Introduction
Non-small cell lung cancer (NSCLC) accounts for
75–80% of all primary lung cancers. A cisplatin-based chemotherapy
regimen has been the main solution for treating advanced NSCLC
(1–4).
Traditionally the first-line chemotherapy regimen includes
cisplatin combined with another third-generation drug, such as
docetaxel, paclitaxel or gemcitabine (5–9). For the
second-line treatment, pemetrexed, docetaxel and target drugs are
constantly used (10–14). The association between histology and a
first-line regimen had not been studied until recently, with
certain studies indicating that overall survival (OS) correlates
with histological subtype. For example, a trial containing 1,725
patients showed that pemetrexed is suitable to be used for tumors
with non-squamous histology, as an improved OS time was recorded
compared with patients with squamous histology, who experienced a
shorter survival time (15–17). Therefore, establishing a treatment
model to mimic the first-line second-line treatment is important,
as it can be used to confirm the known clinical trial information,
to analyze the mechanism and to test other drugs in the future. The
inhibitory effects of docetaxel and pemetrexed on cell
proliferation have been demonstrated (18,19). The
present study compared the inhibitory effect of the
docetaxel-pemetrexed (Doc-Pem) group with that of the
pemetrexed-docetaxel (Pem-Doc) group. Due to certain factors,
including the drug resistance of cisplatin during treatment, the
remission rate is limited to 40–50%. Excision repair
cross-completion gene 1 (ERCC1) is one of the drug resistance
genes, and it plays an important role in DNA repair (20). Overexpression of ERCC1 can cause drug
resistance to cisplatin, and lowering the expression of ERCC1 in
A549 cells can enhance the sensitivity to cisplatin (21,22). The
current study also detected if the combination plan can lower the
expression of ERCC1.
Materials and methods
Materials
The materials used in the present study were as
follows: Docetaxel (Shanghai Yuanye Bio-Technology Co., Ltd.,
Shanghai, China); pemetrexed (National Institutes for Food and Drug
Control, Beijing, China); cisplatin (National Institutes for Food
and Drug Control, Beijing, China); the A549 cell line (JRDUN
Biotechnology, Shanghai, China); Hyclone RPMI 1640 media (GE
Healthcare; Logan, UT, USA); fetal bovine serum (FBS; MRC, Jintan,
Jiangsu, China); 0.25% EDTA-trypsin (Beijing Leagene Biotechnology
Co., Ltd., Beijing, China); CellTiter 96 Aqueous One Solution Cell
Proliferation assay (Promega Corporation, Madison, WI, USA); TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA); RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.); Real Time PCR Easy-SYBR Green I (Foregene Co.,
Ltd., Chengdu, Sichuan, China); primers (Shanghai Generay Biotech
Co., Ltd., Shanghai, China); polyvinylidene difluoride (PVDF)
membrane (EMD Millipore, Billerica, MA, USA); BCIP/NBT alkaline
phosphatase color development kit (Beijing Huamaike Biotechnology,
Beijing, China); and anti-ERCC1 (human) antibody, anti-β-actin
(human) antibody and alkaline phosphatase-conjugated Affinipure
Goat Anti-Rabbit IgG (H+L) antibody (Proteintech Group Inc.,
Rosemont, IL, USA).
Cell culture
A549 cells were cultured in RPMI 1640 medium
containing 10% FBS, and kept in a 37°C incubator maintaining
humidified air with 5% CO2. When the cells reached
80–90% confluence, passaging was performed using 0.25%
EDTA-trypsin.
Cell proliferation assay (CPA) to
determine the half maximal inhibitory concentration
(IC50)
The cells were seeded in 96-well plates at a density
of 2×104/ml (100 µl per well), and were kept in a 37°C
incubator maintaining humidified air with 5% CO2.
Subsequent to 24 h, drugs at different concentrations were added
(docetaxel, 0.2, 0.3 and 0.4 mg/ml; pemetrexed, 0.4, 0.8 and 1.6
mg/ml; cisplatin, 0.265, 0.53 and 1.06 µg/ml), with no addition to
the blank group (3 wells per drug). Following incubation in a 37°C
incubator for 72 h, 20 µl CPA solution was added. Subsequent to 2
h, 25 µl 10% SDS was added to stop the reaction. The optical
density (OD) values were determined using a microplate reader
(detection wave length, 492 nm; reference wave length, 630 nm;
Multiskan MK3; Thermo Fisher Scientific, Inc.). The inhibition
ratio was calculated as follows: Inhibition ratio (100%) = (1 -
ODexperimental / ODblank) × 100. Graphs were
created showing the log-concentration vs. inhibition ratio, and the
IC50 values were calculated.
CPA for combination with cisplatin in
different sequential therapy timings
The cells were cultured as aforementioned and four
groups were then created (3 wells per drug): 0.2 mg/ml docetaxel
plus 0.62 µg/ml cisplatin was added to form the Doc-Pem group; 1.42
mg/ml pemetrexed plus 0.62 µg/ml cisplatin was added to form the
Pem-Doc group; 0.62 µg/ml cisplatin only was added to form the DDP
group; and no addition was made to form the blank group. All
cultures were maintained in an incubator, and subsequent to 24 h
(or 48 h, 1st drug duration as shown in Table I) 1.42 mg/ml pemetrexed was added to
the Doc-Pem group, and 0.2 mg/ml docetaxel was added to the Pem-Doc
group. The medium was replaced for the DDP group and blank group,
the incubation was continued for 24 h (or 48 h, 2nd drug duration
as shown in Table I), and the OD and
inhibition ratio calculated as aforementioned.
 | Table I.Duration of drug treatment in 4
methods. |
Table I.
Duration of drug treatment in 4
methods.
| Method | 1st drug regime and
duration | 2nd drug regime and
duration | Change to fresh
media |
|---|
| 1 | Doc + DDP for 24
h | Pem for 24 h |
|
|
| Pem + DDP for 24
h | Doc for 24 h |
|
|
| DDP for 24 h | Blank for 24 h |
|
|
| Blank for 24 h | Blank for 24 h |
|
| 2 | Doc + DDP for 24
h | Pem for 24 h | 24 h |
|
| Pem + DDP for 24
h | Doc for 24 h | 24 h |
|
|
| DDP for 24 h | Blank for 24 h | 24 h |
|
| Blank for 24 h | Blank for 24 h | 24 h |
| 3 | Doc + DDP for 48
h | Pem for 24 h |
|
|
| Pem + DDP for 48
h | Doc for 24 h |
|
|
| DDP for 48 h | Blank for 24 h |
|
|
| Blank for 48 h | Blank for 24 h |
|
| 4 | Doc + DDP for 24
h | Pem for 48 h |
|
|
| Pem + DDP for 24
h | Doc for 48 h |
|
|
| DDP for 24 h | Blank for 48 h |
|
|
| Blank for 24 h | Blank for 48 h |
|
Quantitative polymerase chain reaction
(qPCR)
Cells of each group were collected and the total RNA
was extracted using a TRIzol kit; the small amount of remaining
genomic DNA was removed using DNase I (Beijing Huamaike
Biotechnology, Beijing, China). The RNA quality was confirmed by
Nanodrop spectrophotometry. Reverse transcription was performed as
described in the manufacturer's instructions. Briefly, total RNA
was mized with Random Hexamer primer (1 µl), made up to a total
volume of 12 µl using nuclease-free water, and then 5X reaction
buffer (4 µl), Ribolock RNase Inhibitor (1 µl), 10 mM dNTP mix (2
µl) and RevertAid M-MuLV RT (1 µl) were added. This was mixed
gently and spun briefly, then incubated for 5 min at 25°C, followed
by 60 min at 42°C. The reaction was terminated by heating at 70°C
for 5 min. The reverse transcription reaction products were stored
at −80°C. qPCR was performed using the StepOne system normalized to
a reference gene by the comparative Cq method
(2−ΔΔCq) (23). The PCR
was conducted using the Real Time PCR Easy SYBR Green kit (Foregene
Co., Ltd.). The cycling conditions were 94°C for 3 min, followed by
40 cycles at 94°C for 10 sec, 65°C for 10 sec and 72°C for 30 sec,
the signal of amplified ERCC1 or β-actin was collected at the end
of each cycle. The PCR mixture containing no template was used as a
negative control. This qPCR experiment was repeated twice. The
primers were as follows: ERCC1 forward, 5′-CTTGTCTTCTGGCTCGAAGG-3′
and reverse, 5′-ACTCAGGAGGCAGTGAATGG-3′; β-actin forward,
5′-CATCGTCCACCGCAAATGCTTC-3′ and reverse,
5′-AACCGACTGCTGTCACCTTCAC-3′.
Western blot analysis
Cells of each group were collected (cells from five
10-cm dishes per group), and 1/4 volume of 5X loading buffer was
added. The cells were subjected to 98°C denaturation for 5 min,
prior to separation by 10% SDS-PAGE. The proteins were transferred
to a PVDF membrane by the semi-dry method, and were blocked for 30
min by 5% skimmed milk. The primary antibodies anti-ERCC1
(Proteintech Group Inc.; catalog no. 14586-1-AP; dilution, 1:300)
and anti-β-actin (Proteintech Group Inc.; catalog no, 20536-1-AP;
dilution, 1:500) were added, and the sheets were incubated at 4°C
overnight, prior to being washed by Tris-buffered saline with
Tween-20 (TBST) 4 times, for 5 min each. The ALP-conjugated
Affinipure Goat Anti-Rabbit IgG(H+L) secondary antibody
(Proteintech Group Inc.; catalog no. SA00002-2; dilution, 1/500)
was then added and incubated at room temperature for 1 h, prior to
being washed by TBST 4 times, for 5 min each. The color was
developed with a BCIP/NBT kit. The bands were analyzed by ImageJ
software (National Institutes of Health, Bethesda, MD, USA) and the
ERCC1/β-actin ratios were calculated using ImageJ software
(National Institutes of Health, Bethesda, MD, USA) to measure the
grayscale value of each band.
Statistical analysis
All analyses were performed with the Statistical
Package for the Social Sciences version 22 (IMB SPSS, Armonk, NY,
USA). One-wayanalysis of variance and the least significant
difference test were used to evaluate the differences between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Inhibitory effect on cell
proliferation is stronger in the Pem-Doc group
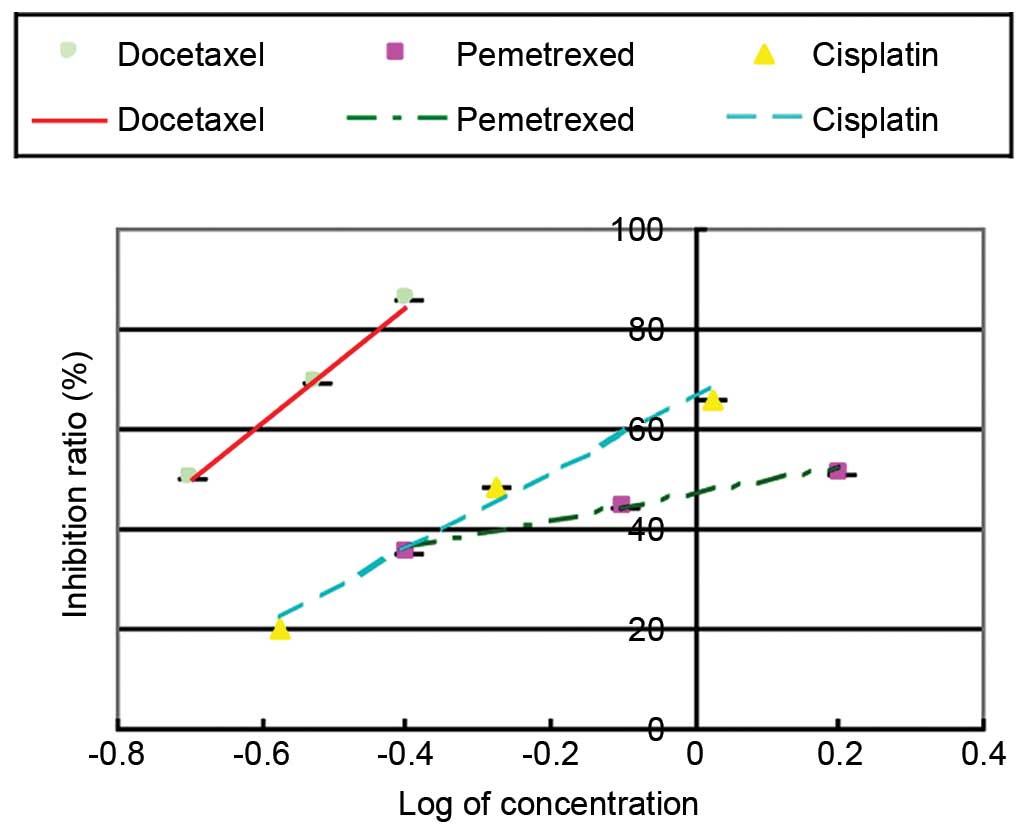
The CPA (Fig. 1)
showed that all 3 drugs, including docetaxel, pemetrexed and
cisplatin, could inhibit the proliferation of the cells; this
inhibitory effect became stronger with increased drug
concentration. The IC50 of docetaxel was 0.2 mg/ml, the
IC50 of pemetrexed was 1.42 mg/ml and the
IC50 of cisplatin was 0.62 µg/ml. To test the effect of
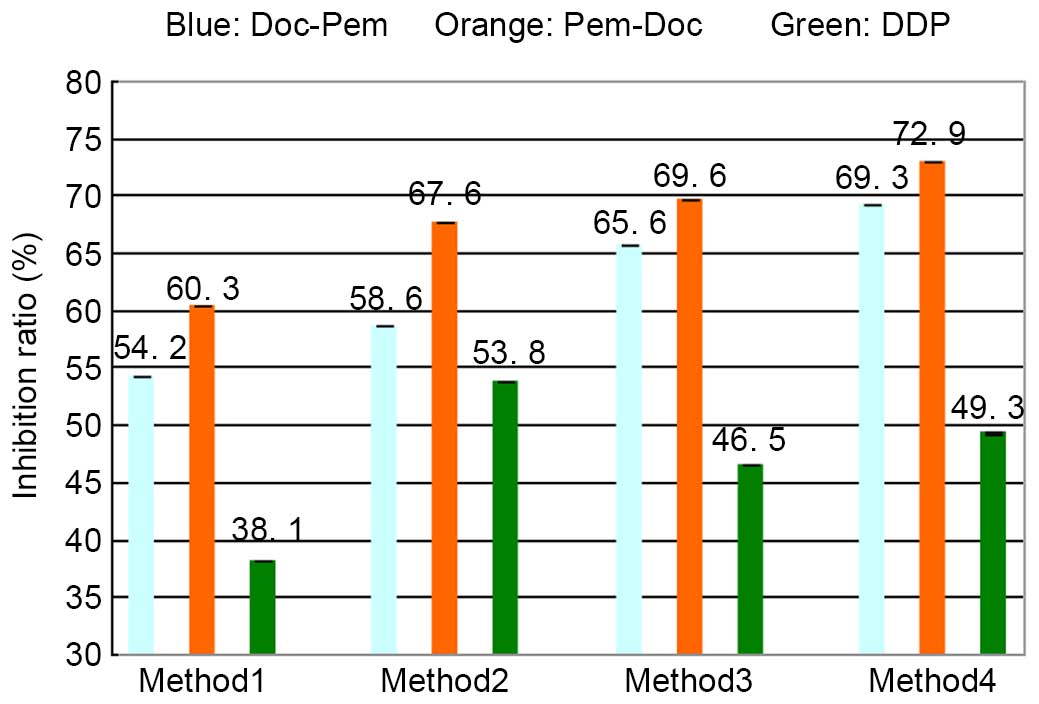
sequential combination, four methods were tested (Table I). The results (Fig. 2) show that in method 1, the inhibition
ratio of the Pem-Doc group (60.3%) and that of the Doc-Pem group
(54.2%) was increased compared with that of the DDP group (38.1%)
(both P<0.001). Additionally, the Pem-Doc group was
significantly increased compared with the Doc-Pem group (P=0.020).
In methods 2, 3 and 4, the inhibition ratio of the Pem-Doc group
and the Doc-Pem group was increased compared with that of the DDP
group (P<0.001, P=0.002, P<0.001, P<0.001, P<0.001 and
P<0.001, respectively), and there were significant differences
between the Pem-Doc group and the Doc-Pem group (P<0.001,
P=0.040 and P=0.040, respectively). This indicated that the
inhibitory effect on cell proliferation in the Pem-Doc group was
stronger compared with that of the Doc-Pem group; furthermore, this
tendency was not affected by the different treatment duration of
the drugs.
ERCC1 gene expression in the Pem-Doc
group is lower than that in the Doc-Pem group
To investigate the association between the
inhibitory effects of two regimes on the cell proliferation and
drug resistance of cisplatin, ERCC1, a drug resistance gene, was
studied by qPCR, with β-actin as the internal control. qPCR
(Fig. 3) showed that the expression
of the ERCC1 gene in the DDP group was ~1.9 times that of the blank
group (without the addition of drugs). The expression in the
Doc-Pem group was slightly decreased compared with that of the
blank group, while the expression in the Pem-Doc group was 0.066
times that of the blank group. This indicates that the Pem-Doc plan
may increase the inhibitory effect by inhibiting the expression of
ERCC1.
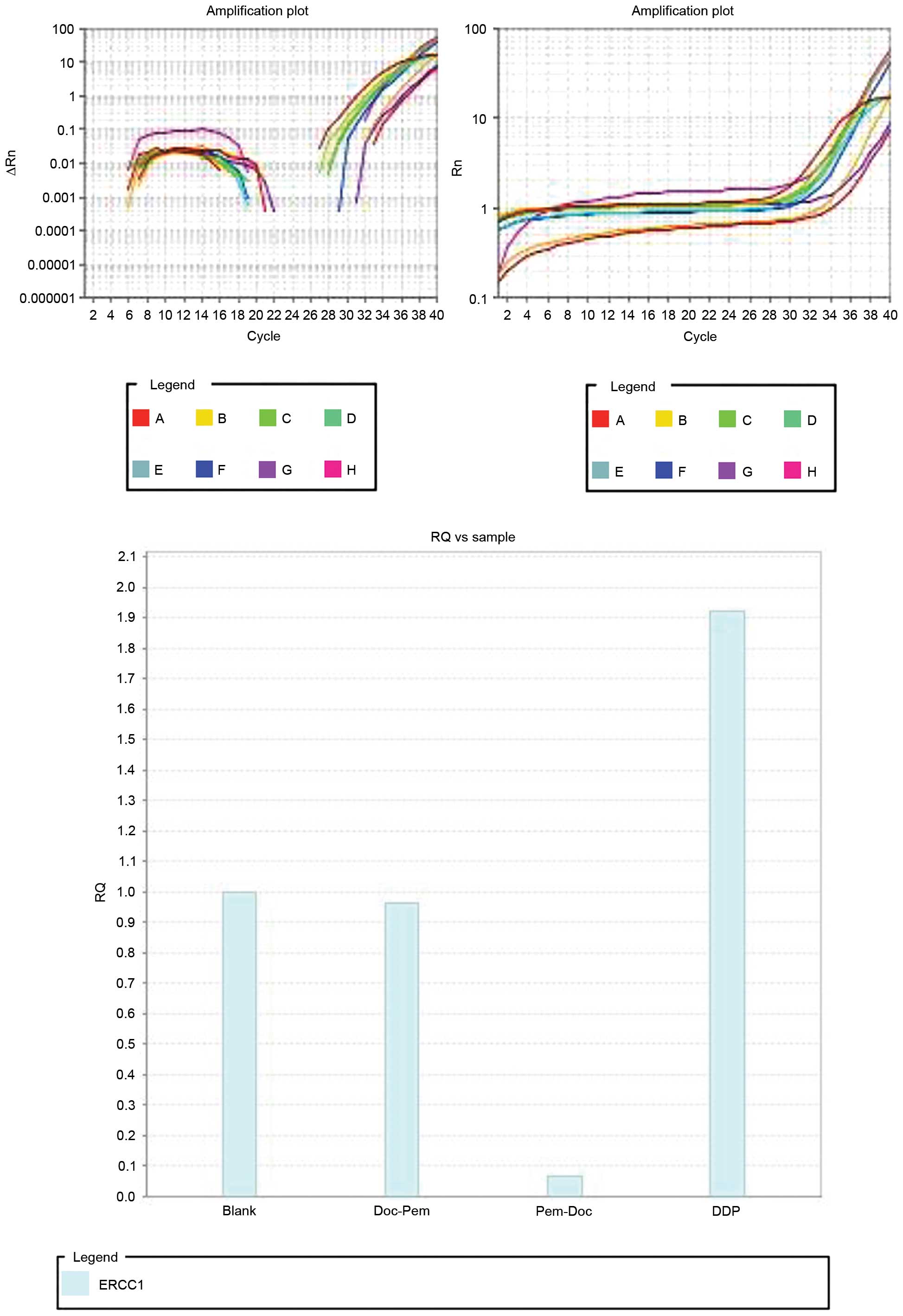 | Figure 3.Expression of ERCC1 by quantitative
polymerase chain reaction. The internal control was β-actin. A to H
represent the samples in row A (numbers 1 to 12) to row H (numbers
1 to 12), respectively (A1, ERCC1 primers, no template; B1, actin
primers, no template; C1, ERCC1 primers, template from blank group;
D1, actin primers, template from blank group; E1, ERCC1 primers,
template from Doc-Pem group; F1, actin primers, template from
Doc-Pem group; G1, ERCC1 primers, template from Pem-Doc group; H1,
actin primers, template from Pem-Doc group; A2, ERCC1 primers,
template from DDP group; B2, actin primers, template from DDP
group. Blank, without addition of drugs in the 1st and 2nd stages;
RQ, relative quantification; Rn, normalized reporter (fluorescence
of the reporter dye divided by the fluorescence of a passive
reference dye (ROX)]; ∆Rn, Rn minus the baseline. |
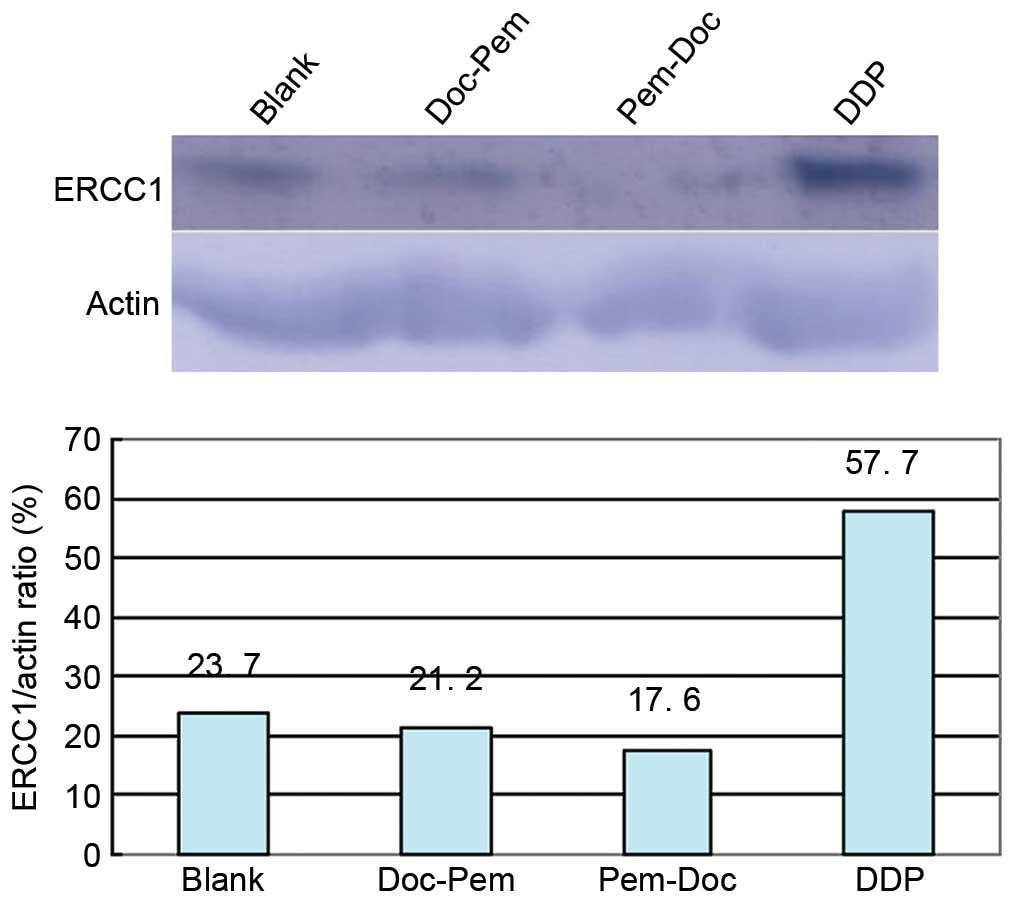
Pem-Doc group inhibits the expression
of the ERCC1 protein more efficiently
To obtain further information on ERCC1 expression,
cells from 4 groups underwent western blotting. The result
(Fig. 4) show that the DDP group
(0.62 µg/ml) markedly promoted the expression of the ERCC1 protein;
the ERCC1/β-actin ratio was 57.7%, which was 2.4 times that of the
blank group. The values of the combination groups were lower than
that of blank group; the value in the Doc-Pem group was 0.89 times
that of the blank group, while it was 0.74 times that of the blank
group in the Pem-Doc group. This indicates that the Pem-Doc plan
may have a better inhibitory effect through inhibiting the
expression of the ERCC1 protein.
Discussion
Docetaxel promotes microtubule polymerization and
inhibits depolymerization, which results in the death of cancer
cells. The combination of docetaxel with cisplatin is constantly
used in the clinic, and patients with non-small cell lung cancer
can benefit from the prolongation of survival (7,10,14). Pemetrexed is a novel drug that can
inhibit the metabolism of folic acid by inhibiting the activity of
required enzymes, including thymidylate, dihydrofolate reductase
and phosphoribosyl glycinamide transtormylase (17). This process results in abnormalities
in folic acid metabolism and nucleotide synthesis, which leads to
the death of cancer cells. In a large trial containing 571 patients
(12), results showed that pemetrexed
had the same effect as docetaxel in treating patients with advanced
stage NSCLC. Additionally, fewer side effects were observed and
pemetrexed may therefore be a useful drug for second-line
treatment. In 2004, pemetrexed was approved for use as a
second-line treatment. In previous years, it was reported that the
combination of pemetrexed with cisplatin was well tolerated and was
the approved standard first-line therapy (24,25).
Although pemetrexed is an ineffective drug for squamous carcinomas,
it has been found to be effective in non-squamous NSCLC (15–17).
Adenocarcinoma is a non-squamous subtype that accounts for ~50% of
lung cancer cases. The inhibitory effect of chemical drugs on cell
proliferation have been researched widely (21,22), and
the present study was designed to establish an adenocarcinoma
treatment model, which includes the combination of cisplatin with
docetaxel or pemetrexed in vitro in different sequential
therapy timings, so that the effect and mechanism could be studied.
The present model can be used to test other drugs, and to compare
the effect against the Pem-Doc group, which acts as a control.
The present results showed that the inhibitory
effect of the combination groups (Doc-Pem and Pem-Doc groups) was
stronger than that of the DDP group. Among the combination groups,
the effect in the Pem-Doc group was stronger compared with that in
the Doc-Pem group. Furthermore, the tendency did not change with
different treatment durations (24→24 h; 48→24 h; 24→48 h). The drug
resistance of cisplatin is an important affecting factor in
chemotherapy, qPCR showed that the expression of the ERCC1 gene in
the Pem-Doc group was inhibited compared with that of the Doc-Pem
group, and the same pattern was identified for the protein level by
western blot analysis. This indicated that Pem-Doc plan may
increase the inhibitory effect more efficiently by inhibiting the
expression of ERCC1, thus lowering the drug resistance of
cisplatin.
Acknowledgements
The present study was supported a grant from
Shenyang Medical College for the Returned Overseas Chinese Scholars
of Lining Wang (no. 20144062).
References
|
1
|
Takita H, Edgerton F, Marabella P, Conway
D and Harguindey S: Platinum-based combination chemotherapy in
non-small cell lung carcinoma. Cancer. 48:1528–1530. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Belani CP: Docetaxel in combination with
platinums in patients with advanced non-small-cell lung cancer.
Oncology (Williston Park). 11(8): Suppl 8. S42–S45. 1997.
|
|
3
|
Stinchcombe TE, Borghaei H, Barker SS,
Treat JA and Obasaju C: Pemetrexed with platinum combination as a
backbone for targeted therapy in non-small-cell lung cancer. Clin
Lung Cancer. 17:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Farsi A and Ellis PM: Treatment
paradigms for patients with metastatic non-small cell lung cancer,
squamous lung cancer: First, second, and third-line. Front Oncol.
4:1572014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gatzemeier U, von Pawel J, Gottfried M,
ten Velde GP, Mattson K, de Marinis F, Harper P, Salvati F, Robinet
G, Lucenti A, et al: Phase III comparative study of high-dose
cisplatin versus a combination of paclitaxel and cisplatin in
patients with advanced non-small cell lung cancer. J Clin Oncol.
18:3390–3399. 2000.PubMed/NCBI
|
|
6
|
Scagliotti GV, De Marinis F, Rinaldi M,
Crinò L, Gridelli C, Ricci S, Matano E, Boni C, Marangolo M, Failla
G, et al: Phase III randomized trial comparing three platinum-based
doublets in advanced non-small-cell lung cancer. J Clin Oncol.
20:4285–4291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smit EF, van Meerbeeck JP, Lianes P,
Debruyne C, Legrand C, Schramel F, Smit H, Gaafar R, Biesma B,
Manegold C, et al: Three-arm randomized study of two
cisplatin-based regimens and paclitaxel plus gemcitabine in
advanced non-small-cell lung cancer: A phase III trial of the
European organisation for research and treatment of cancer lung
cancer group-EORTC 08975. J Clin Oncol. 21:3909–3917. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fossella F, Pereira JR, von Pawel J,
Pluzanska A, Gorbounova V, Kaukel E, Mattson KV, Ramlau R, Szczesna
A, Fidias P, et al: Randomized, multinational, phase III study of
docetaxel plus platinum combination versus vinorelbine plus
cisplatin for advanced non-small-cell lung cancer: The TAX 326
study group. J Clin Oncol. 21:3016–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shepherd FA, Dancey J, Ramlau R, Mattson
K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R,
et al: Prospective randomized trial of docetaxel versus best
supportive care in patients with non-small-cell lung cancer
previously treated with platinum-based chemotherapy. J Clin Oncol.
18:2095–2103. 2000.PubMed/NCBI
|
|
11
|
Fossella FV, DeVore R, Kerr RN, Crawford
J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, et al:
Randomized phase III trial of docetaxel versus vinorelbine or
ifosfamide in patients with advanced non-small-cell lung cancer
previously treated with platinum-containing chemotherapy regimens.
The TAX 320 Non-Small cell lung cancer study group. J Clin Oncol.
18:2354–2362. 2000.PubMed/NCBI
|
|
12
|
Hanna N, Shepherd FA, Fossella FV, Pereira
JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M,
Muller T, et al: Randomized phase 111 trial of pemetrexed versus
doeetaxel in patients with non-small-cell lung cancer previously
treated with chemotherapy. J Clin Onco1. 22:1589–1597. 2004.
View Article : Google Scholar
|
|
13
|
Shepherd FA, Pereira J Rodrigues, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: Erlotinib in previously treated
non-small-cell lung cancer. N Engl J Med. 353:123–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Di Maio M, Perrone F, Chiodini P, Gallo C,
Camps C, Schuette W, Quoix E, Tsai CM and Gridelli C: Individual
patient data meta-analysis of docetaxel administered once every3
weeks compared with once every week second-line treatment of
advanced non-small-cell lung cancer. J Clin Oncol. 25:1377–1382.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peterson P, Park K, Fossella F, Gatzemeier
U, John W and Scagliotti GV: Is pemetrexed more effective in
adenocarcinoma and large cell lung cancer than in squamous cell
carcinoma? A retrospective analysis of a phase III trial of
pemetrexed vs docetaxel in previously treated patients with
advanced non-small cell lung cancer (NSCLC). J Thorac Oncol.
2:(Suppl 4). s8512007. View Article : Google Scholar
|
|
16
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scagliotti G, Hanna N, Fossella F,
Sugarman K, Blatter J, Peterson P, Simms L and Shepherd FA: The
differential efficacy of pemetrexed according to NSCLC histology: A
review of two phase III studies. Oncologist. 14:253–263. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang QC, Jiang SJ, Zhang S and Ma XB:
Histone deacetylase inhibitor trichostatin A enhances antitumor
effects of docetaxel or erlotinib in A549 cell line. Asian Pac J
Cancer Prev. 13:3471–3476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu DM, Zhang P, Xu GC, Tong AP, Zhou C,
Lang JY and Wang CT: Pemetrexed induces G1 phase arrest and
apoptosis through inhibiting Akt activation in human non small lung
cancer cell line A549. Asian Pac J Cancer Prev. 16:1507–1513. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsai MS, Weng SH, Chen HJ, Chiu YF, Huang
YC, Tseng SC, Kuo YH and Lin YW: Inhibition of p38 MAPK-dependent
excision repair cross-complementing 1 expression decreases the DNA
repair capacity to sensitize lung cancer cells to etoposide. Mol
Cancer Ther. 11:561–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu YP, Ling Y, Qi QF, Zhang YP, Zhang CS,
Zhu CT, Wang MH and Pan YD: The effects of ERCC1 expression levels
on the chemosensitivity of gastric cancer cells to platinum agents
and survival in gastric cancer patients treated with
oxaliplatin-based adjuvant chemotherapy. Oncol Lett. 5:935–942.
2013.PubMed/NCBI
|
|
22
|
Cai Y, Yan X, Zhang G, Zhao W and Jiao S:
The predictive value of ERCC1 and p53 for the effect of
panobinostat and cisplatin combination treatment in NSCLC.
Oncotarget. 6:18997–19005. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fleeman N, Bagust A, McLeod C, Greenhalgh
J, Boland A, Dundar Y, Dickson R, Smith C Tudur, Davis H, Green J
and Pearson M: Pemetrexed for the first-line treatment of locally
advanced or metastatic non-small cell lung cancer. Health Technol
Assess. 14:(Suppl 1). S47–S53. 2010. View Article : Google Scholar
|
|
25
|
Pereira JR, Cheng R, Orlando M, Kim JH and
Barraclough H: Elderly subset analysis of randomized phase III
study comparing pemetrexed plus carboplatin with docetaxel plus
carboplatin as first-line treatment for patients with locally
advanced or metastatic non-small cell lung cancer. Drugs R D.
13:289–296. 2013. View Article : Google Scholar : PubMed/NCBI
|