Introduction
The T-box (TBX) gene family is an evolutionarily
ancient gene family, as indicated by phylogenetic analysis
(1), and has been widely studied in
the field of developmental biology (2). TBX genes serve key roles during
organogenesis and pattern formation in vertebrate and invertebrate
embryos (3). Previous studies have
shown that TBX genes encode a group of transcription factors that
are characterized by a highly conserved DNA-binding domain and an
unusual mode of DNA recognition (4–7). Certain
TBX genes are purely activators or repressors, while others contain
activation and repression domains (8). Since the first TBX family member was
discovered in 1927, the TBX family has been increasingly implicated
in the development of various organs and the pathogenesis of a
number of syndromes (9,10).
Over 20 TBX genes have been identified in various
species, ranging from invertebrates, including Drosophila and
Caenorhabditis elegans, to vertebrates, including zebrafish,
Xenopus, mice, chickens and humans (11–13).
Current phylogenetic analysis divides the TBX gene family into five
subfamilies, T, Tbx1, Tbx2, Tbx6 and Tbr1 (8,14). The T
subfamily includes T and TBX19; the Tbx1 subfamily includes TBX1,
TBX10, TBX15, TBX18, TBX20 and TBX22; the Tbx2 subfamily includes
TBX2, TBX3, TBX4 and TBX5; the Tbx6 subfamily includes TBX6 and
Mga; and the Tbr1 subfamily includes TBR1, TBR2 and TBX21. TBX
genes serve significant roles in craniofacial (TBR1, TBX10, TBX15
and TBX22), brain (TBR1 and TBR2), mammary gland (TBX2 and TBX3),
pituitary gland (TBX3, TBX19), thymus (TBX1), liver (TBX3), lung
(TBX2, TBX4 and TBX5) and limb (TBX4 and TBX5) development, in
addition to pigmentation (TBX15) and the immune system (TBX21)
(8,14). Furthermore, TBX proteins function in
proliferation, differentiation, tissue integrity and
epithelial-mesenchymal transition (EMT) (8,14).
Recent studies have shown that defects in, or
overexpression of, certain TBX genes may be involved in the genesis
and progression of a variety of types of cancer (2,15). For
example, unlike the majority of members of the TBX family, which
function as transcriptional activators, TBX2 and TBX3 are the only
mammalian TBX factors that function as transcriptional repressors
(16,17). The role of TBX2 and TBX3 in the
oncogenic process is thought to be associated with an increase in
their level of expression, as they have been found to be
overexpressed in 8 different types of cancer, including breast,
cervical, ovarian, pancreatic, liver and bladder cancer (18,19).
However, there is no comprehensive summary of the molecular and
mechanistic changes to TBX genes during the genesis and progression
of cancer. Therefore, in the present review, the roles of TBX genes
in cancer are discussed. Through increased understanding of TBX
factors at a genetic and molecular level, targeted therapy of these
factors may become a promising therapy for the treatment of
cancer.
TBX genes are involved in tumor cell
invasion and migration
Preliminary evidence suggests that overexpression of
the TBX3 gene is associated with several types of cancer, and that
increased levels of TBX3 are associated with the promotion of tumor
migration and invasion (20). Peres
et al (21) reported that TBX3
knockdown in MCF-7 breast cancer cells and in metastatic melanoma
cells resulted in increased proliferation and reduced migration.
Boyd et al (22) applied a
genome-wide expression profiling approach in an attempt to identify
an association between the expression of B-Raf proto-oncogene
(B-RAF), the transcriptional repressor TBX3 and E-cadherin. The
results of this study demonstrated that B-Raf induced the
expression of TBX3, which potently repressed E-cadherin expression
(Fig. 1). Therefore, TBX3 may act as
a critical regulator of the oncogenic B-Raf signaling pathway and
as a promoter of metastasis in B-RAF-mutant melanomas (22). Furthermore, a previous study has shown
that overexpression of TBX2 and TBX3 in melanoma cells
downregulated endogenous E-cadherin expression, whereas depletion
of TBX3, but not TBX2, increased E-cadherin mRNA and protein
levels, and decreased melanoma invasion in vitro (23). Consistent with these observations,
TBX3 and E-cadherin expression are inversely correlated in melanoma
tissue.
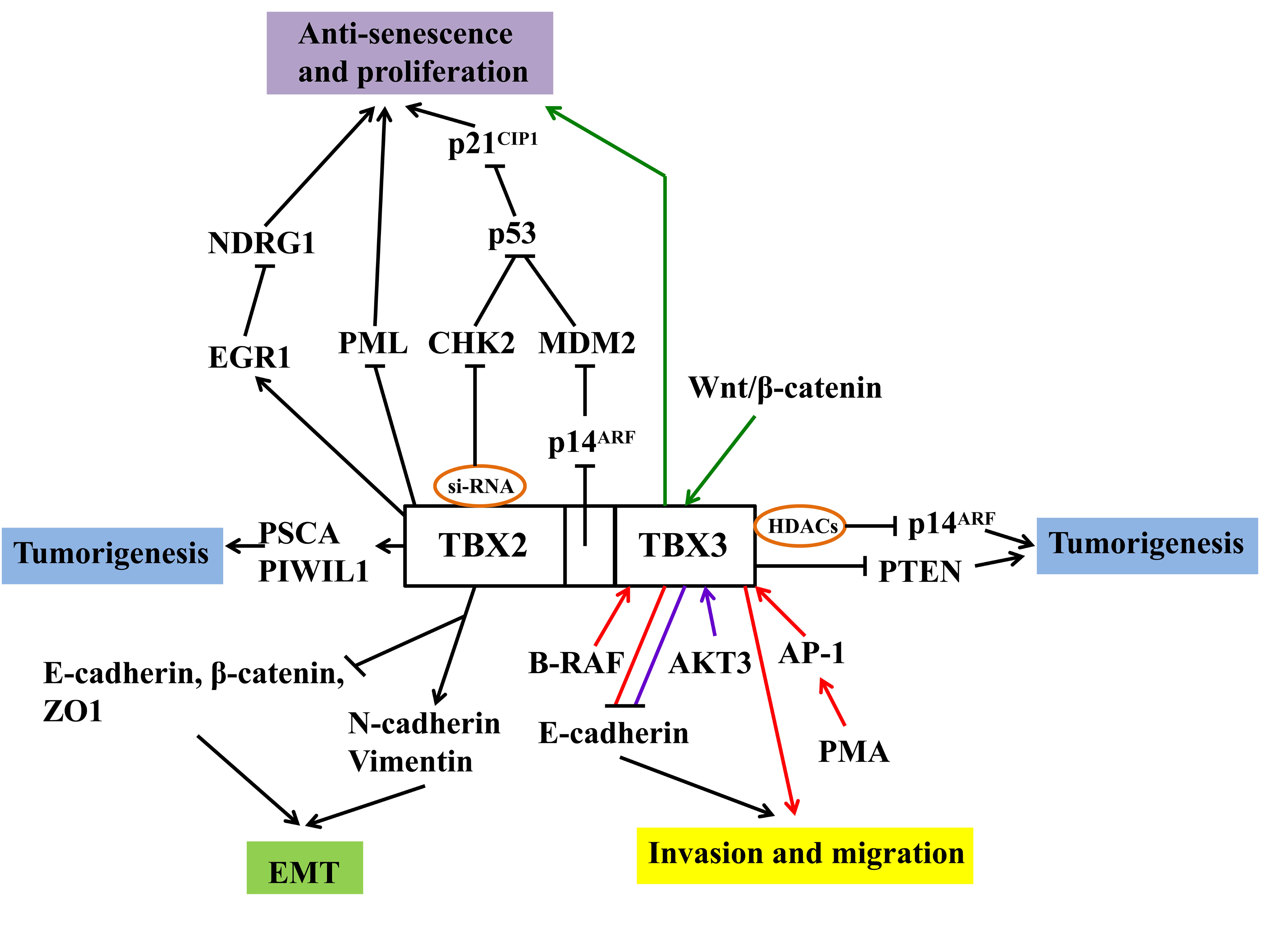 | Figure 1.Roles of TBX2 and TBX3 in
tumorigenesis and tumor progression. Pointed arrowheads indicate
activation; flat arrowheads indicate inhibition. Invasion and
migration: B-RAF induces the expression of TBX3, which represses
E-cadherin expression; AKT3 promotes the expression of TBX3 and
enhances the ability of TBX3 to repress expression of E-cadherin;
PMA increases TBX3 mRNA and protein levels in a protein kinase
C-dependent manner via AP-1. Anti-senescence and proliferation:
Elevated TBX2 levels antagonize PML pro-senescence functions
through direct protein-protein interactions; TBX2 co-represses
NDRG1 through recruitment of EGR1; TBX2 knockdown causes a
reduction in phosphorylated Chk2, p53 and p21CIP1 proteins levels;
TBX2 and TBX3 inhibit p14ARF expression, which inhibits downstrean
MDM2, p53 and p21CIP1 expression; TBX3 is a downstream target gene
of the Wnt/β-catenin signaling pathway. Tumorigenesis: TBX2 may
upregulate the expression of PSCA and PIWIL1; TBX3 interacts with
HDAC1, 2, 3 and 5, and represses p14ARF expression; overexpression
of TBX3 causes a reduction in PTEN mRNA and protein levels. EMT:
Ectopic expression of TBX2 induces loss of epithelial adhesion,
decreased expression of proteins involved in epithelial cell
adhesion (E-cadherin, β-catenin, ZO1) and an abnormal gain of
mesenchymal markers (N-cadherin, Vimentin). TBX, T-box; B-RAF,
B-Raf proto-oncogene; AKT3, Akt serine/threonine kinase 3; PMA,
phorbol-12-myristate-13-acetate; AP-1, activator protein 1; NDRG1,
N-myc downregulated gene 1; EGR1, early growth response protein 1;
CHK2, checkpoint kinase 2; PSCA, prostate stem cell antigen; PIWIL,
piwi-like RNA-mediated gene silencing 1; HDAC, histone deacetylase;
PTEN, phosphatase and tensin homolog; ZO1, zonula occludens-1;
siRNA, small interfering RNA. |
A previous study has shown that the phorbolester
phorbol-12-myristate-13-acetate (PMA) increases TBX3 protein and
mRNA levels in a protein kinase C-dependent manner, via activator
protein 1 (AP-1) (24). Furthermore,
AP-1 mediated activation of the TBX3 gene by binding to a
non-consensus PMA-response element in the TBX3 promoter in
vitro and in vivo (24).
This demonstrates that TBX3 contributes to PMA-induced cell
migration, which has previously been observed in the MCF-7 breast
epithelium cancer cell line (25).
These results provide evidence that increased levels of TBX3
contribute to tumor cell migration (Fig.
1).
TBX genes are involved in tumor cell
immortality and proliferation
TBX genes and promyelocytic leukemia
(PML)
The tumor-suppressing function of PML was identified
through the observation that PML knockout mice were more
tumor-prone (26). PML protein levels
were found to be significantly reduced in a cancers of different
histologic origins, including prostate and colon adenocarcinomas,
breast and lung carcinomas, lymphomas, and central nervous system
and germ cell tumors, compared with corresponding normal tissue
(27). Conversely, excessive TBX2
protein levels have been detected in primary human breast and
pancreatic cancer, and in various cancer cell lines (2). This indicates that there is an inverse
correlation between TBX2 and PML protein levels in cancer. Through
the combination of gene expression profiling, chromatin-binding
analysis and promoter-reporter studies, Martin et al
(28) identified TBX2 as a novel and
direct PML-repressible E2F-target gene involved in the evasion of
senescence. TBX2 gene repression might actively contribute to
senescence, as depletion of TBX2 triggered PML and caused cells to
enter senescence (28). Reciprocally,
elevated TBX2 levels antagonized the pro-senescence functions of
PML through direct protein-protein interaction. These observations
suggest that PML and TBX2 act in an autoregulatory loop to mediate
the effective execution of senescence (Fig. 1).
TBX genes, p14ARF and p21CIP1
During mouse oncogenesis, TBX2 and TBX3 have been
associated with the repression of tumor suppressor genes p19ARF
(p14ARF in humans) and the cyclin-dependent kinase (CDK) inhibitor
p21CIP1 (18). p14ARF increases the
level of p53 by elevating the level of MDM2 proto-oncogene (MDM2).
Increased p53 levels lead to increased levels of p21CIP1, and
subsequent cell cycle arrest in G1 and G2/M (29,30). A
further study demonstrated that p14ARF expression could be induced
by overexpression of various oncogenes or by mitogenic signals
(31). p14ARF can bind to MDM2,
causing stabilization of p53, which initiates a program of gene
expression leading to cell cycle arrest or apoptosis (31). Activated p53, which can initiate
growth arrest in senescent cells, causes activation of p21CIP1,
which is necessary for p53-mediated growth arrest (31). TBX2 and TBX3 suppressed senescence
efficiently in a mouse model through the inhibition of p19ARF
expression (31). Then, inhibition of
p19ARF expression reduced the level of MDM2, blocking stabilization
of p53 and thus inhibiting p53-mediated upregulation of p21CIP1,
which has been implicated in the control of proliferation,
differentiation and senescence (31).
Therefore, it may be necessary for the p14ARF/p19ARF-MDM2-p53-p21
signaling pathway (Fig. 1), a
well-characterized mechanism of cellular senescence, to be targeted
at multiple points for the treatment of cancer.
TBX genes and early growth response
(EGR) 1
The EGR protein family includes zinc-finger
transcription factors involved in cell proliferation and apoptosis.
EGR1 is best characterized as a direct regulator of a number of
signaling pathways, it has significant tumor-suppressing
properties, including the promotion of apoptosis in response to
stress and DNA damage in a number of types of cancer (32,33).
Redmond et al (34) described
a novel mechanism of TBX2 interaction with EGR1, which drove cell
proliferation and survival in breast cancer cell lines. Depletion
of TBX2 or EGR1 resulted in growth inhibition, and the expression
of cell senescence and apoptotic markers, including deleted in
esophageal cancer 1 (Dec1), p21CIP1 and p53 (34). The putative breast cancer suppressor,
N-myc downregulated gene 1 (NDRG1), is implicated in cell
differentiation, apoptosis and senescence (34). TBX2 was found to repress NDRG1 not
through direct promoter interaction, but as a corepressor through
recruitment of EGR1. In this way, TBX2 drove the proliferation of
breast cancer cells (34). NDRG1 was
demonstrated to be a significant effector of growth control
downstream of TBX2-EGR1, and its expression recapitulated growth
inhibition and induced expression of the cell senescence marker
Dec1 (34). These results demonstrate
the significance of the EGR1 signaling pathway in cancer (Fig. 1) and reveal it to be a potential novel
therapeutic target.
TBX genes, histone deacetylase (HDAC)
1 and p21
Rhabdomyosarcomas (RMSs) are the most prevalent soft
tissue sarcomas in children and share many features with developing
skeletal muscle tissue. A recent study by Zhu et al
(35) identified that TBX2 was highly
upregulated in tumor cells of the major RMS subtypes. The results
of this study demonstrated that elevated expression of TBX2
contributes to the pathology of RMS cells. TBX2 inhibited the
regulatory factors myogenic differentiation 1 and myogenin, leading
to the promotion of proliferation (35). Furthermore, TBX2 recruited the histone
deacetylase HDAC1 and inhibited p21, which led to increased
proliferation and decreased differentiation-specific gene
expression (35). Depletion or
inhibition of TBX2 completely inhibited tumor cell growth in RMS
cells (35). These results indicate
that deregulated TBX2 serves as an oncogene in RMS, and may be a
novel diagnostic marker and/or therapeutic target in patients with
RMS.
The CDK inhibitor 2A (CDKN2A) locus, which encodes
p16INK4A and p14ARF, promotes senescence (36). A previous study found that CDKN2A-null
B16 cells exhibited a specific deletion at the CDKN2A locus,
leading to loss of function of p16INK4A and p14ARF (37). In addition, the activation of a
dominant-negative TBX2 (dnTBX2) gene induced senescence in the
CDKN2A-null B16 melanoma cells (37).
Furthermore, senescence was accompanied by increased expression of
the TBX2 target gene p21 and displacement of HDAC1 from the p21
promoter, which markedly reduced proliferation (37). This TBX2-mediated molecular mechanism
of transcriptional repression suggests that continued TBX2 activity
is required to prevent CDKN2A-independent senescence in transformed
cells.
Holt-Oram syndrome is characterized by upper limb
malformations and cardiac septation defects (38). Mutations in the TBX5 gene were found
to cause Holt-Oram syndrome in humans (38). In addition, a previous study reported
that ectopic expression of TBX5 induced apoptosis and decreased the
growth rate of U2OS cells, indicating that TBX5 is a novel
regulator of apoptosis and cell growth (39). These results suggest that the TBX5
gene and protein serve important roles in the development of
certain tissues.
TBX genes are involved in tumorigenesis
Liu et al (40)
investigated the expression and association of prostate stem cell
antigen (PSCA), piwi-like RNA-mediated gene silencing 1 (PIWIL1)
and TBX2 in endometrial adenocarcinoma (EAC). Elevated expression
of PSCA, PIWIL1 and TBX2 was observed significantly more frequently
in EACs compared with normal endometrium (40). TBX2 expression was positively
correlated with PSCA and PIWIL1 expression (40), indicating that TBX2 may upregulate
PSCA and PIWIL1 (Fig. 1). The
association between clinicopathological features and the three
genes (40) revealed that they may
have roles in the development and progression of EAC, and that
PIWIL1 and TBX2 may be candidate markers for early pathological
diagnosis and detection. PSCA, PIWIL1 and TBX2 were not associated
with cancer stage (40), suggesting
an involvement in the initiation of EAC rather than invasion.
However, PSCA and TBX2 were associated with lymph node metastasis,
and may be candidate targets for EAC prognosis and therapy
(40). This study provides a basis
for further investigations into the carcinogenesis of EAC.
HDACs serve an essential role in transcriptional
regulation (41–44) and have been found to be overexpressed
in a number of malignancies, including breast cancer (45,46). The
results of a study by Yarosh et al (47) indicated that overexpression of TBX3
increases the degradation of p53 via downregulating p14ARF
expression, leading to the promotion of breast cancer
tumorigenesis. TBX3 was found to interact with HDAC1, 2, 3 and 5 to
repress their downstream effects on gene expression (47). In the MCF-7 breast cancer cell line,
HDACs were tested for their ability to reverse TBX3 regulation of
p14ARF in a dose-dependent manner (47). TBX3-mediated gene repression may
function by recruiting HDACs to the T-box binding site in the
promoter region of genes and was dependent on HDAC activity
(47). The results of this study
suggest that TBX3-HDAC interaction is important in breast cancer
development (47). Therefore, HDACs
may have an anticancer effect through TBX3-HDAC interaction
(Fig. 1).
A previous study reported that mRNA and protein
levels of TBX3 were increased in head and neck squamous cell
carcinoma (HNSCC) samples compared with their normal tissue
counterparts (48), and another study
found that phosphatase and tensin homolog (PTEN) mRNA levels were
decreased in HNSCC tissues (49).
Furthermore, overexpression of TBX3 in human HeLa and HEK cell
lines was demonstrated to cause a reduction in endogenous PTEN mRNA
and protein levels (48). In
addition, transcription activity assays have shown that TBX3 is
capable of repressing basal and induced activity of PTEN (48). These results suggest that TBX3
represses PTEN and is overexpressed in HNSCC (Fig. 1).
TBX genes are involved in EMT
EMT has been increasingly recognized as a key step
in the progression of primary tumors into metastases (50). EMT interrupts cell-to-cell contact in
a homocellular manner in tumors, allowing the dissemination of
cells from the primary site (51).
During tumor progression, the EMT process appears to be triggered
by genes typically expressed in the early embryo, including TWIST,
SNAIL, SLUG, GOOSECOID and SIP1 (52–55), and
targeting them may prevent tumor invasion and metastasis.
Shimoda et al (51) reported that metastatic ACCS-M green
fluorescent protein cells (ACCS-M GFP cell line), established from
AdCC cells, demonstrated characteristics of EMT, exhibited
sphere-forming ability, and had high expression levels of
EMT-related genes and stem cell and differentiation markers. These
observations suggest that ACCS-MGFP+ cells show characteristics of
cancer stem-like cells (CSCs), which may be involved in the EMT of
adenoid cystic carcinoma (AdCC) cells. Surprisingly, short hairpin
RNA silencing of the TBX transcription factor, T, resulted in
downregulation of EMT and CSC markers in clinical samples of AdCC
(51). Notably, sphere-forming
ability, EMT characteristics and tumorigenicity were simultaneously
lost (51). Therefore, T may be a
regulator of CSC marker expression and EMT in AdCC cells. In
addition, T may be a potential therapeutic target for future
anti-CSC treatment for AdCC.
Wang et al (56) reported that ectopic expression of TBX2
in wild-type HC11 and MCF10A mammary epithelial cells induced
morphological, molecular and behavioral changes characteristic of
EMT. This included loss of expression of proteins involved in
epithelial cell adhesion (E-cadherin, β-catenin and zonula
occludens 1), abnormal gain of mesenchymal markers (N-cadherin,
vimentin), and increased cell motility and invasion (56). Conversely, depletion of overexpressed
endogenous TBX2 in the malignant human breast carcinoma cell lines
MDA-MB-435 and MDA-MB-157 led to the restoration of epithelial
characteristics and loss of mesenchymal markers (56). In addition, chromatin
immunoprecipitation (ChIP) analysis and cell-based reporter assays
revealed that TBX2 directly repressed transcription of E-cadherin,
a tumor suppressor gene, the loss of which is essential for
malignant tumor progression (56).
The results of this study demonstrated an unanticipated link
between TBX2 deregulation in cancer, and the acquisition of EMT and
invasive features in epithelial tumor cells (Fig. 1).
TBX genes are involved in tumorigenic
signaling pathways
ATM serine/threonine kinase
(ATM)-checkpoint kinase 2 (CHK2)-p53 signaling pathway
Wansleben et al (57) demonstrated that silencing of TBX2
reversed several features of transformation in breast cancer and
melanoma cells. In addition, it was shown that ectopic expression
of TBX2 resulted in genetically unstable polyploidy cells with
resistance to cisplatin (57). In an
attempt to identify whether the overexpression of endogenous TBX2
was associated with cisplatin resistance in TBX2-driven cancers,
TBX2 was silenced in a cisplatin-resistant breast cancer cell line
(57). This demonstrated that TBX2
knockdown sensitized the cells to cisplatin by disrupting the
ATM-CHK2-p53 signaling pathway (Fig.
1). Cell cycle analyses demonstrated that TBX2 knockdown led to
a decrease in S-phase arrest, but robust G2/M arrest, which
correlated with a reduction in phosphorylated CHK2 and p53 protein
levels. This knockdown prevented DNA repair, and resulted in
TBX2-deficient cells entering mitosis with damaged DNA and
consequently undergoing mitotic catastrophe (57). These results suggest that targeting
TBX2 in combination with typical chemotherapeutic drugs, including
cisplatin, may improve the efficacy of current anticancer
treatments.
Wnt/β-catenin signaling pathway
The Wnt signaling pathway, a critical regulator of
stem cell function, is involved in numerous aspects of embryonic
development and controls homeostatic self-renewal in a number of
adult tissues (58). Germline
mutations in the Wnt signaling pathway cause several hereditary
diseases, and somatic mutations in this signaling pathway are
associated with cancer of the intestine, liver and a variety of
other tissues (59). Therefore, the
tightly regulated self-renewal mediated by the Wnt signaling
pathway in stem and progenitor cells may be disrupted in cancer
cells, allowing for malignant proliferation.
Using ChIP and reporter assays, a previous study
identified TBX3 as a direct downstream regulatory target gene of
the Wnt/β-catenin signaling pathway that has been implicated in
hepatocarcinogenesis (60).
Furthermore, TBX3 transcription was activated by ectopic expression
of β-catenin in mouse and human tumor cell lines (60) (Fig. 1).
In addition, it has been shown that inhibition of TBX3 by small
interfering RNAs (siRNAs) blocks β-catenin-mediated cell survival
and renders cells sensitive to doxorubicin-induced apoptosis
(60). Conversely, ectopic expression
of TBX3 has been identified to inhibit apoptosis induced by
β-catenin depletion (60). The
results of this study reveal a role for TBX3 as a mediator of
β-catenin-induced cell proliferation and regulator of
hepatocarcinogenesis. Fong et al (61) demonstrated that TBX2 functions within
the context of the Wnt signaling pathway to mediate cell migration
in early embryonic development, indicating that TBX2 may be a
target of the Wnt signaling pathway. However, whether TBX2 is a
direct target of the Wnt signaling pathway in cancer cells remains
unclear.
Akt serine/threonine kinase 3
(AKT)/TBX3/E-cadherin axis
The AKT family includes AKT1, AKT2 and AKT3, among
which AKT3 is the predominant isoform (62,63). There
is growing evidence that AKT3 serves a key role in the
proliferation, invasion and migration of melanomas (64). A previous study demonstrated that
there is a synergistic co-operation between B-RAF-V600E and AKT3 in
promoting melanoma development (65).
In addition, TBX3 may be regulated by AKT3 (65). Notably, Peres et al (66) tested three TBX3 overexpressing
melanoma cell lines, MM200, ME1402 and 501-mel, and observed a
direct correlation between TBX3 mRNA and protein levels, suggesting
that TBX3 may be upregulated at the transcriptional and
post-transcriptional level in cancer. In addition, a direct
positive correlation between phosphorylated AKT levels and TBX3
expression was identified through western blotting (66). Following AKT3 silencing by siRNA in
ME1402 and MM200 melanoma cells in vertical growth phase (VGP),
TBX3 protein levels were reduced (66). Subsequently, site-directed mutagenesis
and in vitro kinase assays demonstrated that TBX3 was
phosphorylated at Ser-720 by AKT3 in vitro (66). Furthermore, it was observed that the
phosphorylation of S720 enhanced the ability of TBX3 to repress its
target gene, E-cadherin (23). TBX3
and E-cadherin protein levels were found to be inversely correlated
in ME1402 VGP melanoma cells (66),
indicating that the AKT/TBX3/E-cadherin axis is involved in
melanoma invasion and migration, and that TBX3 may function
downstream of the AKT3 pathway (Fig.
1). Therefore, TBX3 may become a target gene for the treatment
of advanced melanomas.
TBX genes as targets for cancer therapy
There is a lack of effective long-term cancer
treatments. Therefore, there is a need for novel therapeutic
targets for the treatment of cancer. The studies examined in the
present review indicate that further studies on TBX genes and their
protein products are important, and that any effective anticancer
treatments must inhibit these genes. No other proteins have small
repression domains (RD), so it may be possible to design a drug
targeting the RD, and thus TBX genes, with a high specificity
(67). This may block the function of
TBX proteins in cancer cells. Furthermore, highly specific
treatments have the advantage of possessing low toxicity and a
minimal side-effect profile (68–70).
Through increased understanding of the interaction between TBX
proteins and their signaling partners in various types of cancer,
it may be possible to develop successful TBX RD-specific inhibitors
in the future.
Conclusion
In conclusion, the present review provides an
overview of the roles of TBX genes in the development of various
types of cancer, including tumor cell invasion and migration,
anti-senescence and proliferation, tumorigenesis, EMT and
tumorigenic signaling. In addition, the current review illustrates
the potential to use TBX genes as targets for cancer therapy, which
is currently being examined (1,71). The
theoretical rationale behind this is based on the fact that TBX
genes are overexpressed in various types of cancer and function as
potential oncogenes. An increased understanding of TBX genes and
their protein products will accelerate the development of new
cancer therapies targeting these genes.
Acknowledgements
The present review was supported by the National
Nature Science Foundation of China (grant nos. 81372872, 81402215
and 81320108022), the Nature Science Foundation of Tianjin (grant
no. 16JCYBJC24100) and the program for Innovative University
Research Teams in China (grant no. IRT14R40).
Glossary
Abbreviations
Abbreviations:
|
B-RAF
|
B-Raf proto-oncogene
|
|
PMA
|
phorbol-12-myristate-13-acetate
|
|
AP-1
|
activator protein 1
|
|
PML
|
promyelocytic leukemia
|
|
CDK
|
cyclin-dependent kinase
|
|
MDM2
|
MDM2 proto-oncogene
|
|
EGR
|
early growth response protein
|
|
NDRG1
|
N-myc downregulated gene 1
|
|
HDAC
|
histone deacetylase
|
|
CDKN2A
|
cyclin-dependent kinase inhibitor
2A
|
|
PSCA
|
prostate stem cell antigen
|
|
PIWIL1
|
piwi-like RNA-mediated gene silencing
1
|
|
PTEN
|
phosphatase and tensin homolog
|
|
ATM
|
ATM serine/threonine kinase
|
|
CHK2
|
checkpoint kinase 2
|
|
AKT3
|
Akt serine/threonine kinase 3
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Lu J, Li XP, Dong Q, Kung HF and He ML:
TBX2 and TBX3: The special value for anticancer drug targets.
Biochim Biophys Acta. 1806:268–274. 2010.PubMed/NCBI
|
|
2
|
Abrahams A, Parker MI and Prince S: The
T-box transcription factor Tbx2: Its role in development and
possible implication in cancer. IUBMB Life. 62:92–102.
2010.PubMed/NCBI
|
|
3
|
Takeuchi JK, Koshiba-Takeuchi K, Suzuki T,
Kamimura M, Ogura K and Ogura T: Tbx5 and Tbx4 trigger limb
initiation through activation of the Wnt/Fgf signaling cascade.
Development. 130:2729–2739. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kispert A and Hermann BG: The Brachyury
gene encodes a novel DNA binding protein. EMBO J. 12:4898–4899.
1993.PubMed/NCBI
|
|
5
|
MÜller CW and Herrmann BG:
Crystallographic structure of the T domain-DNA complex of the
Brachyury transcription factor. Nature. 389:884–888. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruvinsky I and Gibson-Brown JJ: Genetic
and developmental bases of serial homology in vertebrate limb
evolution. Development. 127:5233–5244. 2000.PubMed/NCBI
|
|
7
|
Tada M and Smith JC: T-targets: Clues to
understanding the functions of T-box proteins. Dev Growth Differ.
43:1–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papaioannou VE: The T-box gene family:
Emerging roles in development, stem cells and cancer. Development.
141:3819–3833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stein RA: A new TBX gene linked to human
disease. Clin Genet. 76:23–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naiche LA, Harrelson Z, Kelly RG and
Papaioannou VE: T-box genes in vertebrate development. Annu Rev
Genet. 39:219–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morley RH, Lachani K, Keefe D, Gilchrist
MJ, Flicek P, Smith JC and Wardle FC: A gene regulatory network
directed by zebrafish No tail accounts for its roles in mesoderm
formation. Proc Natl Acad Sci USA. 106:3829–3834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gentsch GE, Owens ND, Martin SR,
Piccinelli P, Faial T, Trotter MW, Gilchrist MJ and Smith JC: In
vivo T-box transcription factor profiling reveals joint regulation
of embryonic neuromesodermal bipotency. Cell Rep. 4:1185–1196.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lolas M, Valenzuela PD, Tjian R and Liu Z:
Charting Brachyury-mediated developmental pathways during early
mouse embryogenesis. Proc Natl Acad Sci USA. 111:4478–4483. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Papaioannou VE and Silver LM: The T-box
gene family. Bioessays. 20:9–19. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mowla S, Pinnock R, Leaner VD, Goding CR
and Prince S: PMA-induced up-regulation of TBX3 is mediated by AP-1
and contributes to breast cancer cell migration. Biochem J.
433:145–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lingbeek ME, Jacobs JJ and van Lohuizen M:
The T-box repressors TBX2 and TBX3 specifically regulate the tumor
suppressor gene p14ARF via a variant T-site in the initiator. J
Biol Chem. 277:26120–26127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davenport TG, Jerome-Majewska LA and
Papaioannou VE: Mammary gland, limb and yolk sac defects in mice
lacking Tbx3, the gene mutated in human ulnar mammary syndrome.
Development. 130:2263–2273. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rowley M, Grothey E and Couch FJ: The role
of Tbx2 and Tbx3 in mammary development and tumorigenesis. J
Mammary Gland Biol Neoplasia. 9:109–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan W, Huang X, Chen C, Gray J and Huang
T: TBX3 and its isoform TBX3+2a are functionally distinctive in
inhibition of senescence and are overexpressed in a subset of
breast cancer cell lines. Cancer Res. 64:5132–5139. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Weinberg MS, Zerbini L and Prince S:
The oncogenic TBX3 is a downstream target and mediator of the
TGF-β1 signaling pathway. Mol Biol Cell. 24:3569–3576. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peres J, Davis E, Mowla S, Bennett DC, Li
JA, Wansleben S and Prince S: The highly homologous T-Box
transcription factors, TBX2 and TBX3, have distinct roles in the
oncogenic process. Genes Cancer. 1:272–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boyd SC, Mijatov B, Pupo GM, Tran SL,
Gowrishankar K, Shaw HM, Goding CR, Scolyer RA, Mann GJ, Kefford
RF, et al: Oncogenic B-RAF(V600E) signaling induces the T-Box3
transcriptional repressor to repress E-cadherin and enhance
melanoma cell invasion. J Invest Dermatol. 133:1269–1277. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodriguez M, Aladowicz E, Lanfrancone L
and Goding CR: Tbx3 represses E-cadherin expression and enhances
melanoma invasiveness. Cancer Res. 68:7872–7881. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mowla S, Pinnock R, Leaner VD, Goding CR
and Prince S: PMA-induced up-regulation of TBX3 is mediated by AP-1
and contributes to breast cancer cell migration. Biochem J.
433:145–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jackson D, Zheng Y, Lyo D, Shen Y,
Nakayama K, Nakayama KI, Humphries MJ, Reyland ME and Foster DA:
Suppression of cell migration by protein kinase Cdelta. Oncogene.
24:3067–3072. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang ZG, Delva L, Gaboli M, Rivi R,
Giorgio M, Cordon-Cardo C, Grosveld F and Pandolfi PP: Role of PML
in cell growth and the retinoic acid pathway. Science.
279:1547–1551. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gurrieri C, Capodieci P, Bernardi R,
Scaglioni PP, Nafa K, Rush LJ, Verbel DA, Cordon-Cardo C and
Pandolfi PP: Loss of the tumor suppressor PML in human cancers of
multiple histologic origins. J Natl Cancer Inst. 96:269–279. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin N, Benhamed M, Nacerddine K,
Demarque MD, van Lohuizen M, Dejean A and Bischof O: Physical and
functional interaction between PML and TBX2 in the establishment of
cellular senescence. EMBO J. 31:95–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pomerantz J, Schreiber-Agus N, Liégeois
NJ, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee
HW, et al: The Ink4a tumor suppressor gene product, p19Arf,
interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell.
92:713–723. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stott FJ, Bates S, James MC, McConnell BB,
Starborg M, Brookes S, Palmero I, Ryan K, Hara E, Vousden KH and
Peters G: The alternative product from the human CDKN2A locus,
p14(ARF), participates in a regulatory feedback loop with p53 and
MDM2. EMBO J. 17:5001–5014. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prince S, Carreira S, Vance KW, Abrahams A
and Goding CR: Tbx2 directly represses the expression of the
p21(WAF1) cyclin-dependent kinase inhibitor. Cancer Res.
64:1669–1674. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gitenay D and Baron VT: Is EGR1 a
potential target for prostate cancer therapy? Future Oncol.
5:993–1003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Krones-Herzig A, Adamson E and Mercola D:
Early growth response 1 protein, an upstream gatekeeper of the p53
tumor suppressor, controls replicative senescence. Proc Natl Acad
Sci USA. 100:3233–3238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Redmond KL, Crawford NT, Farmer H, D'Costa
ZC, O'Brien GJ, Buckley NE, Kennedy RD, Johnston PG, Harkin DP and
Mullan PB: T-box 2 represses NDRG1 through an EGR1-dependent
mechanism to drive the proliferation of breast cancer cells.
Oncogene. 29:3252–3262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu B, Zhang M, Byrum SD, Tackett AJ and
Davie JK: TBX2 blocks myogenesis and promotes proliferation in
rhabdomyosarcoma cells. Int J Cancer. 135:785–797. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ruas M and Peters G: The p16INK4a/CDKN2A
tumor suppressor and its relatives. Biochim Biophys Acta.
1378:F115–F177. 1998.PubMed/NCBI
|
|
37
|
Vance KW, Carreira S, Brosch G and Goding
CR: Tbx2 is overexpressed and plays an important role in
maintaining proliferation and suppression of senescence in
melanomas. Cancer Res. 65:2260–2268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Basson CT, Bachinsky DR, Lin RC, Levi T,
Elkins JA, Soults J, Grayzel D, Kroumpouzou E, Traill TA,
Leblanc-Straceski J, et al: Mutations in human TBX5 [corrected]
cause limb and cardiac malformation in Holt-Oram syndrome. Nat
Genet. 15:30–35. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He ML, Chen Y, Peng Y, Jin D, Du D, Wu J,
Lu P, Lin MC and Kung HF: Induction of apoptosis and inhibition of
cell growth by developmental regulator hTBX5. Biochem Biophys Res
Commun. 297:185–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu WK, Jiang XY and Zhang ZX: Expression
of PSCA, PIWIL1, and TBX2 in endometrial adenocarcinoma. Onkologie.
33:241–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Glozak MA, Sengupta N, Zhang X and Seto E:
Acetylation and deacetylation of non-histone proteins. Gene.
363:15–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dokmanovic M and Marks PA: Prospects:
Histone deacetylase inhibitors. J Cell Biochem. 96:293–304. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Puri PL, Iezzi S, Stiegler P, Chen TT,
Schiltz RL, Muscat GE, Giordano A, Kedes L, Wang JY and Sartorelli
V: Class I histone deacetylases sequentially interact with MyoD and
pRb during skeletal myogenesis. Mol Cell. 8:885–897. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang X, Ozawa Y, Lee H, Wen YD, Tan TH,
Wadzinski BE and Seto E: Histone deacetylase 3 (HDAC3) activity is
regulated by interaction with protein serine/threonine phosphatase
4. Genes Dev. 19:827–839. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pan LN, Lu J and Huang B: HDAC inhibitors:
A potential new category of anti-tumor agents. Cell Mol Immunol.
4:337–343. 2007.PubMed/NCBI
|
|
46
|
Hosford SR and Miller TW: Clinical
potential of novel therapeutic targets in breast cancer: CDK4/6,
Src, JAK/STAT, PARP, HDAC, and PI3K/AKT/mTOR pathways.
Pharmgenomics Pers Med. 7:203–215. 2014.PubMed/NCBI
|
|
47
|
Yarosh W, Barrientos T, Esmailpour T, Lin
L, Carpenter PM, Osann K, Anton-Culver H and Huang T: TBX3 is
overexpressed in breast cancer and represses p14 ARF by interacting
with histone deacetylases. Cancer Res. 68:693–699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Burgucu D, Guney K, Sahinturk D, Ozbudak
IH, Ozel D, Ozbilim G and Yavuzer U: Tbx3 represses PTEN and is
over-expressed in head and neck squamous cell carcinoma. BMC
Cancer. 12:4812012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Moral M and Paramio JM: Akt pathway as a
target for therapeutic intervention in HNSCC. Histol Histopathol.
23:1269–1278. 2008.PubMed/NCBI
|
|
50
|
Palena C, Polev DE, Tsang KY, Fernando RI,
Litzinger M, Krukovskaya LL, Baranova AV, Kozlov AP and Schlom J:
The human T-box mesodermal transcription factor Brachyury is a
candidate target for T-cell-mediated cancer immunotherapy. Clin
Cancer Res. 13:2471–2478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shimoda M, Sugiura T, Imajyo I, Ishii K,
Chigita S, Seki K, Kobayashi Y and Shirasuna K: The T-box
transcription factor Brachyury regulates epithelial-mesenchymal
transition in association with cancer stem-like cells in adenoid
cystic carcinoma cells. BMC Cancer. 12:3772012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Grünert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bolós V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with Snail and E47 repressors. J Cell
Sci. 116:499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang B, Lindley LE, Fernandez-Vega V,
Rieger ME, Sims AH and Briegel KJ: The T box transcription factor
TBX2 promotes epithelial-mesenchymal transition and invasion of
normal and malignant breast epithelial cells. PLoS One.
7:e413552012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wansleben S, Davis E, Peres J and Prince
S: A novel role for the anti-senescence factor TBX2 in DNA repair
and cisplatin resistance. Cell Death Dis. 4:e8462013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Renard CA, Labalette C, Armengol C, Cougot
D, Wei Y, Cairo S, Pineau P, Neuveut C, de Reyniès A, Dejean A, et
al: Tbx3 is a downstream target of the Wnt/beta-catenin pathway and
a critical mediator of beta-catenin survival functions in liver
cancer. Cancer Res. 67:901–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fong SH, Emelyanov A, Teh C and Korzh V:
Wnt signalling mediated by Tbx2b regulates cell migration during
formation of the neural plate. Development. 132:3587–3596. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Brazil DP, Park J and Hemmings BA: PKB
binding proteins. Getting in on the Akt. Cell. 111:293–303. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Stahl JM, Sharma A, Cheung M, Zimmerman M,
Cheng JQ, Bosenberg MW, Kester M, Sandirasegarane L and Robertson
GP: Deregulated Akt3 activity promotes development of malignant
melanoma. Cancer Res. 64:7002–7010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cheung M, Sharma A, Madhunapantula SV and
Robertson GP: Akt3 and mutant V600E B-Raf cooperate to promote
early melanoma development. Cancer Res. 68:3429–3439. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Peres J, Mowla S and Prince S: The T-box
transcription factor, TBX3, is a key substrate of AKT3 in
melanomagenesis. Oncotarget. 6:1821–1833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Paxton C, Zhao H, Chin Y, Langner K and
Reecy J: Murine Tbx2 contains domains that activate and repress
gene transcription. Gene. 283:117–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhou Y, Du W, Koretsky T, Bagby GC and
Pang Q: TAT-mediated intracellular delivery of NPM-derived peptide
induces apoptosis in leukemic cells and suppresses leukemogenesis
in mice. Blood. 112:2474–2483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fåhraeus R, Laín S, Ball KL and Lane DP:
Characterization of the cyclin-dependent kinase inhibitory domain
of the INK4 family as a model for a synthetic tumour suppressor
molecule. Oncogene. 16:587–596. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bonfanti M, Taverna S, Salmona M,
D'Incalci M and Broggini M: p21WAF1-derived peptides linked to an
internalization peptide inhibit human cancer cell growth. Cancer
Res. 57:1442–1446. 1997.PubMed/NCBI
|
|
71
|
Douglas NC and Papaioannou VE: The T-box
transcription factors TBX2 and TBX3 in mammary gland development
and breast cancer. J Mammary Gland Biol Neoplasia. 18:143–147.
2013. View Article : Google Scholar : PubMed/NCBI
|















