|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Atlas of cancer mortality in the European
union and the European Economic Area: 1993–1997. IARC Sci Publ.
1–259. 2008.
|
|
3
|
Besse B, Adjei A, Baas P, Meldgaard P,
Nicolson M, Paz-Ares L, Reck M, Smit EF, Syrigos K, Stahel R, et
al: 2nd ESMO consensus conference on lung cancer: Non-small-cell
lung cancer first-line/second and further lines of treatment in
advanced disease. Ann Oncol. 25:1475–1484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
NCCN: National Comprehensive Cancer
Network (NCCN) Clinical Practice Guidelines in Oncology, .
Non-Small Cell Lung Cancer. Version I. 2015.https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
|
|
5
|
National Cancer Institute, .
PDQ® Non-Small Cell Lung Cancer Treatment. Bethesda, MD:
National Cancer Institute; http://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdqAccessed
Nov 26, 2014.
|
|
6
|
Azzoli CG, Baker S Jr, Temin S, Pao W,
Aliff T, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, et
al: American society of clinical oncology clinical practice
guideline update on chemotherapy for stage IV non-small-cell lung
cancer. J Clin Oncol. 27:6251–6266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmid-Bindert G: Update on antiangiogenic
treatment of advanced non-small cell lung cancer (NSCLC). Target
Oncol. 8:15–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel JD, Hensing TA, Rademaker A, Hart
EM, Blum MG, Milton DT and Bonomi PD: Phase II study of pemetrexed
and carboplatin plus bevacizumab with maintenance pemetrexed and
bevacizumab as first-line therapy for nonsquamous non-small-cell
lung cancer. J Clin Oncol. 27:3284–3289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stevenson JP, Langer CJ, Somer RA, Evans
TL, Rajagopalan K, Krieger K, Jacobs-Small M, Dyanick N, Milcarek
B, Coakley S, et al: Phase 2 trial of maintenance bevacizumab alone
after bevacizumab plus pemetrexed and carboplatin in advanced,
nonsquamous nonsmall cell lung cancer. Cancer. 118:5580–5587. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spigel DR, Hainsworth JD, Shipley DL,
Ervin TJ, Kohler PC, Lubiner ET, Peyton JD, Waterhouse DM, Burris
HA III and Greco FA: A randomized phase II trial of
pemetrexed/gemcitabine/bevacizumab or
pemetrexed/carboplatin/bevacizumab in the first-line treatment of
elderly patients with advanced non-small cell lung cancer. J Thorac
Oncol. 7:196–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patel JD, Socinski MA, Garon EB, Reynolds
CH, Spigel DR, Olsen MR, Hermann RC, Jotte RM, Beck T, Richards DA,
et al: PointBreak: A randomized phase III study of pemetrexed plus
carboplatin and bevacizumab followed by maintenance pemetrexed and
bevacizumab versus paclitaxel plus carboplatin and bevacizumab
followed by maintenance bevacizumab in patients with stage IIIB or
IV nonsquamous non-small-cell lung cancer. J Clin Oncol.
31:4349–4357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokoi T, Torii Y, Katashiba Y, Sugimoto H,
Tanijiri T, Ogata M, Inagaki N, Kibata K, Hayashi M, Niki M, et al:
Phase II study of pemetrexed and carboplatin plus bevacizumab,
followed by maintenance pemetrexed and bevacizumab in Japanese
patients with non-squamous non-small cell lung cancer. Oncol Lett.
8:2453–2457. 2014.PubMed/NCBI
|
|
15
|
Malhotra B, Evans T, Weiss J, Eaby B,
Stonehouse-Lee S, Sherry V and Langer CJ:
Carboplatin/pemetrexed/bevacizumab in the treatment of patients
with advanced non-small-cell lung cancer: A single-institution
experience. Clin Lung Cancer. 11:192–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura H, Satoh H, Kaburagi T, Nishimura
Y, Shinohara Y, Inagaki M, Endo T, Saito T, Hayashihara K, Hizawa
N, et al: Bevacizumab-containing chemotherapy for non-small cell
lung cancer patients: A population-based observational study by the
Ibaraki thoracic integrative (POSITIVE) research group. Med Oncol.
29:3202–3206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
NCCN: National Comprehensive Cancer
Network (NCCN) Clinical Practice Guidelines in Oncology, . Central
Nervous System Cancers. Version 2. 2014.https://www.nccn.org/professionals/physician_gls/PDF/cns.pdf
|
|
18
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
NIH, . Common Terminology Criteria for
Adverse Events (CTCAE) Version 4.0. simpleevs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
|
|
20
|
Compton CC, Byrd DR, Garcia-Aguilar J,
Kurtzman SH, Olawaiye A and Washington MK: AJCC Cancer Staging
Atlas: A Companion to the Seventh Editions of the AJCC Cancer
Staging Manual and HandbookAJCC Cancer Staging Atlas: A Companion
to the Seventh Editions of the AJCC Cancer Staging Manual and
Handbook. 2nd. Springer; New York: 2012, View Article : Google Scholar
|
|
21
|
Parkin DM and Hakulinen T: Cancer
registration: Principles and methods. Analysis of survival. IARC
Sci Publ. 159–176. 1991.PubMed/NCBI
|
|
22
|
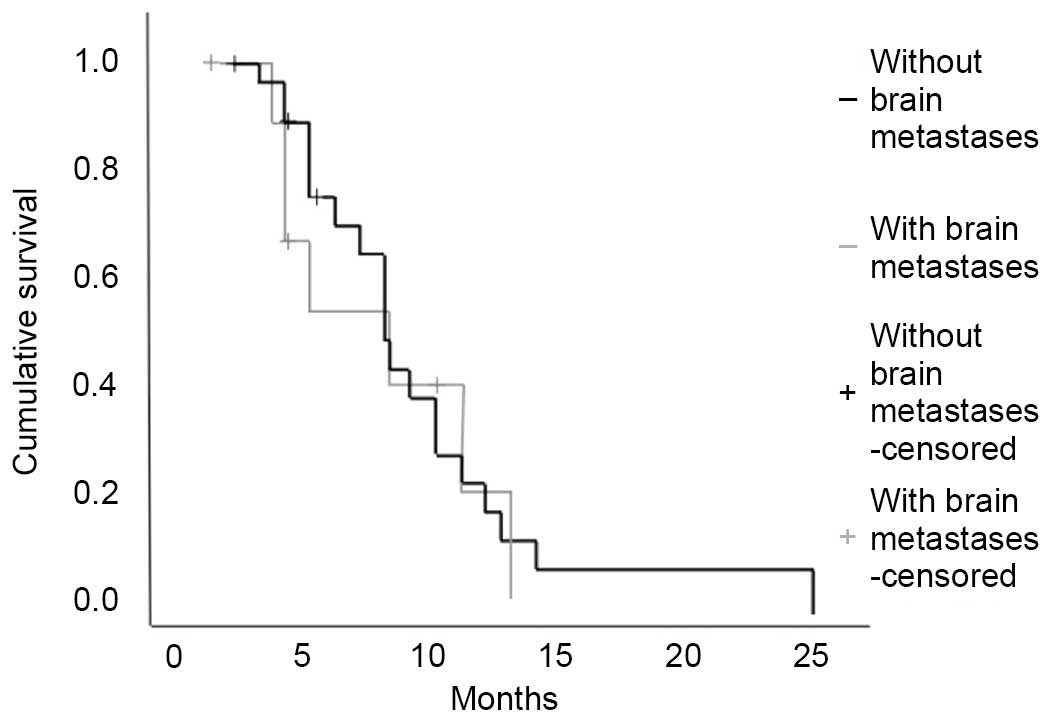
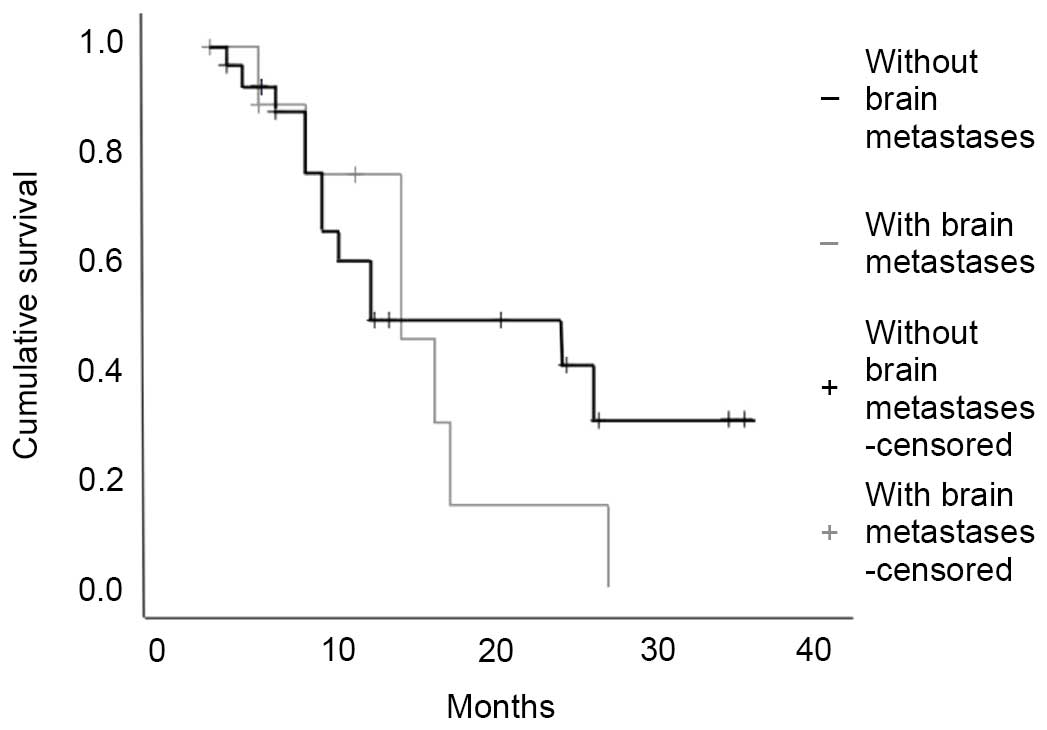
Ali A, Goffin JR, Arnold A and Ellis PM:
Survival of patients with non-small-cell lung cancer after a
diagnosis of brain metastases. Curr Oncol. 20:e300–e306. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davis FG, Dolecek TA, McCarthy BJ and
Villano JL: Toward determining the lifetime occurrence of
metastatic brain tumors estimated from 2007 United States cancer
incidence data. Neuro Oncol. 14:1171–1177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patchell RA, Tibbs PA, Walsh JW, Dempsey
RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS and Young
B: A randomized trial of surgery in the treatment of single
metastases to the brain. N Engl J Med. 322:494–500. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Louie AV, Rodrigues G, Yaremko B, Yu E,
Dar AR, Dingle B, Vincent M, Sanatani M, Younus J, Malthaner R and
Inculet R: Management and prognosis in synchronous solitary
resected brain metastasis from non-small-cell lung cancer. Clin
Lung Cancer. 10:174–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schuette W: Treatment of brain metastases
from lung cancer: Chemotherapy. Lung Cancer. 45:(Suppl 2).
S253–S257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zimmermann S, Dziadziuszko R and Peters S:
Indications and limitations of chemotherapy and targeted agents in
non-small cell lung cancer brain metastases. Cancer Treat Rev.
40:716–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
D'Antonio C, Passaro A, Gori B, Del
Signore E, Migliorino MR, Ricciardi S, Fulvi A and de Marinis F:
Bone and brain metastasis in lung cancer: Recent advances in
therapeutic strategies. Ther Adv Med Oncol. 6:101–114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bailon O, Chouahnia K, Augier A, Bouillet
T, Billot S, Coman I, Ursu R, Belin C, Zelek L, Des Guetz G, et al:
Upfront association of carboplatin plus pemetrexed in patients with
brain metastases of lung adenocarcinoma. Neuro Oncol. 14:491–495.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barlesi F, Gervais R, Lena H, Hureaux J,
Berard H, Paillotin D, Bota S, Monnet I, Chajara A and Robinet G:
Pemetrexed and cisplatin as first-line chemotherapy for advanced
non-small-cell lung cancer (NSCLC) with asymptomatic inoperable
brain metastases: A multicenter phase II trial (GFPC 07–01). Ann
Oncol. 22:2466–2470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bearz A, Garassino I, Tiseo M, Caffo O,
Soto-Parra H, Boccalon M, Talamini R, Santoro A, Bartolotti M,
Murgia V, et al: Activity of pemetrexed on brain metastases from
non-small cell lung cancer. Lung Cancer. 68:264–268. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bernardo G, Cuzzoni Q, Strada MR, Bernardo
A, Brunetti G, Jedrychowska I, Pozzi U and Palumbo R: First-line
chemotherapy with vinorelbine, gemcitabine, and carboplatin in the
treatment of brain metastases from non-small-cell lung cancer: A
phase II study. Cancer Invest. 20:293–302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Edelman MJ, Belani CP, Socinski MA, Ansari
RH, Obasaju CK, Chen R, Monberg MJ and Treat J: Alpha Oncology
Research Network: Outcomes associated with brain metastases in a
three-arm phase III trial of gemcitabine-containing regimens versus
paclitaxel plus carboplatin for advanced non-small cell lung
cancer. J Thorac Oncol. 5:110–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Besse B, Lasserre SF, Compton P, Huang J,
Augustus S and Rohr UP: Bevacizumab safety in patients with central
nervous system metastases. Clin Cancer Res. 16:269–278. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gordon MS, Margolin K, Talpaz M, Sledge GW
Jr, Holmgren E, Benjamin R, Stalter S, Shak S and Adelman D: Phase
I safety and pharmacokinetic study of recombinant human
anti-vascular endothelial growth factor in patients with advanced
cancer. J Clin Oncol. 19:843–850. 2001.PubMed/NCBI
|
|
36
|
Morgensztern D and Govindan R: Treatment
of patients excluded from Eastern Cooperative Oncology Group 4599
and AVAiL studies: Focus on brain metastasis and squamous
histology. Clin Lung Cancer. 9:(Suppl 2). S57–S61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oh Y and Stewart DJ: Systemic therapy for
lung cancer brain metastases: A rationale for clinical trials.
Oncology (Williston Park). 22:168–178; discussion 178, 183, 188
passim. 2008.PubMed/NCBI
|
|
38
|
Socinski MA, Langer CJ, Huang JE, Kolb MM,
Compton P, Wang L and Akerley W: Safety of bevacizumab in patients
with non-small-cell lung cancer and brain metastases. J Clin Oncol.
27:5255–5261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lynch TJ Jr, Spigel DR, Brahmer J,
Fischbach N, Garst J, Jahanzeb M, Kumar P, Vidaver RM, Wozniak AJ,
Fish S, et al: Safety and effectiveness of bevacizumab-containing
treatment for non-small-cell lung cancer: Final results of the
ARIES observational cohort study. J Thorac Oncol. 9:1332–1339.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Crinò L, Dansin E, Garrido P, Griesinger
F, Laskin J, Pavlakis N, Stroiakovski D, Thatcher N, Tsai CM, Wu YL
and Zhou C: Safety and efficacy of first-line bevacizumab-based
therapy in advanced non-squamous non-small-cell lung cancer (SAiL,
MO19390): A phase 4 study. Lancet Oncol. 11:733–740. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Reck M, von Pawel J, Zatloukal P, Ramlau
R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N and
Manegold C: Phase III trial of cisplatin plus gemcitabine with
either placebo or bevacizumab as first-line therapy for nonsquamous
non-small-cell lung cancer: AVAil. J Clin Oncol. 27:1227–1234.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
European Medicines Agency, . Committee for
Medicinal Products for Human Use Post-Authorisation Summary of
Positive Opinion for Avastin. Doc. Ref. No. EMEA/CHMP/121120/2009.
http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/000582/WC500059420.pdf
|
|
43
|
European Medicines Agency, . Assessment
Report for Avastin. Doc. Ref. No. EMEA/274499/2009. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000582/WC500029270.pdf
|