Introduction
Endometrial cancer (EC) is the most common
malignancy of the female genital system, with a mortality rate only
less than that of ovarian cancer in the United States in 2013
(1). In China, there has been a
growing tendency for this disease to increasingly occur in younger
women (2,3). Although the majority of patients are
initially diagnosed at an early stage, 15–20% of high risk cases
are found to suffer from relapse (4,5). The use
of lymphadenectomy for EC has remained debatable since the
International Federation of Gynecology and Obstetrics (FIGO)
(6) proposed surgical staging in
1988. Previously, two randomized controlled trials reported that
there was no benefit from systematic lymph node excision in terms
of survival in early-stage EC (7,8). In
addition, it was shown by a retrospective review that nodal
resection was not found to have a survival benefit in the low-risk
group, whereas in the medium- and high-risk group, complete staging
was correlated with improved 5-year survival rates (9). It is well known that clinical stage I
disease consists of not only low-risk, but also intermediate- and
high-risk patients. Therefore, lymphadenectomy must be tailored via
pre-operative risk assessments to ensure its therapeutic effect,
and to avoid unwanted invasiveness and complications such as lymph
cyst formation and lower limb lymphedema. Thus far, literature
regarding the prediction of lymph node metastasis prior to surgery
in EC has been limited and each study has had limitations (10–12).
Identification of novel and more reliable molecular markers in
pre-operative curettage specimens would be conducive to
individualized and more accurate surgical treatment for EC
patients.
MicroRNAs (miRNAs/miRs) are small, non-coding RNA
sequences containing 19–25 nucleotides, which usually control gene
expression by binding to the 3′-untranslated region of their target
mRNAs at a post-transcriptional level, either resulting in the
degradation of the mRNA transcript or leading to translational
repression (13,14). Numerous miRNAs have been revealed to
be overexpressed or downregulated in various types of human tumors,
thus demonstrating their roles as oncogenes or tumor suppressors
(15–18). The altered expression of miRNAs in the
majority of tumor types suggests that they can be used as
diagnostic or prognostic biomarkers in cancer (19,20).
Moreover, the superior stability of miRNAs in formalin-fixed
paraffin-embedded (FFPE) tissues and their relative stability even
in curettage specimens make the clinical utility of miRNAs feasible
(21).
It has previously been revealed that miR-205 shows
altered expression in a variety of malignancies. In certain cancer
types, such as ovarian cancer and nasopharyngeal carcinoma, it
serves as an oncogene (22,23), while in prostate cancer, it exerts a
tumor suppressive function (24). For
EC, however, several studies have consistently achieved results
showing that miR-205 is upregulated in EC compared with normal
endometrial tissues or plasma samples (25,26).
The goal of the present study was to validate the
role of miR-205 as a prognostic marker and to determine its
correlation with clinicopathological parameters, including lymph
node status, in EC. Notably, the study aimed to investigate whether
the overexpression of miR-205 in curettage samples of EC could
identify patients who are more likely to develop lymph node
metastases prior to surgery, so that a tailored lymphadenectomy
could be offered to patients who required it the most and survival
benefits could be obtained from the procedure of lymph node
dissection.
Materials and methods
Patients and samples
A total of 360 eligible EC patients admitted to the
General Hospital Affiliated to Ningxia Medical University
(Yinchuan, Ningxia, China) between January 2006 and December 2010
were enrolled in the current study. All the EC patients were
females and the median age was 59 years (range, 35–78). The study
was approved by the Ethics Committee of the General Hospital
Affiliated to Ningxia Medical University. For the inclusion
criteria, all patients had received primary surgical treatment
mainly consisting of a hysterectomy, bilateral
salpingo-oophorectomy and pelvic lymphadenectomy. Para-aortic lymph
node sampling was conducted when suspicious nodes were identified
or encountered during the surgery. Patients who were subjected to
hormonal, chemical or radiation therapy prior to surgical treatment
were ruled out according to the exclusion criteria. The clinical
stage was confirmed according to the 1971 FIGO criteria (27). For surgical staging, the 1988 FIGO
(28) criteria were applied.
Clinicopathological data and follow-up information with regard to
survival were retrieved. The date of the last follow-up was
December 31, 2014, with a median follow-up time of 55 months
(range, 0–96 months). A total of 86 patients succumbed to the EC.
The characteristics of the patients are summarized in Table I. The archival FFPE tissue blocks used
in this study were collected from the Department of Pathology at
the General Hospital Affiliated to Ningxia Medical University. For
RNA extraction, tumor samples were obtained from EC patients who
underwent curettage and hysterectomy, while normal control
endometrial tissues were acquired from patients receiving
hysterectomy due to myoma of the uterus at the General Hospital
Affiliated to Ningxia Medical University.
 | Table I.Clinicopathological characteristics of
patients with endometrial cancer. |
Table I.
Clinicopathological characteristics of
patients with endometrial cancer.
| Characteristics | n (%) |
|---|
| Age, years |
|
|
<60 | 111 (30.8) |
| ≥60 | 249 (69.2) |
| Menopause |
|
| No | 107 (29.7) |
|
Yes | 253 (70.3) |
| FIGO
staginga |
|
| I | 267 (74.2) |
| II | 39 (10.8) |
|
III | 48 (13.3) |
| IV | 6 (1.7) |
| Histological
type |
|
|
Endometrioid | 321 (89.2) |
|
Non-endometrioid | 39 (10.8) |
| Histological
grade |
|
| 1 and
2 | 310 (86.1) |
| 3 | 50 (13.9) |
| Myometrial
invasion |
|
| No or
<1/2 | 302 (83.9) |
|
≥1/2 | 58 (16.1) |
| Lymph node
metastasis |
|
|
Negative | 314 (87.2) |
|
Positive | 46 (12.8) |
RNA extraction
Using archival FFPE tissues, tumor and normal
endometrium were identified using the corresponding hematoxylin and
eosin-stained sections, the width of which was 2.0 cm. Total RNA
was isolated using a miRNeasy FFPE kit (Qiagen, Hilden, Germany)
based on the manufacturer's protocols. The quantity of the RNA was
assessed using a NanoDrop 1000 spectrophotometer (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA).
Reverse transcription and quantitative
polymerase chain reaction (PCR)
Reverse transcription (cDNA synthesis) was fulfilled
by the High Capacity cDNA Synthesis kit (Applied Biosystems, CA,
USA) with miRNA specific primers, using 2 µl total RNA, performed
according to the manufacturer's instructions. The miR-205 and the
internal control U6 small nuclear RNA (U6) specific primers was
synthesized by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai,
China). The primer sequences were as follows: miR-205 forward,
5′-ACACTCCAGCTGGCTCCTTCATTCCACCGGAG-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′; and U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′. Quantitative PCR was performed
on a Roche LightCycler® 480-II. All reactions were
performed in a final volume of 20 µl reaction mixture containing 2
µl synthesized cDNA, 2 µl of each primer (100 µM), 10 µl 2X Roche
LightCycler® SYBR Green I Master (Roche Applied Science,
Mannheim, Germany), and 6 µl water, which was a type of tailor-made
solvent of the LightCycler® 480 SYBR Green I Master mix.
The PCR parameters were a first pre-incubation step at 95°C for 5
min, followed by an amplification step with 45 cycles at 95°C for
10 sec, 60°C for 5 sec and 72°C for 5 sec. A melting curve was
generated to evaluate the specificity of the PCR products. U6 was
used as an internal control to normalize the amount of miR-205.
Relative expression level of miR-205 was calculated using the ∆∆Ct
method (29). Each sample was
detected in triplicate.
Statistical analyses
All statistical analyses were performed using SPSS
program version 17.0 (SPSS, Chicago, IL, USA). The relative
expression of miR-205 in different groups was compared by a
Mann-Whitney U test. Analyses for associations between miR-205
expression and clinicopathological parameters were made using
Pearson's χ2 test. Binary logistic regression was used
to evaluate odds ratios (OR) for lymph node metastasis. A receiver
operating characteristic (ROC) curve was generated to find a cutoff
of miR-205 with optimal diagnostic sensitivity and specificity.
Disease-specific survival time was defined as the time from surgery
to mortality from endometrial carcinoma. The analysis of
disease-specific survival was performed by the Kaplan-Meier method
and compared using the Log-rank test. Cox's proportional hazard
model was used for multivariate survival analysis and the
assessment of prognostic factors. All statistical tests were
two-sided and P<0.05 was considered to indicate a statistically
significant difference.
Results
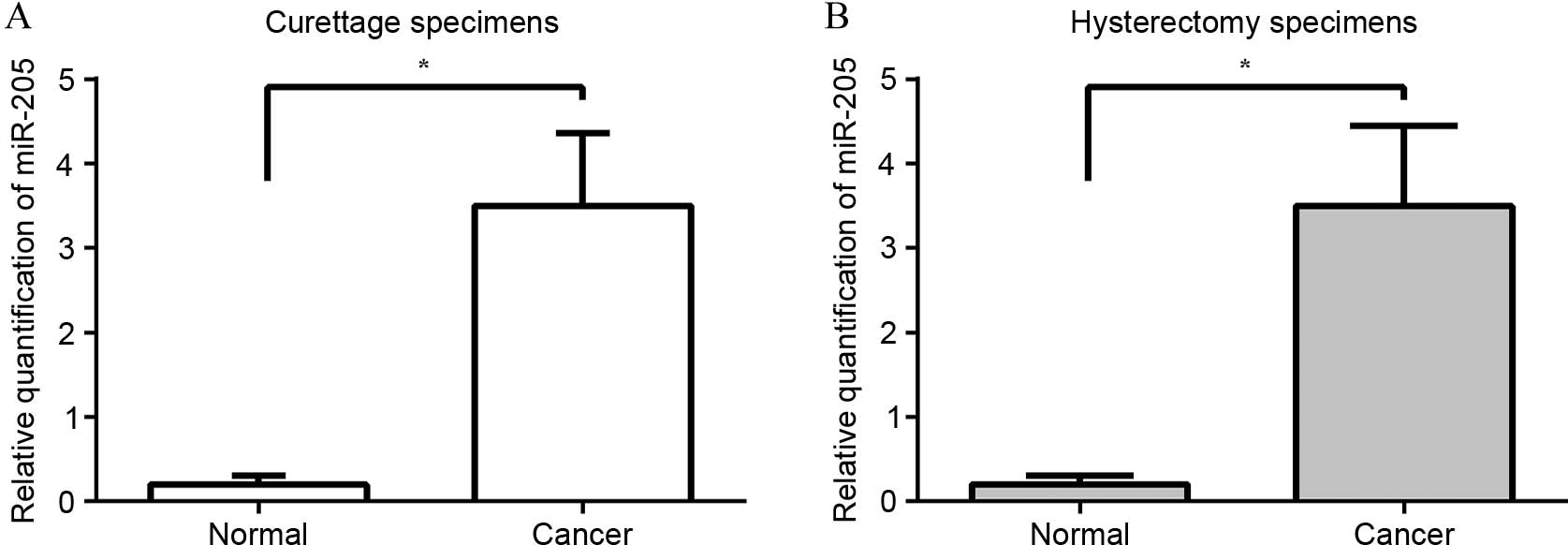
Expression of miR-205 is elevated in
curettage and hysterectomy samples of EC
It had been previously revealed by certain studies
that miR-205 plays a role as an oncogene in EC (25,26). The
present study examined the relative expression of miR-205 in
curettage and hysterectomy specimens of EC and in corresponding
normal endometrial tissues by quantitative PCR. The median miR-205
expression level in the endometrial biopsies of EC and in the
normal endometrium were 3.5 (range, 1.8–5.0) and 0.2 (range,
0.04–0.37), respectively. The median miR-205 expression level in
the hysterectomy samples of EC and in the normal endometrium were
3.5 (range, 1.6–5.4) and 0.2 (range, 0.03–0.40), respectively. The
median expression level of miR-205 was upregulated consistently in
the curettage and hysterectomy cancer tissues by 17.5-fold when
compared with their normal counterparts (P<0.0001) (Fig. 1).
Correlation between overexpression of miR-205 and
clinicopathological parameters in EC. The overexpression of miR-205
was found in 129 (36%) of the curettage and the hysterectomy
specimens. The increased expression of miR-205 in the curettage and
hysterectomy samples was significantly correlated with
pre-operative histological characteristics, including subtype and
grade (both P<0.001; Table II).
The upregulated expression of miR-205 in the curettage and
hysterectomy samples of EC was also significantly correlated with
the presence of lymph node metastasis (both P<0.001; Table II).
 | Table II.Overexpression of microRNA-205 in
curettage and hysterectomy specimens of patients with endometrial
cancer associated with clinicopathological variables. |
Table II.
Overexpression of microRNA-205 in
curettage and hysterectomy specimens of patients with endometrial
cancer associated with clinicopathological variables.
|
| Curettage
specimens | Hysterectomy
specimens |
|---|
|
|
|
|
|---|
| Variable | n (%) | P-value | n (%) | P-value |
|---|
| Histological
subtype |
| <0.001 |
| <0.001 |
|
Endometrioid | 105/323 (32.5) |
| 104/321 (32.3) |
|
|
Non-endometrioid |
24/37 (64.9) |
|
25/39 (64.1) |
|
| Histological
grade |
| <0.001 |
| <0.001 |
| 1 and
2 | 101/313 (32.3) |
| 99/310 (31.9) |
|
| 3 |
28/47 (59.6) |
| 30/50
(60.0) |
|
| Lymph node
metastasis |
| <0.001 |
| <0.001 |
|
Absent | 94/314
(29.9) |
| 95/314
(30.2) |
|
|
Present |
35/46 (76.1) |
|
34/46 (73.9) |
|
The expression of miR-205 in the curettage and
hysterectomy specimens was totally concordant. In addition, there
were no statistically significant differences between pre-operative
and post-operative histological characteristics (subtype and
grade), as presented in Table II.
The concordance of histological subtype was 94.9%, with 2 patients
diagnosed as endometrioid by endometrial biopsy, but validated by
final pathological report as non-endometrioid. The concordance of
histological grade was 94.0%, with 3 patients diagnosed as grade 1
or 2 prior to surgery and subsequently confirmed as grade 3 by
post-operative pathological result.
Overexpression of miR-205 can predict
lymph node metastasis for patients with EC
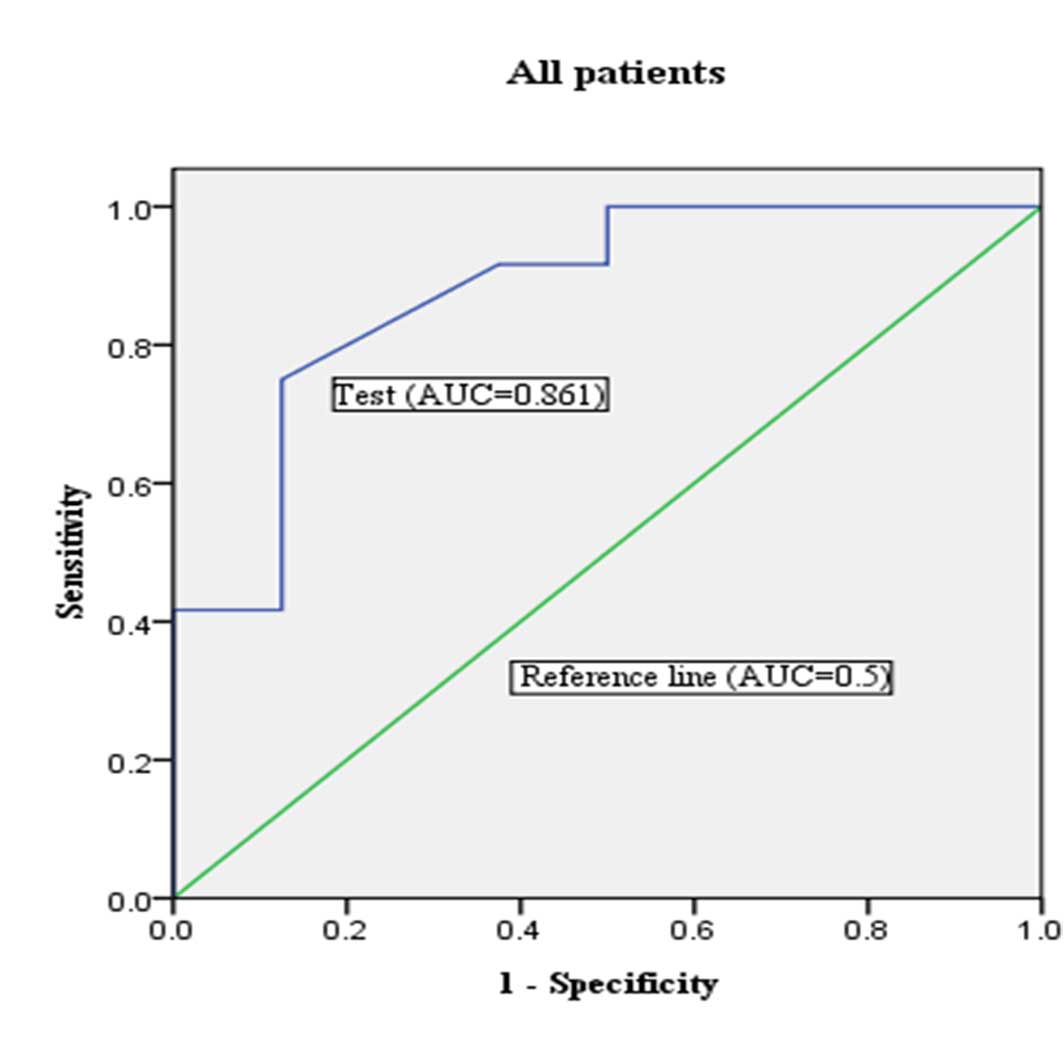
In order to investigate the predictive potential of
miR-205 expression for lymph node metastasis, an ROC curve of
miR-205 expression was made by comparing patients with and without
lymph node metastasis. For all EC patients (n=360), 46 patients
with lymph node metastasis had been validated by final pathological
reports. Lymph node metastasis could be identified with a
sensitivity of 50.0% and a specificity of 85.0% by pre-operative
histological characteristics (Table
III). In this study population, the best cut-off of miR-205
expression was a fold-change of 16.0. The expression of miR-205
with a fold-change of >16.0 in curettage specimens demonstrated
a sensitivity of 71.4%, a specificity of 80.2%, a positive
likelihood ratio of 3.55, a negative likelihood ratio of 0.36 and
an accuracy of 79.4% [area under curve (AUC)=0.861; P=0.003; 95%
CI, 0.709–1.000] (Table III;
Fig. 2). Based on a combination of
pre-operative histological characteristics and miR-205 expression,
the predictive ability of lymph node metastasis could be enhanced
to a sensitivity of 80.4%, a specificity of 80.3%, a positive
likelihood ratio of 4.00, a negative likelihood ratio of 0.25 and
an accuracy of 80.3% (Table
III).
 | Table III.Prediction of lymph node metastasis
according to histological variables and expression of miR-205
(cut-off of 16.0 FC) in curettage specimens for all endometrial
cancer patients. |
Table III.
Prediction of lymph node metastasis
according to histological variables and expression of miR-205
(cut-off of 16.0 FC) in curettage specimens for all endometrial
cancer patients.
|
|
| Univariate | Multivariate |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variable | n | OR | 95%CI | P-value | OR | 95%CI | P-value | SNS | SP | LR+ | LR- |
|---|
| Curettage
histology |
|
|
| 0.002 |
|
| 0.01 | 0.50 | 0.85 | 3.33 | 0.59 |
|
Low-risk | 291 | 1.00 |
|
| 1.00 |
|
|
|
|
|
|
| High-risk | 69 | 3.01 | 1.77–5.08 |
| 2.76 | 1.22–3.84 |
|
|
|
|
|
| miR-205
expression |
|
|
| <0.001 |
|
| 0.02 | 0.71 | 0.80 | 3.55 | 0.36 |
| miR-205<16.0
FC | 266 | 1.00 |
|
| 1.00 |
|
|
|
|
|
|
| miR-205>16.0
FC | 94 | 3.11 | 1.56–4.63 |
| 2.14 | 1.17–4.15 |
|
|
|
|
|
| Curettage
histology+ miR-205 |
|
|
| 0.001 |
|
| 0.01 | 0.80 | 0.80 | 4.00 | 0.25 |
|
Low-risk+miR-205<16.0 FC | 261 | 1.00 |
|
| 1.00 |
|
|
|
|
|
|
| High-risk or/and
miR-205>16.0 FC | 99 | 3.06 | 1.10–4.99 |
| 2.55 | 1.18–3.97 |
|
|
|
|
|
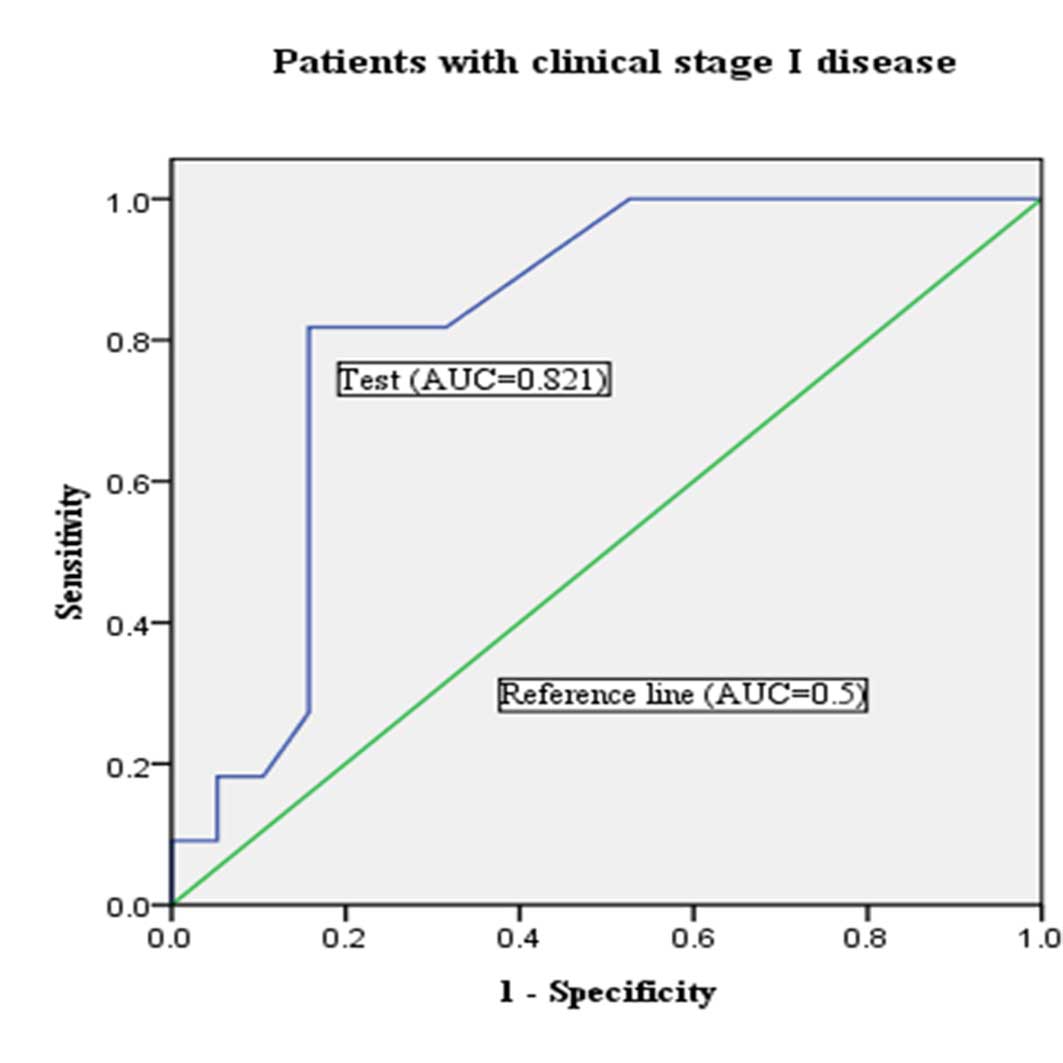
According to the 1971 FIGO clinical staging, the EC
patients with clinical stage I and II–IV were 270 (75.0%) and 90
(25.0%), respectively. On the basis of the final pathological
reports, 29 patients with lymph node metastasis had been found
among the patients with clinical stage I disease. Lymph node
metastasis could be predicted with a sensitivity of 45.0% and a
specificity of 88.0% by pre-operative characteristics (Table IV). When the best cutoff of miR-205
was evaluated in the endometrial biopsies for predicting lymph node
metastasis in this group using a ROC curve, the miR-205 level of
14.5 fold-change was the best, with a sensitivity of 72.5%, a
specificity of 84.2%, a positive likelihood ratio of 4.56, a
negative likelihood ratio of 0.32 and an accuracy of 83.0%
(AUC=0.821; P=0.004; 95% CI, 0.666–0.975) (Table IV; Fig.
3). When combining pre-operative histological characteristics
with miR-205 expression, the predictive value of lymph node
metastases could be improved to a sensitivity of 82.7%, a
specificity of 83.8%, a positive likelihood ratio of 5.19, a
negative likelihood ratio of 0.20 and an accuracy of 83.7%
(Table IV).
 | Table IV.Expression of miR-205 (cut-off of
14.5 FC) and histological parameters in curettage specimens predict
lymph node metastasis of endometrial cancer patients with clinical
stage I disease. |
Table IV.
Expression of miR-205 (cut-off of
14.5 FC) and histological parameters in curettage specimens predict
lymph node metastasis of endometrial cancer patients with clinical
stage I disease.
|
|
| Univariate | Multivariate |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Variable | n | OR | 95%CI | P-value | OR | 95%CI | P-value | SNS | SP | LR+ | LR- |
|---|
| Curettage
histology |
|
|
| 0.002 |
|
| 0.02 | 0.45 | 0.88 | 3.75 | 0.62 |
|
Low-risk | 228 | 1.00 |
|
| 1.00 |
|
|
|
|
|
|
|
High-risk | 42 | 3.03 | 1.81–4.78 |
| 2.67 | 1.17–3.64 |
|
|
|
|
|
| miR-205
expression |
|
|
| 0.001 |
|
| 0.01 | 0.73 | 0.84 | 4.56 | 0.32 |
|
miR-205<14.5 FC | 211 | 1.00 |
|
| 1.00 |
|
|
|
|
|
|
|
miR-205>14.5 FC | 59 | 3.12 | 1.97–5.01 |
| 2.09 | 1.05–4.74 |
|
|
|
|
|
| Curettage
histology+ miR-205 |
|
|
| <0.001 |
|
| 0.01 | 0.83 | 0.84 | 5.19 | 0.20 |
|
Low-risk+miR-205<14.5
FC | 207 | 1.00 |
|
| 1.00 |
|
|
|
|
|
|
|
High-risk or/and
miR-205>14.5 FC | 63 | 3.07 | 1.08–4.96 |
| 2.47 | 1.12–3.89 |
|
|
|
|
|
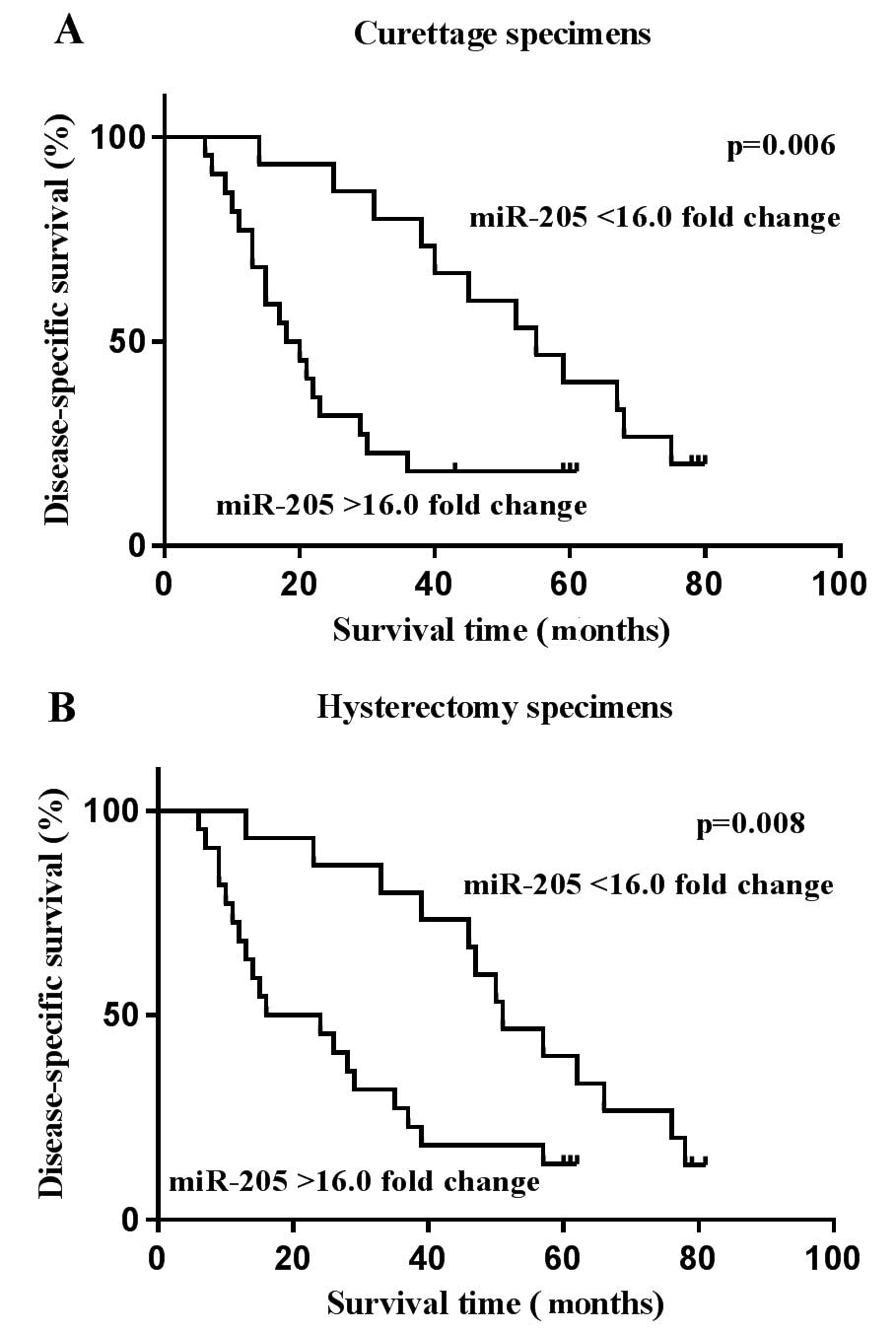
Upregulated miR-205 is associated with
poor prognosis in patients with EC
It has been reported that the overexpression of
miR-205 is negatively correlated with the prognosis of patients
with EC (30). To further validate
the prognostic significance of miR-205 in EC, Kaplan-Meier survival
analysis was performed to assess disease-specific survival. The
results revealed that the overexpression of miR-205 was
significantly correlated with poor patient survival. As shown in
Table V and Fig. 4, patients with a relatively high
expression level of miR-205 yielded worse survival times than
patients with a low level of miR-205 in the curettage (P=0.006) and
hysterectomy (P=0.008) groups, respectively. These findings
indicated that the increased expression of miR-205 in tumor tissues
could identify EC patients with unfavorable survival times.
 | Table V.Multivariate survival analyses for
all patients with endometrial cancer. |
Table V.
Multivariate survival analyses for
all patients with endometrial cancer.
| Variable | n | Adjusted HR | 95% CI | P-value |
|---|
| Age, years |
|
|
| 0.017 |
|
<60 | 111 | 1.00 |
|
|
|
≥60 | 249 | 1.14 | 1.05–1.19 |
|
| FIGO
stagea |
|
|
| 0.001 |
| I and
II | 306 | 1.00 |
|
|
| III and
IV | 54 | 4.32 | 1.77–7.84 |
|
| Histological
grade |
|
|
| 0.003 |
| 1 and
2 | 310 | 1.00 |
|
|
| 3 | 50 | 3.02 | 1.56–5.04 |
|
| Lymph node
metastasis |
|
|
| 0.002 |
|
Negative | 314 | 1.00 |
|
|
|
Positive | 46 | 2.97 | 1.14–3.89 |
|
| miR-205
curettage |
|
|
| 0.006 |
|
miR-205<16.0 FC | 266 | 1.00 |
|
|
|
miR-205>16.0 FC | 94 | 2.23 | 1.08–3.54 |
|
| miR-205
hysterectomy |
|
|
| 0.008 |
|
miR-205<16.0 FC | 268 | 1.00 |
|
|
|
miR-205>16.0 FC | 92 | 2.16 | 1.01–3.23 |
|
Discussion
The individualization of lymphadenectomy remains
challenging in the surgical management of EC, particularly in
clinical stage I disease where lymph node excision has been
suggested to provide a survival benefit for high-risk patients,
whereas the omission of lymphadenectomy can be adopted in the
low-risk group (31,32). Owing to the relatively low incidence
of lymph node metastasis in low-risk EC patients, randomized
surgical trials with high efficacy are difficult to implement
unless an adequate population is available. By contrast, the
assessment of novel tools holding potential for identifying
patients with a high risk for lymph node involvement is regarded
more reasonable, as this pattern would decrease the required sample
size in a clinical trial of lymphadenectomy, meanwhile avoiding
unnecessary complications from sampling low-risk patients (33).
The pre-operative evaluation of lymph node
metastasis represents a crucial step to decide the extent of
surgery in EC. Endometrial biopsy is considered to be the
cornerstone of the diagnosis and primary surgical treatment
planning for EC (34). It has
previously been reported that pre-operative histological subtype
and grade determined through endometrial biopsy-derived samples are
independent predictors for lymph node metastasis in EC (35,36).
However, the predictive efficacy of these two basic histological
parameters is not robust enough and remains to be improved.
Of the miRNAs studied that are associated with EC,
miR-205 is one of those that is consistently overexpressed. The
upregulation of miR-205 was previously reported to be significantly
correlated with advanced-stage disease, the occurrence of relapse
and poor survival rates in EC (37,38,30). It
was revealed that the reduced expression of miR-205 was useful in
predicting lymph node involvement in triple-negative breast cancer
patients (39), but this result was
based on the final pathological reports, which could only be
obtained when surgery had been performed.
As pre-operative endometrial biopsy-based detection
is a minimally invasive and cost-effective approach, and in view of
the stability of miRNAs in FFPE tissues, miR-205 expression was
examined in the curettage and hysterectomy specimens of patients
with EC in the present study. A good concordance was found
concerning the expression of miR-205 between pre-operative and
post-operative detection. In line with previous studies, it was
demonstrated that the overexpression of miR-205 in the curettage
and excised uterus specimens was inversely correlated with
disease-specific survival in EC. Moreover, in the current pilot
study, the predictive value of miR-205 was first investigated by
detecting its expression in curettage specimens to identify EC
patients at high risk for lymph node metastasis, thus trying to
allocate only patients who could potentially benefit from
lymphadenectomy to undergo this surgical procedure. The results
revealed that lymph node metastasis could be identified by the
overexpression of miR-205 for all EC patients, with a sensitivity
of 71.4%, a specificity of 80.2%, a positive likelihood ratio of
3.55, a negative likelihood ratio of 0.36 and an accuracy of 79.4%.
Based on a combination of pre-operative histological
characteristics and miR-205 expression, the predictive ability of
lymph node metastasis could be enhanced to a sensitivity of 80.4%,
a specificity of 80.3%, a positive likelihood ratio of 4.00, a
negative likelihood ratio of 0.25 and an accuracy of 80.3%.
In particular, the present study focused on the
patients with clinical stage I disease, where the role of systemic
lymph node excision is not yet well defined. It was found that for
clinical stage I disease, lymph node metastasis could be predicted
by the overexpression of miR-205, with a sensitivity of 72.5%, a
specificity of 84.2%, a positive likelihood ratio of 4.56, a
negative likelihood ratio of 0.32 and an accuracy of 83.0%. When
combining pre-operative histological characteristics with miR-205
expression, the predictive value of lymph node metastases could be
improved to a sensitivity of 82.7%, a specificity of 83.8%, a
positive likelihood ratio of 5.19, a negative likelihood ratio of
0.20 and an accuracy of 83.7%. These findings demonstrated that
overexpressed miR-205 in curettage specimens could provide
additional and useful information for reinforcing risk
stratification prior to surgery. On the other hand, the enhanced
sensitivity and positive likelihood ratio are beneficial for
identifying patients at high risk for lymph node metastasis.
There are also certain limitations to the present
study. Although the probability of isolated para-aortic nodal
involvement in patients without pelvic nodal metastasis was only
~1% (40), the fact that only a small
proportion of the patients were subjected to pelvic and para-aortic
node excision may have affected the results. Furthermore, other
factors that can identify high-risk patients, such as myometrial
invasion, were not studied, as pre-operative magnetic resonance
imaging (MRI) data were available for only a limited number of
patients due to the relatively high cost. In fact, pre-operative
MRI also has its limitations for the judgment of myometrial
invasion, in other words, interobserver variability or
inconsistency. Additionally, the present study was limited as it
was unicentric. However, we consider that the findings obtained are
promising for further studies.
In summary, the present results suggested again that
the overexpression of miR-205 was inversely correlated with patient
survival. More importantly, the present study has demonstrated, for
the first time, that the increased expression of miR-205 in
curettage specimens could predict lymph node metastasis with high
accuracy in EC, particularly in clinical stage I disease. Moreover,
when combined with conventional histological parameters,
overexpressed miR-205 could provide additional and useful
information, and therefore improve the risk assessment prior to
surgical treatment. Further studies with multicenter large cohorts
are required to fully validate the potential of miR-205 as a
biomarker for the prediction of lymph node metastasis in EC
patients.
Acknowledgements
This study was supported by Ningxia Natural Science
Foundation (grant no. NZ14129) and was also partly funded by the
National Natural Science Foundation of China (grant no. 81372808),
the Science and Technology Development Planning Project of Jinan
(grant no. 201303035) and the Technology Development Planning
Project of Shandong (grant nos. 2012G0021823 and 2011GSF12122).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong YF, Cheung TH, Lo KW, Yim SF, Siu NS,
Chan SC, Ho TW, Wong KW, Yu MY, Wang VW, et al: Identification of
molecular markers and signaling pathway in endometrial cancer in
Hong KongChinese women by genome-wide gene expression profiling.
Oncogene. 26:1971–1982. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Liu Z, Yu X, Zhang X, Lü S, Chen
X and Lü B: The association between metabolic abnormality and
endometrial cancer: A large case-control study inChina. Gynecol
Oncol. 117:41–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morrow CP, Bundy BN, Kurman RJ, Creasman
WT, Heller P, Homesley HD and Graham JE: Relationship between
surgical-pathological risk factors and outcome in clinical stage I
and II carcinoma of the endometrium: A Gynecologic oncology Group
study. Gynecol Oncol. 40:55–65. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abeler VM and Kjørstad KE: Endometrial
adenocarcinoma in Norway. A study of a total population. Cancer.
67:3093–3103. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim HS and Song YS: International
Federation of Gynecology and Obstetrics (FIGO) staging system
revised: What should be considered critically for gynecologic
cancer? J Gynecol Oncol. 20:135–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
ASTEC study group, ; Kitchener H, Swart
AM, Qian Q, Amos C and Parmar MK: Efficacy of systematic pelvic
lymphadenectomy in endometrial cancer (MRC ASTEC trial): A
randomized study. Lancet. 373:125–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Panici P Benedetti, Basile S, Maneschi F,
Lissoni A Alberto, Signorelli M, Scambia G, Angioli R, Tateo S,
Mangili G, et al: Systematic pelvic lymphadenectomy vs. No
lymphadenectomy in early-stage endometrial carcinoma: Randomized
clinical trial. J Natl Cancer Lnst. 100:1707–1716. 2008. View Article : Google Scholar
|
|
9
|
Chan JK, Cheung MK, Huh WK, Osann K,
Husain A, Teng NN and Kapp DS: Therapeutic role of lymph node
resection in endometrioid corpus cancer: A study of 12,333
patients. Cancer. 107:1823–1830. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trovik J, Wik E, Stefansson IM,
Marcickiewicz J, Tingulstad S, Staff AC and Njolstad TS: MoMaTec
Study Group, Vandenput I, Amant F, et al: Stathmin overexpression
identifies high-risk patients and lymph node metastasis in
endometrial cancer. Clin Cancer Res. 17:3368–3377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng G, Wang X, Cao X, Shen L and Zhu J:
ZEB1 expression in endometrial biopsy predicts lymph node
metastases in patient with endometrial cancer. Dis Markers.
2014:6803612014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang T, Huang L and Zhang S: Preoperative
serum CA125: A useful marker for surgical management of endometrial
cancer. BMC Cancer. 15:3962015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bansal N, Yendluri V and Wenham RM: The
molecular biology of endometrial cancers and the implications for
pathogenesis, classification, and targeted therapies. Cancer
Control. 16:8–13. 2009.PubMed/NCBI
|
|
14
|
Zhang B, Wang Q and Pan X: MicroRNAs and
their regulatory roles inanimals and plants. J Cell Physiol.
210:279–289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao G, Cai C, Yang T, Qiu X, Liao B, Li
W, Ji Z, Zhao J, Zhao H, Guo M, et al: MicroRNA-221 induces cell
survival and cisplatin resistance through PI3K/Akt pathway in human
osteosarcoma. PLoS One. 8:e539062013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Go H, Jang JY, Kim PJ, Kim YG, Nam SJ,
Paik JH, Kim TM, Heo DS, Kim CW and Jeon YK: MicroRNA-21 plays an
oncogenic role by targeting FOXO1 and activating the PI3K/AKT
pathway in diffuse large B-cell lymphoma. Oncotarget.
6:15035–15049. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, VandenBoom TG II, Kong D, Wang Z,
Ali S, Philip PA and Sarkar FH: Up-regulation of miR-200 and let-7
by natural agents leads to the reversal of
epithelial-to-mesenchymal transition in gemcitabine-resistant
pancreatic cancer cells. Cancer Res. 69:6704–6712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng Y, Liu YM, Li LC, Wang LL and Wu XL:
microRNA-503 inhibits gastric cancer cell growth and
epithelial-to-mesenchymal transition. Oncol Lett. 7:1233–1238.
2014.PubMed/NCBI
|
|
19
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee TS, Jeon HW, Kim YB, Kim YA, Kim MA
and Kang SB: Aberrant microRNA expression in endometrial carcinoma
using formalin-fixed paraffin-embedded (FFPE) tissues. PLoS One.
8:e814212013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qu C, Liang Z, Huang J, Zhao R, Su C, Wang
S, Wang X, Zhang R, Lee MH and Yang H: miR-205 determines the
radioresistance of human nasopharyngeal carcinoma by directly
targeting PTEN. Cell Cycle. 11:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gandellini P, Folini M, Longoni N, Pennati
M, Binda M, Colecchia M, Salvioni R, Supino R, Moretti R, Limonta
P, et al: miR-205 Exerts tumor-suppressive functions in human
prostate through down-regulation of protein kinase Cepsilon. Cancer
Res. 69:2287–2295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boren T, Xiong Y, Hakam A, Wenham R, Apte
S, Wei Z, Kamath S, Chen DT, Dressman H and Lancaster JM: MicroRNAs
and their target messenger RNAs associated with endometrial
carcinogenesis. Gynecol Oncol. 110:206–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hiroki E, Akahira J, Suzuki F, Nagase S,
Ito K, Suzuki T, Sasano H and Yaegashi N: Changes in microRNA
expression levels correlate with clinicopathological features and
prognoses in endometrial serous adenocarcinomas. Cancer Sci.
101:241–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Campbell K, Nuss RC and Benrubi GI: An
evaluation of the clinical staging of endometrial cancer. J Reprod
Med. 33:8–10. 1988.PubMed/NCBI
|
|
28
|
Creasman WT, Morrow CP, Bundy BN, Homesley
HD, Graham JE and Heller PB: Surgical pathologic spread patterns of
endometrial cancer. A gynecologic oncology group study. Cancer.
60:(8 Suppl). 2035–2041. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karaayvaz M, Zhang C, Liang S, Shroyer KR
and Ju J: Prognostic significance of miR-205 in endometrial cancer.
PLoS One. 7:e351582012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Todo Y, Kato H, Kaneuchi M, Watari H,
Takeda M and Sakuragi N: Survival effect of para-aortic
lymphadenectomy in endometrial cancer (SEPAL study): A
retrospective cohort analysis. Lancet. 375:1165–1172. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mitamura T, Watari H, Todo Y, Kato T,
Konno Y, Hosaka M and Sakuragi N: Lymphadenectomy can be omitted
for low-risk endometrial cancer based on preoperative assessments.
J Gynecol Oncol. 25:301–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trovik J, Wik E, Werner HM, Krakstad C,
Helland H, Vandenput I, Njolstad TS, Stefansson IM, Marcickiewicz
J, Tingulstad S, et al: Hormone receptor loss in endometrial
carcinoma curettage predicts lymph node metastasis and poor outcome
in prospective multicentre trial. Eur J Cancer. 49:3431–3441. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dinkelspiel HE, Wright JD, Lewin SN and
Herzog TJ: Contemporary clinical management of endometrial cancer.
Obstet Gynecol Int. 2013:5838912013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Todo Y, Sakuragi N, Nishida R, Yamada T,
Ebina Y, Yamamoto R and Fujimoto S: Combined use of magnetic
resonance imaging, CA 125 assay, histologic type, and histologic
grade in the prediction of lymph node metastasis in endometrial
carcinoma. Am J Obstet Gynecol. 188:1265–1272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Todo Y, Okamoto K, Hayashi M, Minobe S,
Nomura E, Hareyama H, Takeda M, Ebina Y, Watari H and Sakuragi N: A
validation study of a scoring system to estimate the risk of lymph
node metastasis for patients with endometrial cancer for tailoring
the indication of lymphadenectomy. Gynecol Oncol. 104:623–628.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chung TK, Cheung TH, Huen NY, Wong KW, Lo
KW, Yim SF, Siu NS, Wong YM, Tsang PT, Pang MW, et al: Dysregulated
microRNAs and their predicted targets associated with endometrioid
endometrial adenocarcinoma in Hong Kong women. Int J Cancer.
124:1358–1365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Torres A, Torres K, Pesci A, Ceccaroni M,
Paszkowski T, Cassandrini P, Zamboni G and Maciejewski R:
Diagnostic and prognostic significance of miRNA signatures in
tissues and plasma of endometrioid endometrial carcinoma patients.
Int J Cancer. 132:1633–1645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Berber U, Yilmaz I, Narli G, Haholu A,
Kucukodaci Z and Demirel D: miR-205 and miR-200c: Predictive micro
RNAs for lymph node metastasis in triple negative breast cancer. J
Breast Cancer. 17:143–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abu-Rustum NR, Gomez JD, Alektiar KM,
Soslow RA, Hensley ML, Leitao MM Jr, Gardner GJ, Sonoda Y, Chi DS
and Barakat RR: The incidence of isolated paraaortic nodal
metastasis in surgically staged endometrial cancer patients with
negative pelvic lymph nodes. Gynecol Oncol. 115:236–238. 2009.
View Article : Google Scholar : PubMed/NCBI
|