Introduction
Sorafenib (or nexavar), is an oral multi-kinase
inhibitor that inhibits tumor signal transduction in multi-targets
and blocks tumor cell proliferation, differentiation and migration
(?). It also exerts the effect of inhibiting angiogenesis and
inducing apoptosis (1). Sorafenib is
an effective molecular-targeted drug in advanced renal cell
carcinoma (RCC) and hepatocellular carcinoma (2).
Targeted therapy restricts medicine or the
therapeutic effect to specific target cells, tissues or organs.
This therapy is obviously superior to traditional radiotherapy and
chemotherapy, with fewer adverse effects in advanced malignant
tumors (3). It can also improve
treatment results and prolong the survival period in advanced
differentiated thyroid, lung, gastric, pancreatic and ovarian
cancer (4). However, although there
has been a focus on advanced tumor, to the best of our knowledge,
few studies have been conducted on early-stage resectable tumor
(5).
The aim of the present study was to examine whether
sorafenib was capable of improving the postoperative effect of
resectable tumor in RCC through an analysis of a small sample and a
clinical control study at the Zhumadian Central Hospital (Henan,
China), to provide a valuable reference for clinical treatment.
Patients and methods
Patients
A total of 133 patients diagnosed with renal clear
cell carcinoma at the Zhumadian Central Hospital from June, 2013 to
June, 2015 were selected consecutively. The inclusion criteria for
the present study were: T1-2N0M0 period in American Joint Committee
on Cancer staging of RCCs, no secondary tumor in kidney or kidney
stones, patients having completed surgical removal of the tumor and
cytokines or sorafenib therapies, no participation in other
clinical trials, good compliance and complete clinical data. The
exclusion criteria for the study were: impaired kidney function,
high level (doubled more than normal) of serum creatinine and urea
nitrogen, hemoglobin <100 g/l; history of renal trauma, isolated
kidney, solitary kidney, renal tuberculosis; serious hypertension
and poor diabetes control, and disease of cardiovascular combined
with cerebrovascular, liver function damage, and other underlying
diseases intolerable of surgery.
Informed consent was obtained from patients and
their families. The ethics committee at the Zhumadian Central
Hospital approved the study protocol. The patients were divided
into the surgery alone group (40 cases), surgery combined with
cytokine group (45 cases) and surgery combined with sorafenib group
(48 cases) according to the different treatments. The surgery alone
group included 25 men and 15 women, aged 46–68 years with an
average age of 57.5±10.3 years; tumor numbers were 1–3 with 1.6±0.5
on average; 32 cases of unilateral and 8 of bilateral; and maximum
tumor diameters were 2–7.5 cm with 4.8±1.9 cm on average. The
surgery combined with cytokines group included 28 men and 17 women,
aged 43–69 years with an average age of 56.2±11.4 years; tumor
numbers were 1–3 with 1.4±0.6 on average; 36 cases of unilateral
and 9 of bilateral; and maximum tumor diameters were 2.5–8.0 cm
with 4.9±1.6 cm on average. The surgery combined with sorafenib
group included 29 men and 19 women, aged 44–72 years with an
average age of 59.3±14.2 years; tumor numbers were 1–3 with 1.7±0.8
on average; 39 cases of unilateral and 9 of bilateral; and maximum
diameters of the tumor were 2.5–8.5 cm with 5±1.7 cm on average.
Differences of gender, age, tumor number, location and diameter in
the 3 groups had no statistical significance (P>0.05).
Treatment methods
The operative methods included radical resection and
nephron sparing surgery, and open and laparoscopic surgery, which
were carried out according to the standard of medical procedures
performed by one surgical and nursing team. There was no
statistically significance in the different surgeries of the 3
groups (P>0.05). In the cytokine group, interleukin (IL)-2 was
selected for an intravenous injection of 72,000 IU/kg·8 h, used for
5 days followed with 2 days of break, for a total of ≤4-12 weeks.
In the sorafenib group, sorafenib was injected at 400 mg each time,
twice a day, for a total of 4–12 weeks. Any adverse reactions led
to drugs being withdrawn.
Observation index
The follow-up was continued to January, 2016 and the
2 groups were followed-up 6–30 months; average of 20 months.
Recurrence rates, survival rates, serum creatinine, hemoglobin
levels, the levels of serum vascular endothelial growth factor
(VEGF) and tumor necrosis factor (TNF)-α, and drug adverse reaction
rates of the 2 groups were compared as per the commonly used
chemotherapy drug toxicity standard CTC 3.0 version. Serum
creatinine and hemoglobin levels were detected using conventional
biochemical assay, and VEGF and TNF-α were detected using ELISA.
Kits were purchased from R&D Systems, Inc. (Minneapolis, MN,
USA) and used as per the manufacturer's instructions.
Statistical analysis
SPSS 20.0 (IBM SPSS, Armonk, NY, USA) was used for
data analysis and processing. Quantitative data were presented as
mean ± standard deviation and groups were compared with single
factor analysis of ANOVA. Qualitative data were expressed as cases
number or percentage, and groups were compared using the
χ2 test. Survival time was compared using the
Kaplan-Meier method (log-rank test). P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of recurrence rate and
survival rate
The Kaplan-Meier method was used to determine the
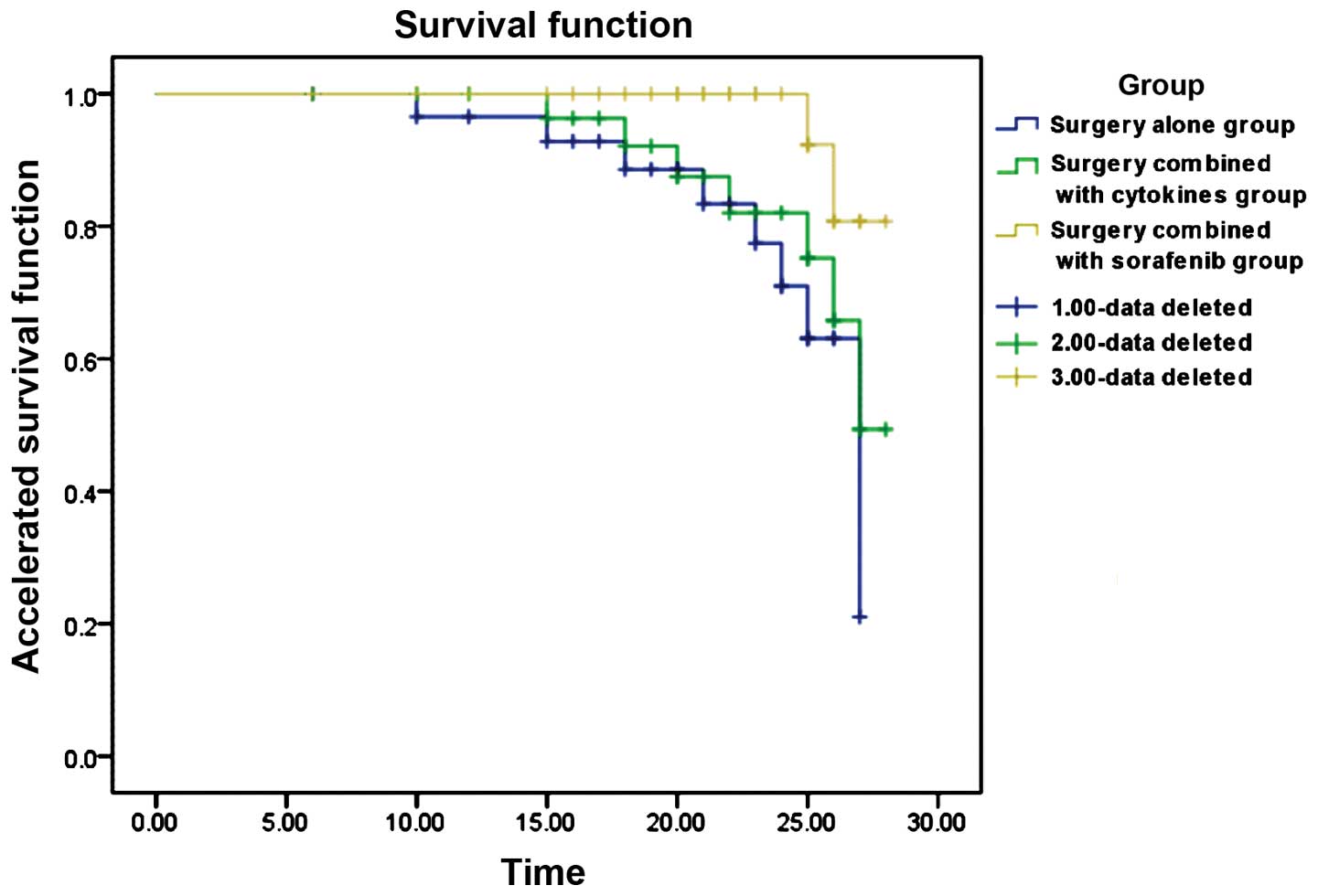
recurrence and survival rates of different treatments. The results
revealed that the surgery combined with sorafenib group had a
significantly lower recurrence and higher survival rates (Fig. 1) (P<0.05). The median survival
period in the sorafenib group was >30 months while that in the
other 2 groups was 27 months, with a significant statistical
difference (χ2=6.214, P=0.045) (Table I).
 | Table I.Tumor recurrence and survival rates
[cases no. (%)]. |
Table I.
Tumor recurrence and survival rates
[cases no. (%)].
| Group | Cases | Recurrence rate | Survival rate |
|---|
| Surgery | 40 | 11 (27.5) | 31 (77.5) |
| Surgery combined with
cytokines | 45 | 11 (24.4) | 38 (84.4) |
| Surgery combined with
sorafenib | 48 | 4 (8.3) | 46 (95.8) |
| χ2 |
| 6.133 | 6.504 |
| P-value |
| 0.047 | 0.039 |
Comparison of serum creatinine and
hemoglobin levels
Differences of preoperative serum creatinine and
hemoglobin levels were not statistically significant (P>0.05).
In 3 months of follow-up, creatinine levels were significantly
elevated while hemoglobin levels were significantly decreased. The
sorafenib group had decreased creatinine and higher hemoglobin
levels than those in the other 2 groups (P<0.05) (Table II).
 | Table II.Comparison of serum creatinine and
hemoglobin level. |
Table II.
Comparison of serum creatinine and
hemoglobin level.
| Group | Preoperative
creatinine, µmol/l | Creatinine in 3
months | Preoperative
hemoglobin, g/l | Hemoglobin in 3
months |
|---|
| Surgery alone | 82.5±26.3 | 243.6±43.2 | 105.3±13.2 | 92.4±12.8 |
| Surgery combined with
cytokines | 84.3±24.7 | 225.7±46.9 | 103.4±14.6 | 93.5±13.2 |
| Surgery combined with
sorafenib | 85.2±22.5 | 163.4±35.2 | 102.8±13.8 | 96.6±12.7 |
| F | 0.321 | 5.624 | 0.632 | 5.128 |
| P-value | 0.465 | 0.037 | 0.597 | 0.044 |
Comparison of VEGF and TNF-α
Differences of preoperative VEGF and TNF-α levels
were not statistically significant (P>0.05). In the 3 months of
follow-up, VEGF levels were reduced but TNF-α levels were
increased. The sorafenib group had significantly lower VEGF and
higher TNF-α levels than those in other 2 groups (P<0.05)
(Table III).
 | Table III.Comparison of VEGF and TNF-α
(pg/ml). |
Table III.
Comparison of VEGF and TNF-α
(pg/ml).
| Group | Preoperative
VEGF | VEGF in 3 months | Preoperative
TNF-α | TNF-α in 3
months |
|---|
| Surgery alone | 326.4±52.6 | 159.9±32.6 | 265.4±46.2 | 342.5±65.4 |
| Surgery combined with
cytokines | 331.5±54.2 | 136.4±33.4 | 246.7±47.8 | 367.8±69.3 |
| Surgery combined with
sorafenib | 329.8±51.3 |
79.6±25.8 | 253.2±46.3 | 532.9±72.5 |
| F | 0.258 | 5.352 | 0.825 | 5.764 |
| P-value | 0.137 | 0.039 | 0.634 | 0.035 |
Comparison of the incidence of adverse
reactions
The surgery combined with cytokine group had 6 cases
of severe abdominal pain and diarrhea, 4 cases of headache, 5 cases
of multiple organ dysfunction (such as myocardial ischemia,
hypotension, respiratory difficulties, liver and kidney
dysfunction, thrombocytopenia, anemia and mental disorders), with a
total incidence of 33.3%. The surgery combined with sorafenib group
had 3 cases of severe hand or foot skin reaction, 2 cases of
hypertension, 1 case of diarrhea, 1 case of fatigue and loss of
appetite, with a total incidence of 33.3%, which was significantly
lower than those in the cytokine group (χ2=4.521,
P=0.033).
Discussion
VEGF and platelet-derived growth factor is the most
important regulatory factor for the promotion of angiogenesis.
Sorafenib inhibits tyrosine kinase receptor activity contributing
to angiogenesis and tumor development, thus blocking the formation
of tumor angiogenesis and cuts off the supply of nutrients to tumor
cells and therefore inhibits tumor cell growth indirectly (6). Sorafenib suppresses the Ras/Raf/MEK/ERK
signaling pathway, which directly inhibits tumor cell proliferation
(7). Sorafenib also suppresses
cytokines, such as FLT-3 and C-KIT, which contribute to tumor cell
evolution and proliferation, thus directly inhibiting tumor cell
proliferation (8). Sorafenib induces
tumor cells into the apoptotic process directly (9).
Phase II clinical studies conducted by Kondo et
al (10) and phase III clinical
trials from Mori et al (11)
confirmed that sorafenib may improve the therapeutic effect of
advanced RCC and increase median progression-free survival. The
multicenter and non-control open clinical study (II T) on advanced
RCC in China also confirmed the safety and effect of sorafenib.
Therefore, sorafenib was the first targeted drug used for RCC
(12). The killing effect of
G250-DC-CIK cells combined with sorafenib on RCC cells indicated
that immune therapy combined with targeted drugs, not only had a
direct inhibitory effect on tumor cells, but also killed tumor
cells indirectly by regulating the immune system (13).
RCC in early stage has a high surgical resection
rate, whereas 30% of the 3-year recurrence rate and 75% of the
survival rate has a poor effect (14). In addition, renal carcinoma is not
sensitive to radiotherapy and chemotherapy, which leads to
treatment failure (15). Cytokine
adjuvant therapy including IL-2 and interferon was confirmed not to
improve the survival rate with more adverse drug reactions, which
reduces patient quality of life (16). The study applied targeted therapy of
sorafenib in early stage resectable RCC and found that the surgery
alone group had a recurrence rate of 27.5% and survival rate of
77.5%, with no significant difference to the surgery combined with
cytokine group. The surgery combined with sorafenib group had a
significantly lower recurrence rate (8.3%) and higher survival rate
(95.8%), prolonging the median survival time (>30 months, >27
months) with slight impaired renal function, which may be due to
the biological activity on inhibition of tumor (17). VEGF levels were significantly lower
whereas TNF-α levels were higher. It was suggested that sorafenib
exhibited the mechanism of inhibiting tumor growth for early stage
RCC, which had a guiding significance to increase the application
scope of sorafenib (18). The
incidence of adverse drug reactions decreased significantly, with a
range of 1–2, and may be alleviated after symptomatic treatment,
without drug withdrawal. However, the applying dose and pathway of
cytokines, applying time of cytokines and sorafenib may affect
study results (19,20). The innovation point of the present
study is that the application of sorafenib in early stage RCC also
has great therapeutic effect and leads to novel ideas regarding
targeted therapy.
In summary, sorafenib can improve the postoperative
survival rate of early stage RCC. It prolongs survival time and
reduces the recurrence rate without increasing adverse reactions.
Sorafenib improves renal function and decreases the level of VEGF
and elevates the level of TNF-α.
References
|
1
|
Flaherty KT: Sorafenib: Delivering a
targeted drug to the right targets. Expert Rev Anticancer Ther.
7:617–626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ratain MJ, Eisen T, Stadler WM, Flaherty
KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, et
al: Phase II placebo-controlled randomized discontinuation trial of
sorafenib in patients with metastatic renal cell carcinoma. J Clin
Oncol. 24:2505–2512. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hampton T: Cancer drug trials show modest
benefit: Drugs target liver, gastric, head and neck cancers. JAMA.
298:273–275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Strumberg D: Preclinical and clinical
development of the oral multikinase inhibitor sorafenib in cancer
treatment. Drugs Today (Barc). 41:773–784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abou-Alfa GK, Schwartz L, Ricci S, Amadori
D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz
B, et al: Phase II study of sorafenib in patients with advanced
hepatocellular carcinoma. J Clin Oncol. 24:4293–4300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43–9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pagano M, Sierra NM, Panebianco M, Rossi
G, Gnoni R, Bisagni G and Boni C: Sorafenib efficacy in thymic
carcinomas seems not to require c-KIT or PDGFR-alpha mutations.
Anticancer Res. 34:5105–5110. 2014.PubMed/NCBI
|
|
9
|
Shi WH, Bian YH, Song XH and Wu JC:
Co-administration of Sorafenib with adriamycin inhibits cell
proliferation in hepatocellular carcinoma cells HepG2. Prog Mod
Biomed. 24:4845–3848. 2011.
|
|
10
|
Kondo T, Nakazawa H, Oya M, Kimura G,
Fujii Y, Hatano T, Kawata N, Kume H, Morita M, Nakajima K, et al:
Clinical efficacy and prognostic factors of tumor progression in
Japanese patients with advanced renal cell carcinoma treated with
sorafenib. Jpn J Clin Oncol. 45:274–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mori Y, Cai K, Cheng Y, Wang S, Paun B,
Hamilton JP, Jin Z, Sato F, Berki AT, Kan T, et al: A genome-wide
search identifies epigenetic silencing of somatostatin,
tachykinin-1, and 5 other genes in colon cancer. Gastroenterology.
131:797–808. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J, Zhu Y, Zhang C, Wang X, He H, Wang
H, Wu Y, Zhou W and Shen Z: Sorafenib or sunitinib as postoperative
adjuvant therapy for Chinese patients with locally advanced clear
cell renal cell carcinoma at high risk for disease recurrence. Urol
Oncol. 31:1800–1805. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang ZY, Sun T and Ma M: The effects of
G250-DC-CIK cells combined with sorafenib on renal tumor cell. Chin
J Biochem Pharm. 32:225–228. 2011.
|
|
14
|
Gaudino M, Lau C, Cammertoni F, Vargiu V,
Gambardella I, Massetti M and Girardi LN: Surgical treatment of
renal cell carcinoma with cavoatrial involvement: A systematic
review of the literature. Ann Thorac Surg. 101:1213–1221. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Antonelli A, Sodano M and Tardanico R:
Response to editorial comment to features, risk factors and
clinical outcome of ‘very late’ recurrences after surgery for
localized renal carcinoma: a retrospective evaluation of a cohort
with a minimum of 10 years of follow up. Int J Urol. 23:412016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Randall JM, Millard F and Kurzrock R:
Molecular aberrations, targeted therapy, and renal cell carcinoma:
Current state-of-the-art. Cancer Metastasis Rev. 33:1109–1124.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miyake H, Muramaki M, Imai S, Harada KI
and Fujisawa M: Changes in renal function of patients with
metastatic renal cell carcinoma during treatment with
molecular-targeted agents. Target Oncol. 27:45–47. 2015.
|
|
18
|
Choi KH, Yu YD, Kang MH and Park DS:
Sorafenib treatment for recurrent stage T1 bilateral renal cell
carcinoma in patients with Von Hippel-Lindau disease: A case report
and literature review. Can Urol Assoc J. 9:E651–E653. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin CH, Yuan HJ, Wang K, Wu JT, Liu QZ, Yu
SQ, Men CP, Gao ZL and Wang J: Initial experience of sorafenib
neoadjuvant therapy combined with retroperitoneoscopy in treating
T2 large renal carcinoma. BioMed Res Int. 2015:6095492015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eriksen KW, Søndergaard H, Woetmann A,
Krejsgaard T, Skak K, Geisler C, Wasik MA and Odum N: The
combination of IL-21 and IFN-alpha boosts STAT3 activation,
cytotoxicity and experimental tumor therapy. Mol Immunol.
46:812–820. 2009. View Article : Google Scholar : PubMed/NCBI
|