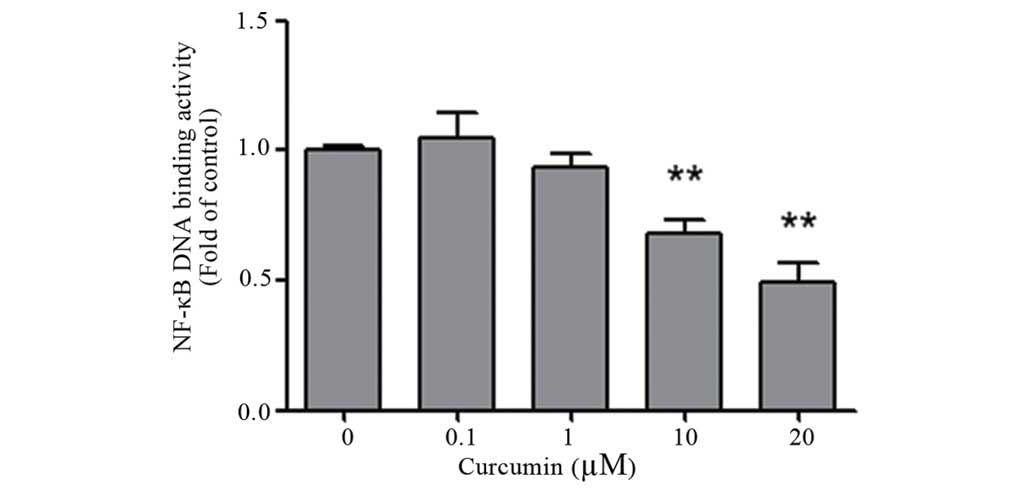
Curcumin inhibits NF-κB activation in
colon cancer LoVo and SW480 cells
The impact of curcumin on the NF-κB pathway has been
observed in multiple human carcinomas (33). To investigate whether curcumin affects
NF-κB activation, which is critical for transcriptional activity,
the DNA binding activity of NF-κB was analyzed by ELISA. Colon
cancer LoVo and SW480 cells were pretreated with various
concentrations of curcumin (0–20 µM) for 24 h. As shown in Fig. 4, curcumin significantly decreased
NF-κB activation in a dose-dependent manner for the two cells lines
(P=0.002).
Curcumin inhibits NF-κB, uPA and MMP9
in colon cancer SW480 cells via AMPK activation
To investigate the effect of curcumin on the
signaling pathway that regulates NF-κB activation, the present
study investigated the expression of various proteins in SW480
cells using western blot analysis. SW480 cells was treated with 10
µM curcumin at various time points (0, 4, 8, 12 and 24 h). As
indicated in Fig. 5A, curcumin
increased AMPK phosphorylation at Thr172 and downregulated NF-κB
p65 in a time-dependent manner.
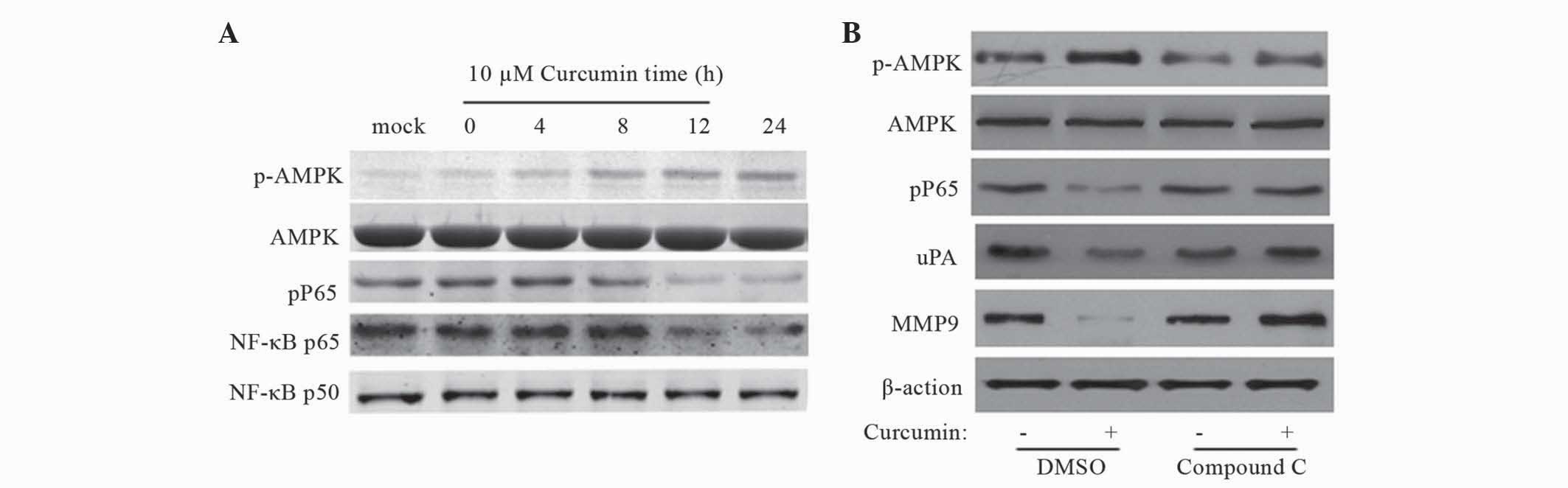 | Figure 5.AMPK activation mediates
curcumin-induced inhibition of NF-κB, uPA and MMP9. Human colon
cancer SW480 cells were cultured until 70% confluence. (A) Cells
were treated with curcumin (10 µM) for 0–24 h to investigate the
effect on the NF-κB signaling pathway. (B) Cells were treated with
curcumin (10 µM) in the absence or presence of 10 µM compound C, an
AMPK inhibitor. Data are representative of three experiemnts. Band
weights (kDa): uPA, 52 kDa; MMP-9, 92 kDa; β-actin, 45 kDa; p-AMPK,
62 kDa; AMPK, 62 kDa; pP65, 65 kDa; NF-κB p65, 65 kDa; NF-κB p50,
50kDa AMPK, 5′ AMP-activated protein kinase; NF-κB, nuclear
factor-κB; uPA, urokinase plasminogen activator; MMP9, matrix
metalloproteinases; DMSO, dimethyl sulfoxide. |
To further delineate the role of AMPK in
curcumin-induced inhibition of uPA and MMP9, pharmacological
inhibition of AMPK was performed using compound C. The inhibitory
effect of compound C on AMPK activity was confirmed by western blot
analysis. As shown in Fig. 5B,
compound C abolished curcumin-induced inhibition of NF-κB, uPA and
MMP9, suggesting that AMPK activation is responsible for
curcumin-mediated NF-κB, uPA and MMP9 inhibition.
Curcumin inhibits p65 NF-κB DNA
binding to the uPA and MMP9 promoter and its subsequent
transactivation
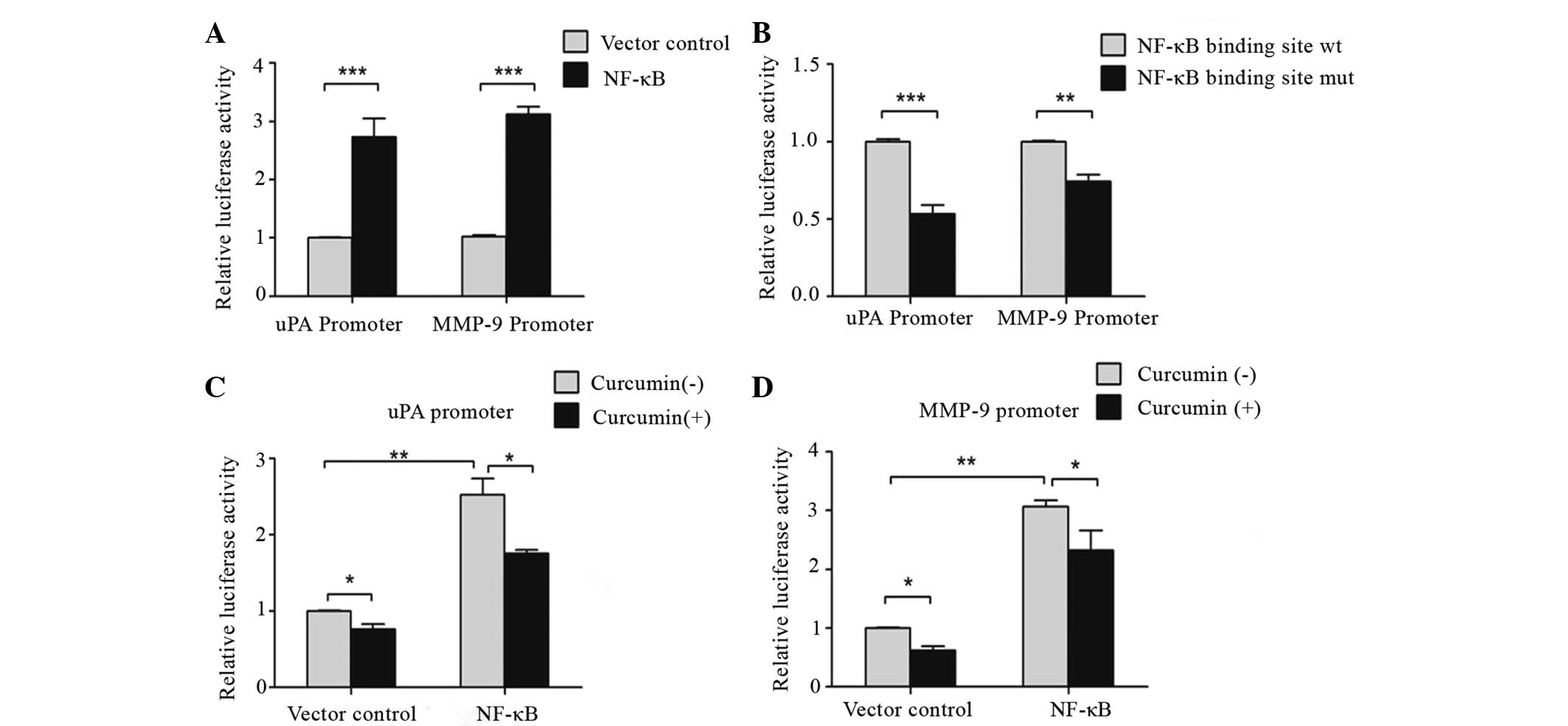
Since several studies have demonstrated that NF-κB
is key in the transactivation of uPA and MMP9 (34–36), the
present study investigated whether the activation of uPA and MMP9
may be attributed to NF-κB. As shown in Fig. 6A, uPA- and MMP9-specific promoter
region activity significantly increased in NF-κB-induced SW480
cells compared with control cells (P=0.0006). Following mutations
in the NF-κB binding site TTCC in uPA and MMP9 promoter regions,
uPA and MMP9 activity decreased significantly compared with the
control. This suggests that NF-κB binds to the uPA and MMP9
promoter sequence TTCC (P=0.0006 and P=0.003; Fig. 6B).
To further confirm the possibility that
downregulation of uPA or MMP9 transactivation, resulting from
curcumin treatment, was caused by reduced binding of NF-κB to the
uPA and MMP9 promoter sequence, wild-type uPA and MMP9 plasmids
were transfected into SW480 cells for 8 h, and the cells were
incubated with 10 µM curcumin for 16 h. As shown in Fig. 6C and D, uPA- and MMP9-specific
promoter region activity significantly decreased following curcumin
treatment (P=0.012; Fig. 6C), and
treatment with NF-κB significantly upregulated uPA- and
MMP9-specific promoter region activity (P=0.008; Fig. 6D). Overall, these results indicate
that curcumin has a significant inhibitory role in NF-κB-mediated
binding transactivation of uPA and MMP9.
Discussion
Tumor cell metastasis is known as a complex cascade
of events, which involves cell adhesion, ECM component degradation
and tumor cell migration. Therefore, blocking one or more of these
steps is required for anti-metastatic therapy (2). Curcumin, which has been demonstrated to
be anti-tumorigenic, has well-documented chemopreventive properties
in a number of cell types, including colon cancer cells (21–25,37–40).
The present study provides clear evidence that curcumin is capable
of inhibiting the adhesion and invasion of human colon cancer cells
through NF-κB-dependent uPA and MMP9 activation and expression in a
dose-dependent manner. This suggests that curcumin may possess
anti-metastatic potential in human colon cancer.
The present data confirms that curcumin
significantly inhibits colon cancer cell growth, particularly at a
high concentration (41). Previous
studies have revealed that NF-κB activation is critical for the
proliferation, survival and metastasis of colon cancer cells
(26,41,42);
therefore, inhibition of NF-κB activation is a potential antitumor
strategy (43–46). The present study observed that
curcumin dose-dependently suppresses constitutive NF-κB activation
in colon cancer LoVo and SW480 cells. These results are in
agreement with previous studies, which demonstrate that curcumin is
a potent inhibitor of NF-κB activation (19,20).
However, the potential mechanisms concerning NF-κB inhibition by
curcumin leading to the suppression of colon cancer cell
proliferation requires additional investigation.
NF-κB activation proceeds sequentially through the
activation of IKK, phosphorylation of IkBα, degradation of IkBα,
release of NF-κB, p65 phosphorylation and p65 nuclear translocation
(47). The present study investigated
the expression of the different proteins in the NF-κB activation
pathway by western blot analysis. Notably, the present study
demonstrated that curcumin inhibited NF-κB via the activation of
AMPK. The AMPK signaling pathway is important in maintaining
cellular survival during stress by regulating metabolic homeostasis
(48). Consistent with the present
findings, previous studies have suggested that the activation of
the AMPK pathway suppresses the function of the NF-κB pathway
(49–54). AMPK has certain direct phosphorylation
targets (55); however, it is
possible that AMPK inhibits NF-κB signaling through its downstream
effectors, including peroxisome proliferator-activated receptor γ
co-activator 1α and sirtuin (silent mating type information
regulation 2 homolog) 1, which suppress inflammatory factors.
Since little is known concerning the function of
curcumin in colon cancer metastatic progression, the present study
determined whether the colon cancer metastatic process is
associated with the suppression of NF-κB. The present cell-adhesion
assay revealed that curcumin significantly reduced LoVo and SW480
cell adhesion to Matrigel (reconstituted basement membrane). This
result led to additional investigation, which revealed that ECM
molecules, including uPA and MMP9, were inhibited by curcumin.
Numerous studies indicate that the key proteases involved in ECM
degradation are MMP and serine proteases, including uPA and MMP9
(7,11). The present study investigated uPA and
MMP9 expression in colon cancer LoVo and SW480 cells. The present
results demonstrated that curcumin significantly decreased the
expression of uPA and MMP9 in a dose-dependent manner, which led to
inhibitory effects on colon cancer cell adhesion and invasion.
Since the level of p65 NF-κB phosphorylation was decreased and
phosphorylation of p65 is required for NF-κB transcriptional
activity (56), the present study
further investigated the mechanism by which curcumin controls uPA
and MMP9 activation and expression via activation of NF-κB in colon
cancer cells. As expected, curcumin suppressed the binding of p65
NF-κB to the uPA and MMP9 promoter. These results clearly indicate
that curcumin may inhibit uPA and MMP9 transcription by suppressing
NF-κB DNA binding activity to uPA and MMP9 promoter region.
Therefore, the anti-metastatic effects of curcumin in human colon
cancer may be mediated by inhibition of the NF-κB signaling
pathway.
In summary, the present preliminary investigation
has revealed that the anti-metastatic effect of curcumin in colon
cancer cells may be mediated by the decrease of uPA and MMP9
expression via NF-κB activation. The NF-κB signaling pathway
promotes the activation of uPA and MMP9 signaling to regulate colon
cancer cell invasion. In addition, curcumin dose-dependently
suppresses the activation of NF-κB, and this effect may attribute
to a dose-dependent decrease in uPA and MMP9 protein levels by
binding to their promoter regions. The present study provides
additional evidence that curcumin inhibits metastatic activity in
cancer cells and reveals a novel therapeutic potential for curcumin
for anti-metastatic therapy.
References
|
1
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brooks SA, Lomax-Browne HJ, Carter TM,
Kinch CE and Hall DM: Molecular interactions in cancer cell
metastasis. Acta Histochem. 112:3–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raghu H, Sodadasu PK, Malla RR, Gondi CS,
Estes N and Rao JS: Localization of uPAR and MMP-9 in lipid rafts
is critical for migration, invasion and angiogenesis in human
breast cancer cells. BMC Cancer. 10:6472010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andreasen PA, Egelund R and Petersen HH:
The plasminogen activation system in tumor growth, invasion, and
metastasis. Cell Mol Life Sci. 57:25–40. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vihinen P, Ala-aho R and Kähäri VM: Matrix
metalloproteinases as therapeutic targets in cancer. Curr Cancer
Drug Targets. 5:203–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hildenbrand R, Gandhari M, Stroebel P,
Marx A, Allgayer H and Arens N: The urokinase-system - role of cell
proliferation and apoptosis. Histol Histopathol. 23:227–236.
2008.PubMed/NCBI
|
|
7
|
Andreasen PA, Kjøller L, Christensen L and
Duffy MJ: The urokinase-type plasminogen activator system in cancer
metastasis: A review. Int J Cancer. 72:1–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Newell KJ, Witty JP, Rodgers WH and
Matrisian LM: Expression and localization of matrix-degrading
metalloproteinases during colorectal tumorigenesis. Mol Carcinog.
10:199–206. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crowley CW, Cohen RL, Lucas BK, Liu G,
Shuman MA and Levinson AD: Prevention of metastasis by inhibition
of the urokinase receptor. Proc Natl Acad Sci USA. 90:5021–5025.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baichwal VR: Activate NF-kappa B or die?
Curr Biol. 7:R94–R96. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beg AA and Baltimore D: An essential role
for NF-kappaB in preventing TNF-alpha-induced cell death. Science.
274:782–784. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Verma IM and Stevenson J: IkappaB kinase:
Beginning, not the end. Proc Natl Acad Sci USA. 94:11758–11760.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen ZJ, Parent L and Maniatis T:
Site-specific phosphorylation of IkappaBalpha by a novel
ubiquitination-dependent protein kinase activity. Cell. 84:853–862.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shishodia S and Aggarwal BB: Nuclear
factor-kappaB activation: A question of life or death. J Biochem
Mol Biol. 35:28–40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: From ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aggarwal BB, Kumar A and Bharti AC:
Anticancer potential of curcumin: Preclinical and clinical studies.
Anticancer Res. 23:363–398. 2003.PubMed/NCBI
|
|
19
|
Singh S and Aggarwal BB: Activation of
transcription factor NF-kappa B is suppressed by curcumin
(diferuloylmethane) [corrected]. J Biol Chem. 270:24995–25000.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar A, Dhawan S, Hardegen NJ and
Aggarwal BB: Curcumin (Diferuloylmethane) inhibition of tumor
necrosis factor (TNF)-mediated adhesion of monocytes to endothelial
cells by suppression of cell surface expression of adhesion
molecules and of nuclear factor-kappaB activation. Biochem
Pharmacol. 55:775–783. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi M, Cai Q, Yao L, Mao Y, Ming Y and
Ouyang G: Antiproliferation and apoptosis induced by curcumin in
human ovarian cancer cells. Cell Biol Int. 30:221–226. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawamori T, Lubet R, Steele VE, Kelloff
GJ, Kaskey RB, Rao CV and Reddy BS: Chemopreventive effect of
curcumin, a naturally occurring anti-inflammatory agent, during the
promotion/progression stages of colon cancer. Cancer Res.
59:597–601. 1999.PubMed/NCBI
|
|
23
|
Mukhopadhyay A, Bueso-Ramos C, Chatterjee
D, Pantazis P and Aggarwal BB: Curcumin downregulates cell survival
mechanisms in human prostate cancer cell lines. Oncogene.
20:7597–7609. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bharti AC, Donato N and Aggarwal BB:
Curcumin (diferuloylmethane) inhibits constitutive and
IL-6-inducible STAT3 phosphorylation in human multiple myeloma
cells. J Immunol. 171:3863–3871. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Plummer SM, Holloway KA, Manson MM, Munks
RJ, Kaptein A, Farrow S and Howells L: Inhibition of
cyclo-oxygenase 2 expression in colon cells by the chemopreventive
agent curcumin involves inhibition of NF-kappaB activation via the
NIK/IKK signalling complex. Oncogene. 18:6013–6020. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mohan R, Sivak J, Ashton P, Russo LA, Pham
BQ, Kasahara N, Raizman MB and Fini ME: Curcuminoids inhibit the
angiogenic response stimulated by fibroblast growth factor-2,
including expression of matrix metalloproteinase gelatinase B. J
Biol Chem. 275:10405–10412. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan MH, Lin-Shiau SY and Lin JK:
Comparative studies on the suppression of nitric oxide synthase by
curcumin and its hydrogenated metabolites through down-regulation
of IkappaB kinase and NFkappaB activation in macrophages. Biochem
Pharmacol. 60:1665–1676. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jobin C, Bradham CA, Russo MP, Juma B,
Narula AS, Brenner DA and Sartor RB: Curcumin blocks
cytokine-mediated NF-kappaB activation and proinflammatory gene
expression by inhibiting inhibitory factor I-kappa B kinase
activity. J Immunol. 163:3474–3483. 1999.PubMed/NCBI
|
|
30
|
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF,
Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al: Phase I
clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
31
|
Al-Tubuly AA: SDS-PAGE and western
blotting. Methods Mol Med. 40:391–405. 2000.PubMed/NCBI
|
|
32
|
Livak KI and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sandur SK, Deorukhkar A, Pandey MK, Pabón
AM, Shentu S, Guha S, Aggarwal BB and Krishnan S: Curcumin
modulates the radiosensitivity of colorectal cancer cells by
suppressing constitutive and inducible NF-kappaB activity. Int J
Radiat Oncol Biol Phys. 75:534–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Killeen SD, Wang JH, Andrews EJ and
Redmond HP: Bacterial endotoxin enhances colorectal cancer cell
adhesion and invasion through TLR-4 and NF-kappaB-dependent
activation of the urokinase plasminogen activator system. Br J
Cancer. 100:1589–1602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sliva D, English D, Lyons D and Lloyd FP
Jr: Protein kinase C induces motility of breast cancers by
upregulating secretion of urokinase-type plasminogen activator
through activation of AP-1 and NF-kappaB. Biochem Biophys Res
Commun. 290:552–557. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsunoda K, Kitange G, Anda T, Shabani HK,
Kaminogo M, Shibata S and Nagata I: Expression of the
constitutively activated RelA/NF-kappaB in human astrocytic tumors
and the in vitro implication in the regulation of urokinase-type
plasminogen activator, migration, and invasion. Brain Tumor Pathol.
22:79–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bharti AC, Donato N, Singh S and Aggarwal
BB: Curcumin (diferuloylmethane) down-regulates the constitutive
activation of nuclear factor-kappa B and IkappaBalpha kinase in
human multiple myeloma cells, leading to suppression of
proliferation and induction of apoptosis. Blood. 101:1053–1062.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zong H, Wang F, Fan QX and Wang LX:
Curcumin inhibits metastatic progression of breast cancer cell
through suppression of urokinase-type plasminogen activator by
NF-kappa B signaling pathways. Mol Biol Rep. 39:4803–4808. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shishodia S, Amin HM, Lai R and Aggarwal
BB: Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB
activation, induces G1/S arrest, suppresses proliferation, and
induces apoptosis in mantle cell lymphoma. Biochem Pharmacol.
70:700–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shishodia S, Potdar P, Gairola CG and
Aggarwal BB: Curcumin (diferuloylmethane) down-regulates cigarette
smoke-induced NF-kappaB activation through inhibition of
IkappaBalpha kinase in human lung epithelial cells: Correlation
with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis.
24:1269–1279. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Johnson SM, Gulhati P, Arrieta I, Wang X,
Uchida T, Gao T and Evers BM: Curcumin inhibits proliferation of
colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer
Res. 29:3185–3190. 2009.PubMed/NCBI
|
|
42
|
Chen H, Zhang ZS, Zhang YL and Zhou DY:
Curcumin inhibits cell proliferation by interfering with the cell
cycle and inducing apoptosis in colon carcinoma cells. Anticancer
Res. 19:3675–3680. 1999.PubMed/NCBI
|
|
43
|
Baeuerle PA and Baichwal VR: NF-kappa B as
a frequent target for immunosuppressive and anti-inflammatory
molecules. Adv Immunol. 65:111–137. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Garg A and Aggarwal BB: Nuclear
transcription factor-kappaB as a target for cancer drug
development. Leukemia. 16:1053–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang S, Pettaway CA, Uehara H, Bucana CD
and Fidler IJ: Blockade of NF-kappaB activity in human prostate
cancer cells is associated with suppression of angiogenesis,
invasion, and metastasis. Oncogene. 20:4188–4197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hideshima T, Chauhan D, Richardson P,
Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A,
Palombella V, et al: NF-kappa B as a therapeutic target in multiple
myeloma. J Biol Chem. 277:16639–16647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109 Suppl:S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Salminen A, Hyttinen JM and Kaarniranta K:
AMP-activated protein kinase inhibits NF-kappaB signaling and
inflammation: Impact on healthspan and lifespan. J Mol Med (Berl).
89:667–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bai A, Ma AG, Yong M, Weiss CR, Ma Y, Guan
Q, Bernstein CN and Peng Z: AMPK agonist downregulates innate and
adaptive immune responses in TNBS-induced murine acute and
relapsing colitis. Biochem Pharmacol. 80:1708–1717. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang Z, Kahn BB, Shi H and Xue BZ:
Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK)
antagonizes fatty acid-induced inflammation through SIRT1. J Biol
Chem. 285:19051–19059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sag D, Carling D, Stout RD and Suttles J:
Adenosine 5′-monophosphate-activated protein kinase promotes
macrophage polarization to an anti-inflammatory functional
phenotype. J Immunol. 181:8633–8641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu
C, Viollet B, Yan D and Zou MH: AMPKalpha2 deletion causes aberrant
expression and activation of NAD(P)H oxidase and consequent
endothelial dysfunction in vivo: Role of 26S proteasomes. Circ Res.
106:1117–1128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu X, Mahadev K, Fuchsel L, Ouedraogo R,
Xu SQ and Goldstein BJ: Adiponectin suppresses IkappaB kinase
activation induced by tumor necrosis factor-alpha or high glucose
in endothelial cells: Role of cAMP and AMP kinase signaling. Am J
Physiol Endocrinol Metab. 293:E1836–E1844. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hattori Y, Suzuki K, Hattori S and Kasai
K: Metformin inhibits cytokine-induced nuclear factor kappaB
activation via AMP-activated protein kinase activation in vascular
endothelial cells. Hypertension. 47:1183–1188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cantó C and Auwerx J: AMP-activated
protein kinase and its downstream transcriptional pathways. Cell
Mol Life Sci. 67:3407–3423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhong H, Voll RE and Ghosh S:
Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional
activity by promoting a novel bivalent interaction with the
coactivator CBP/p300. Mol Cell. 1:661–671. 1998. View Article : Google Scholar : PubMed/NCBI
|