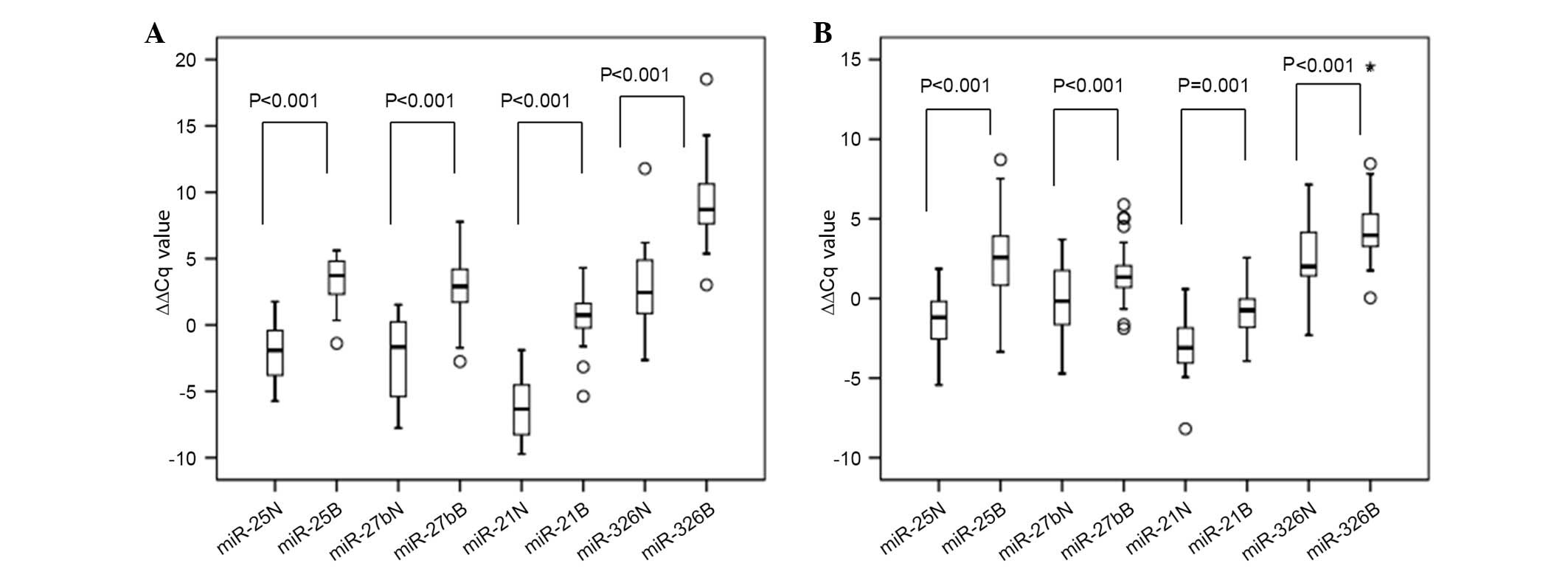
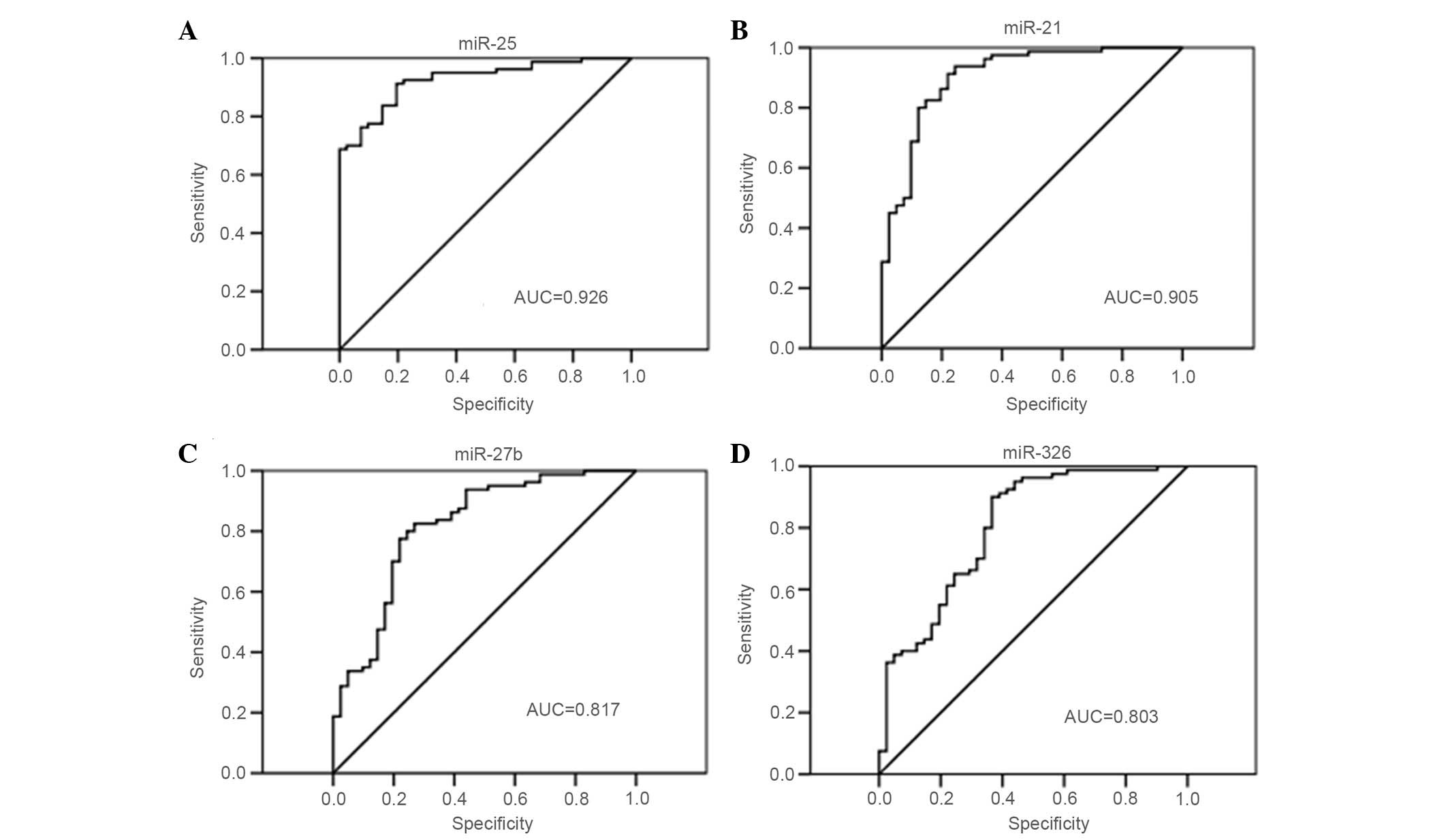
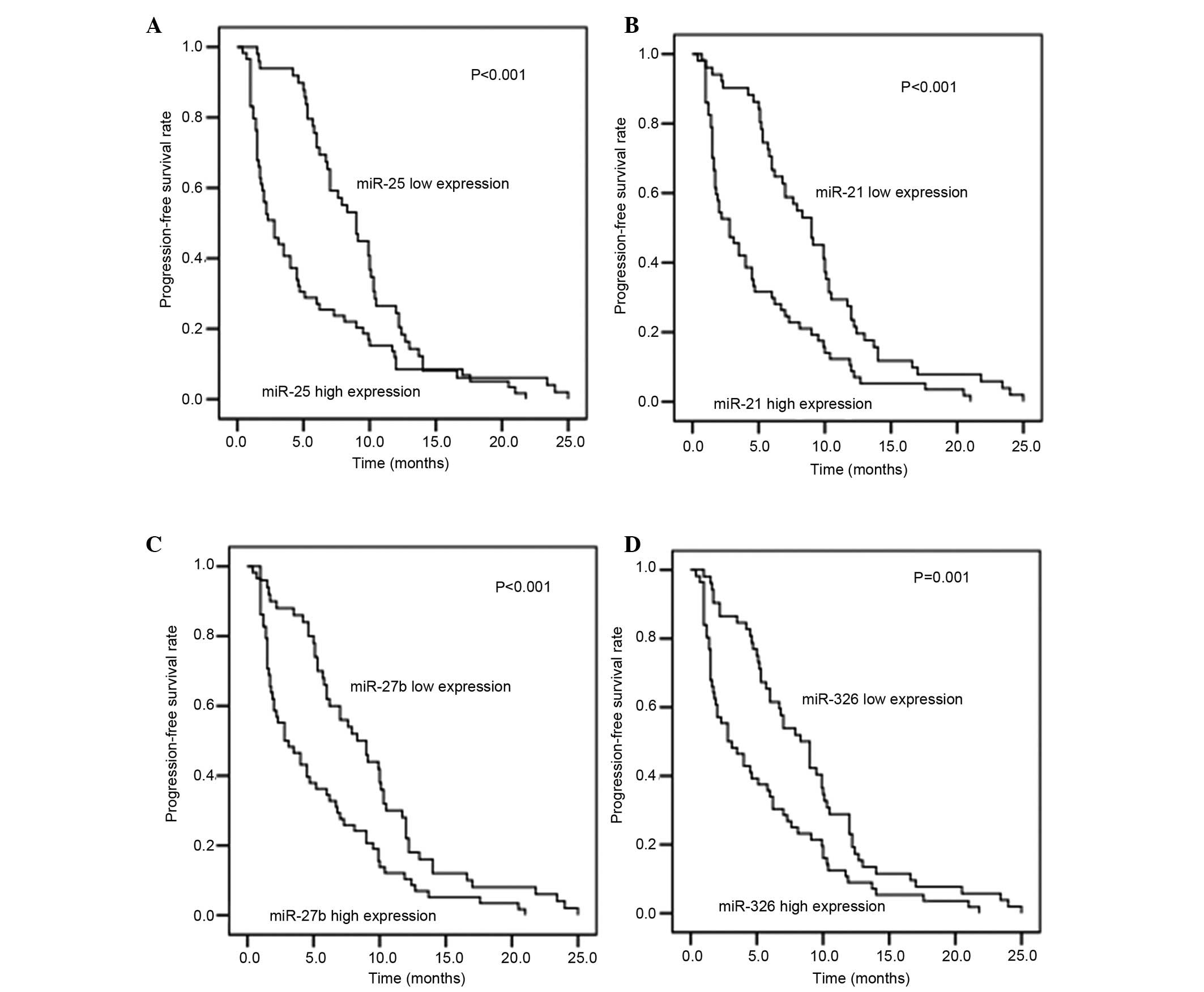
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JK, Hahn S, Kim DW, Suh KJ, Keam B,
Kim TM, Lee SH and Heo DS: Epidermal growth factor receptor
tyrosine kinase inhibitors vs conventional chemotherapy in
non-small cell lung cancer harboring wild-type epidermal growth
factor receptor: A meta-analysis. JAMA. 311:1430–1437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scagliotti G, Brodowicz T, Shepherd FA,
Zielinski C, Vansteenkiste J, Manegold C, Simms L, Fossella F,
Sugarman K and Belani CP: Treatment-by-histology interaction
analyses in three phase III trials show superiority of pemetrexed
in nonsquamous non-small cell lung cancer. J Thorac Oncol. 6:64–70.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pennell NA: Selection of chemotherapy for
patients with advanced non-small cell lung cancer. Cleve Clin J
Med. 79:Electronic (Suppl 1). eS46–eS50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim YH, Hirabayashi M, Togashi Y, Hirano
K, Tomii K, Masago K, Kaneda T, Yoshimatsu H, Otsuka K, Mio T, et
al: Phase II study of carboplatin and pemetrexed in advanced
non-squamous, non-small-cell lung cancer: Kyoto thoracic oncology
research group trial 0902. Cancer Chemother Pharmacol. 70:271–276.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hartmann JT and Lipp HP: Toxicity of
platinum compounds. Expert Opin Pharmacother. 4:889–901. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gota V, Kavathiya K, Doshi K, Gurjar M,
Damodaran SE, Noronha V, Joshi A and Prabhash K: High plasma
exposure to pemetrexed leads to severe hyponatremia in patients
with advanced non small cell lung cancer receiving
pemetrexed-platinum doublet chemotherapy. Cancer Manag Res.
6:261–265. 2014.PubMed/NCBI
|
|
10
|
Olaussen KA, Dunant A, Fouret P, Brambilla
E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH,
et al: DNA repair by ERCC1 in non-small-cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cobo M, Isla D, Massuti B, Montes A,
Sanchez JM, Provencio M, Viñolas N, Paz-Ares L, Lopez-Vivanco G,
Muñoz MA, et al: Customizing cisplatin based on quantitative
excision repair cross-complementing 1 mRNA expression: A phase III
trial in non-small-cell lung cancer. J Clin Oncol. 25:2747–2754.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boukovinas I, Papadaki C, Mendez P, Taron
M, Mavroudis D, Koutsopoulos A, Sanchez-Ronco M, Sanchez JJ,
Trypaki M, Staphopoulos E, et al: Tumor BRCA1, RRM1 and RRM2 mRNA
expression levels and clinical response to first-line gemcitabine
plus docetaxel in non-small-cell lung cancer patients. PLoS One.
3:e36952008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carser JE, Quinn JE, Michie CO, O'Brien
EJ, McCluggage WG, Maxwell P, Lamers E, Lioe TF, Williams AR,
Kennedy RD, et al: BRCA1 is both a prognostic and predictive
biomarker of response to chemotherapy in sporadic epithelial
ovarian cancer. Gynecol Oncol. 123:492–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nicolson MC, Fennell DA, Ferry D, O'Byrne
K, Shah R, Potter V, Skailes G, Upadhyay S, Taylor P, André V, et
al: Thymidylate synthase expression and outcome of patients
receiving pemetrexed for advanced nonsquamous non-small-cell lung
cancer in a prospective blinded assessment phase II clinical trial.
J Thorac Oncol. 8:930–939. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Friboulet L, Olaussen KA, Pignon JP,
Shepherd FA, Tsao MS, Graziano S, Kratzke R, Douillard JY, Seymour
L, Pirker R, et al: ERCC1 isoform expression and DNA repair in
non-small-cell lung cancer. N Engl J Med. 368:1101–1110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bepler G, Williams C, Schell MJ, Chen W,
Zheng Z, Simon G, Gadgeel S, Zhao X, Schreiber F, Brahmer J, et al:
Randomized international phase III trial of ERCC1 and RRM1
expression-based chemotherapy versus gemcitabine/carboplatin in
advanced non-small-cell lung cancer. J Clin Oncol. 31:2404–2412.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moran T, Wei J, Cobo M, Qian X, Domine M,
Zou Z, Bover I, Wang L, Provencio M, Yu L, et al: Two
biomarker-directed randomized trials in European and Chinese
patients with nonsmall-cell lung cancer: The BRCA1-RAP80 Expression
Customization (BREC) studies. Ann Oncol. 25:2147–2155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kerr KM, Bubendorf L, Edelman MJ,
Marchetti A, Mok T, Novello S, O'Byrne K, Stahel R, Peters S and
Felip E: Panel Members; Panel Members: Second ESMO consensus
conference on lung cancer: Pathology and molecular biomarkers for
non-small-cell lung cancer. Ann Oncol. 25:1681–1690. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Tian X, Han R, Zhang X, Wang X,
Shen H, Xue L, Liu Y, Yan X, Shen J, et al: Downregulation of
miR-486-5p contributes to tumor progression and metastasis by
targeting protumorigenic ARHGAP5 in lung cancer. Oncogene.
33:1181–1189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fuse M, Kojima S, Enokida H, Chiyomaru T,
Yoshino H, Nohata N, Kinoshita T, Sakamoto S, Naya Y, Nakagawa M,
et al: Tumor suppressive microRNAs (miR-222 and miR-31) regulate
molecular pathways based on microRNA expression signature in
prostate cancer. J Hum Genet. 57:691–699. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Selcuklu SD, Donoghue MT and Spillane C:
miR-21 as a key regulator of oncogenic processes. Biochem Soc
Trans. 37:918–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rabinowits G, Gerçel-Taylor C, Day JM,
Taylor DD and Kloecker GH: Exosomal microRNA: A diagnostic marker
for lung cancer. Clin Lung Cancer. 10:42–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Z, Huang D, Ni S, Peng Z, Sheng W
and Du X: Plasma microRNAs are promising novel biomarkers for early
detection of colorectal cancer. Int J Cancer. 127:118–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toiyama Y, Hur K, Tanaka K, Inoue Y,
Kusunoki M, Boland CR and Goel A: Serum miR-200c is a novel
prognostic and metastasis-predictive biomarker in patients with
colorectal cancer. Ann Surg. 259:735–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eichelser C, Flesch-Janys D, Chang-Claude
J, Pantel K and Schwarzenbach H: Deregulated serum concentrations
of circulating cell-free microRNAs miR-17, miR-34a, miR-155, and
miR-373 in human breast cancer development and progression. Clin
Chem. 59:1489–1496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu C, Ren C, Han J, Ding Y, Du J, Dai N,
Dai J, Ma H, Hu Z, Shen H, et al: A five-microRNA panel in plasma
was identified as potential biomarker for early detection of
gastric cancer. Br J Cancer. 110:2291–2299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang C, Wang C, Chen X, Chen S, Zhang Y,
Zhi F, Wang J, Li L, Zhou X, Li N, et al: Identification of seven
serum microRNAs from a genome-wide serum microRNA expression
profile as potential noninvasive biomarkers for malignant
astrocytomas. Int J Cancer. 132:116–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng H, Zhang L, Zhao Y, Yang D, Song F,
Wen Y, Hao Q, Hu Z, Zhang W and Chen K: Plasma miRNAs as diagnostic
and prognostic biomarkers for ovarian cancer. PLoS One.
8:e778532013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nolen BM, Marks JR, Ta'san S, Rand A,
Luong TM, Wang Y, Blackwell K and Lokshin AE: Serum biomarker
profiles and response to neoadjuvant chemotherapy for locally
advanced breast cancer. Breast Cancer Res. 10:R452008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hansen TF, Sørensen FB, Lindebjerg J and
Jakobsen A: The predictive value of microRNA-126 in relation to
first line treatment with capecitabine and oxaliplatin in patients
with metastatic colorectal cancer. BMC Cancer. 12:832012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seidman AD, O'Shaughnessy J and Misset JL:
Single-agent capecitabine: A reference treatment for
taxane-pretreated metastatic breast cancer. Oncologist 7: (Suppl
6). 20–28. 2002. View Article : Google Scholar
|
|
36
|
Cecchin E, Innocenti F, D'Andrea M, Corona
G, De Mattia E, Biason P, Buonadonna A and Toffoli G: Predictive
role of the UGT1A1, UGT1A7, and UGT1A9 genetic variants and their
haplotypes on the outcome of metastatic colorectal cancer patients
treated with fluorouracil, leucovorin, and irinotecan. J Clin
Oncol. 27:2457–2465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th.
Springer-Verlag; New York, NY: 2010
|
|
38
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Churchill GA: Fundamentals of experimental
design for cDNA microarrays. Nat Genet. 32:(Suppl). 490–495. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Youden WJ: Index for rating diagnostic
tests. Cancer. 3:32–35. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ng TK, Carballosa CM, Pelaez D, Wong HK,
Choy KW, Pang CP and Cheung HS: Nicotine alters MicroRNA expression
and hinders human adult stem cell regenerative potential. Stem
Cells Dev. 22:781–790. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu ZL, Wang H, Liu J and Wang ZX:
MicroRNA-21 (miR-21) expression promotes growth, metastasis, and
chemo- or radioresistance in non-small cell lung cancer cells by
targeting PTEN. Mol Cell Biochem. 372:35–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao W, Lu X, Liu L, Xu J, Feng D and Shu
Y: MiRNA-21: A biomarker predictive for platinum-based adjuvant
chemotherapy response in patients with non-small cell lung cancer.
Cancer Biol Ther. 13:330–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu XG, Zhu WY, Huang YY, Ma LN, Zhou SQ,
Wang YK, Zeng F, Zhou JH and Zhang YK: High expression of serum
miR-21 and tumor miR-200c associated with poor prognosis in
patients with lung cancer. Med Oncol. 29:618–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dong Z, Ren L, Lin L and Li J, Huang Y and
Li J: Effect of microRNA-21 on multidrug resistance reversal in
A549/DDP human lung cancer cells. Mol Med Rep. 11:682–690.
2015.PubMed/NCBI
|
|
47
|
Xu L, Huang Y, Chen D, He J, Zhu W, Zhang
Y and Liu X: Downregulation of miR-21 increases cisplatin
sensitivity of non-small-cell lung cancer. Cancer Genet.
207:214–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang H, Zuo Z, Lu X, Wang L, Wang H and
Zhu Z: MiR-25 regulates apoptosis by targeting Bim in human ovarian
cancer. Oncol Rep. 27:594–598. 2012.PubMed/NCBI
|
|
49
|
Li Q, Zou C, Zou C, Han Z, Xiao H, Wei H,
Wang W, Zhang L, Zhang X, Tang Q, et al: MicroRNA-25 functions as a
potential tumor suppressor in colon cancer by targeting Smad7.
Cancer Lett. 335:168–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang T, Chen T, Li Y, Gao L, Zhang S, Wang
T and Chen M: Downregulation of miR-25 modulates non-small cell
lung cancer cells by targeting CDC42. Tumour Biol. 36:1903–1911.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang T, Chen T, Li Y, Gao L, Zhang S, Wang
T and Chen M: Downregulation of miR-25 modulates non-small cell
lung cancer cells by targeting CDC42. Tumour Biol. 36:1903–1911.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu FX, Su YL, Zhang H, Kong JY, Yu H and
Qian BY: Prognostic implications for high expression of MiR-25 in
lung adenocarcinomas of female non-smokers. Asian Pac J Cancer
Prev. 15:1197–1203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rasmussen MH, Jensen NF, Tarpgaard LS,
Qvortrup C, Rømer MU, Stenvang J, Hansen TP, Christensen LL,
Lindebjerg J, Hansen F, et al: High expression of microRNA-625-3p
is associated with poor response to first-line oxaliplatin based
treatment of metastatic colorectal cancer. Mol Oncol. 7:637–646.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Park YT, Jeong JY, Lee MJ, Kim KI, Kim TH,
Kwon YD, Lee C, Kim OJ and An HJ: MicroRNAs overexpressed in
ovarian ALDH1-positive cells are associated with chemoresistance. J
Ovarian Res. 6:182013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bera A, VenkataSubbaRao K, Manoharan MS,
Hill P and Freeman JW: A miRNA signature of chemoresistant
mesenchymal phenotype identifies novel molecular targets associated
with advanced pancreatic cancer. PLoS One. 9:e1063432014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Buffa FM, Camps C, Winchester L, Snell CE,
Gee HE, Sheldon H, Taylor M, Harris AL and Ragoussis J:
microRNA-associated progression pathways and potential therapeutic
targets identified by integrated mRNA and microRNA expression
profiling in breast cancer. Cancer Res. 71:5635–5645. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Goto Y, Kojima S, Nishikawa R, Enokida H,
Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T and Seki
N: The microRNA-23b/27b/24-1 cluster is a disease progression
marker and tumor suppressor in prostate cancer. Oncotarget.
5:7748–7759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jiang J, Lv X, Fan L, Huang G, Zhan Y,
Wang M and Lu H: MicroRNA-27b suppresses growth and invasion of
NSCLC cells by targeting Sp1. Tumour Biol. 35:10019–10023. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shen J, Todd NW, Zhang H, Yu L, Lingxiao
X, Mei Y, Guarnera M, Liao J, Chou A, Lu CL, et al: Plasma
microRNAs as potential biomarkers for non-small-cell lung cancer.
Lab Invest. 91:579–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kefas B, Comeau L, Floyd DH, Seleverstov
O, Godlewski J, Schmittgen T, Jiang J, diPierro CG, Li Y, Chiocca
EA, et al: The neuronal microRNA miR-326 acts in a feedback loop
with notch and has therapeutic potential against brain tumors. J
Neurosci. 29:15161–15168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ferretti E, De Smaele E, Miele E, Laneve
P, Po A, Pelloni M, Paganelli A, Di Marcotullio L, Caffarelli E,
Screpanti I, et al: Concerted microRNA control of Hedgehog
signalling in cerebellar neuronal progenitor and tumour cells. EMBO
J. 27:2616–2627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhou J, Xu T, Yan Y, Qin R, Wang H, Zhang
X, Huang Y, Wang Y, Lu Y, Fu D and Chen J: MicroRNA-326 functions
as a tumor suppressor in glioma by targeting the Nin one binding
protein (NOB1). PLoS One. 8:e684692013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang
K, Wagar N, Yoon Y, Cho HT, Scala S and Shim H: Involvement of
miR-326 in chemotherapy resistance of breast cancer through
modulating expression of multidrug resistance-associated protein 1.
Biochem Pharmacol. 79:817–824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang S, Lu S, Geng S, Ma S, Liang Z and
Jiao B: Expression and clinical significance of microRNA-326 in
human glioma miR-326 expression in glioma. Med Oncol. 30:3732013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang R, Chen XF and Shu YQ: Prediction of
non-small cell lung cancer metastasis-associated microRNAs using
bioinformatics. Am J Cancer Res. 5:32–51. 2014.eCollection 2015.
PubMed/NCBI
|
|
67
|
Valencia K, Martín-Fernández M, Zandueta
C, Ormazábal C, Martínez-Canarias S, Bandrés E, de la Piedra C and
Lecanda F: miR-326 associates with biochemical markers of bone
turnover in lung cancer bone metastasis. Bone. 52:532–539. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kjersem JB, Ikdahl T, Lingjaerde OC, Guren
T, Tveit KM and Kure EH: Plasma microRNAs predicting clinical
outcome in metastatic colorectal cancer patients receiving
first-line oxaliplatin-based treatment. Mol Oncol. 8:59–67. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gomez GG, Wykosky J, Zanca C, Furnari FB
and Cavenee WK: Therapeutic resistance in cancer: microRNA
regulation of EGFR signaling networks. Cancer Biol Med. 10:192–205.
2013.PubMed/NCBI
|
|
70
|
Seike M, Goto A, Okano T, Bowman ED,
Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, Gemma
A, Kudoh S, Croce CM and Harris CC: MiR-21 is an EGFR-regulated
anti-apoptotic factor in lung cancer in never-smokers. Proc Natl
Acad Sci USA. 106:12085–12090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chiyomaru T, Seki N, Inoguchi S, Ishihara
T, Mataki H, Matsushita R, Goto Y, Nishikawa R, Tatarano S, Itesako
T, et al: Dual regulation of receptor tyrosine kinase genes EGFR
and c-Met by the tumor-suppressive microRNA-23b/27b cluster in
bladder cancer. Int J Oncol. 46:487–496. 2015.PubMed/NCBI
|