Introduction
Circulating tumor cells (CTCs) refer to tumor cells
existing in the circulatory system, and they have been increasingly
investigated recently due to the belief of their responsibility for
tumor metastasis (1). Tumor
metastasis is the major cause of mortality in patients with
malignancies (2). Although the
mechanisms of tumor metastasis are largely unknown, CTCs were
considered crucial for tumor metastasis rather than local
advancement (3), and in particular,
certain subgroups of CTCs can initiate metastases in ectopic organs
(4). Several steps were artificially
classified for the purpose of a clear understanding of tumor
metastasis (5,6), that is, invasion into the circulation
system, survival and shift in the circulating system, and
extravasation into ectopic organs. There are a number of hypotheses
regarding each step of tumor metastasis respectively (7), although none of them have been able to
elucidate the whole processes of metastasis.
CellSearch® is now the only certificated
method for CTC detection approved by the US Food and Drug
Administration, and epithelial cell adhesion molecule (EpCAM) is
the most widely used biomarker of CTCs in a variety of methods for
CTC detection. However, the validity of EpCAM as the CTC biomarker
has been challenged by the identification of CTCs lacking
epithelial characteristics (8,9).
Epithelial-mesenchymal transition (EMT) has also been noted in CTCs
(10), indicating the heterogeneity
of the cells. EMT and its reversal process, mesenchymal-epithelial
transition, were considered necessary for tumor metastasis
(11,12). However, the status of post-EMT tumor
cells in the circulatory system is vague and requires further
investigation to elucidate the EMT-based metastatic hypotheses.
Notably, CTCs can be found in the peripheral blood
in the early stages of numerous malignancies (13–15), while
metastasis is considered as concrete evidence of late-stage cancer.
This discrepancy suggests that the formation of macrometastasis led
by CTC is difficult, even though the critical role of CTCs in tumor
metastasis has been widely accepted. On the other hand, persistent
release of tumor cells must exist and a CTC pool in the circulatory
system is progressively established during tumor progression. The
CTC pool implies that the CTCs have a long-term effect on tumor
cell dissemination and an indeterminable boundary for solid tumors,
even those with an intact capsule.
The imperfection of all existing models of tumor
metastasis is the mechanism of CTC extravasation and its inducers
[such as C-X-C motif chemokine ligand 12, C-C motif chemokine
ligand 2, vascular endothelial growth factor and transforming
growth factor-β (TGF-β)]. Since tumor cells undergoing EMT have a
better capacity for invasion and migration, EMT is believed to be a
crucial event during extravasation (16). However, it has not been clearly
elucidated as to whether EMT is indispensable and the inducer of
EMT.
The sites of CTC extravasation are also confusing.
Although certain cancer types have preferable metastatic organs
(17), non-specific organ metastasis
also contributes to considerable cases. The lungs are one of the
organs with a high incidence of metastasis from other locations,
and the majority of metastases locate in the outer regions of the
lungs. A lower bloodstream velocity and a thinner blood vessel
diameter are possible reasons for this. Actually, CTCs are bigger
in size than the majority of blood cells (18), and their interception by vessels can
be observed in vivo (19).
Hypothesis
We hypothesize that i) extravasation of CTC clusters
and single CTCs from vessels induced by interception in relatively
small vessels and ii) extravasation-dependent and -independent
mechanisms are of importance for tumor metastasis.
A CTC cluster-based model
Multicellular CTC clusters have been identified
(18,20), with up to 100 tumor cells found in one
cluster (21). Currently, only a few
studies have reported the successful detection of CTC clusters,
while single CTCs have been universally found in corresponding
studies (20–22). We believe that the amount of CTC
clusters is largely underestimated by current detection methods, as
the majority of these methods use a strategy of surface
protein-targeted antibody-based capture. Since the ratio of the
superficial area to the volume decreases as the volume increases,
the efficiency of CTC capture by antibody-targeting surface
proteins is greatly reduced for CTC clusters compared with a single
CTC. Therefore, the actual amount of CTC clusters in a patient is
underestimated, and the role of CTC clusters in tumor metastasis is
largely unknown. CTC detection methods applying novel principles
may improve the efficacy of CTC cluster capture and shed light on
an in-depth understanding of CTCs in tumor metastasis.
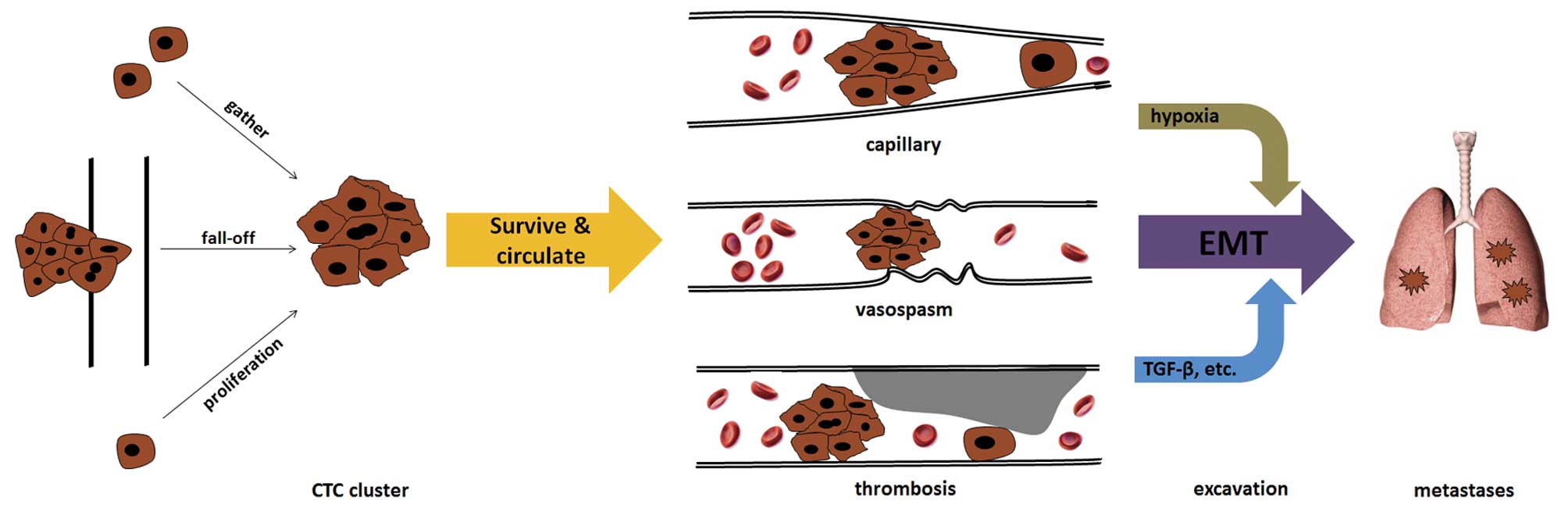
The origin of CTC clusters remains elusive. At
present, there are at least three possible origins (Fig. 1). The first is that CTC clusters
directly break off from the primary tumor as a result of the
shearing force of the blood. Tumor emboli can often be found in
patients with late-stage cancer. Solid tumors tend to invade
vessels to obtain abundant oxygen and nutrients for cell metabolism
and proliferation-associated biosynthesis. Clinical studies have
confirmed that patients with vessel invasion by tumors had an
increased risk of recurrence and a poorer prognosis compared with
those who had tumor-free vessels in a range of cancers, including
breast cancer, hepatocellular carcinoma and papillary thyroid
carcinoma (23–25). In addition, in patients without
pre-operative metastasis, resection of the tumor-invaded vessels
conferred a survival benefit, with a similar long-term prognosis
compared with patients without vessel invasion by tumors (26,27). Thus,
removal of tumor emboli and tumor-invaded vessels can eliminate a
critical origin of CTCs and minimize the possibility and extent of
tumor metastasis, as well as tumor-associated mortality.
The second origin of CTC clusters can be the result
of single CTC proliferation. Although there is a lack of strong
evidence regarding the potential of CTCs to resist immune
clearance, CTCs were previously found to be associated with
impaired immune function in patients (28,29). In
addition, certain single CTCs can survive for a considerable time
in the circulatory system due to their probable potential of
passing through capillaries and proliferating to form CTC clusters.
Thus, for certain single CTCs, this process may continue until a
big enough CTC cluster has been established and intercepted by
vessels with small diameters.
The third origin of CTC clusters is the aggregation
of single CTCs (30). Although it is
difficult to monitor the formation of CTC clusters by single CTCs
in real time in vivo, existing evidence may indirectly
support this idea. For instance, normal cells are programmed to die
if they are detached from neighboring cells and the extracellular
matrix, this is known as anoikis (31). The proportion of non-apoptotic CTCs is
essential to the formation of metastases (13). However, tumor cells resist anoikis
partially by forming cell clusters (32,33).
Notably, characteristics of EMT, including upregulation of vimentin
expression and downregulation of E-cadherin expression, were found
to be associated with anoikis (34,35).
Meanwhile, EMT was also shown to play an important role in CTC
cluster formation (21). These
complicated phenomena suggest that CTC cluster formation may be a
strategy adopted by tumor cells to resist anoikis. On the other
hand, it should also be noted that the existence of CTCs is
significantly associated with vessel thrombosis (36), which leads to embolism or thrombosis,
particularly in microvessels. The formation and special biological
characteristics of CTCs have been proven to be responsible for the
hypercoagulability and subsequent thrombosis of patients (36); however, the unique physical trait of
being cell cluster is also logically associated with a difficulty
in passing through the capillaries. In addition, thrombosis and
thrombolysis in microvessels can frequently occur in patients with
hypercoagulability. Ongoing thrombosis can induce stenosis of
vessel lumina, which tends to intercept CTC clusters. Numerous
changes occur under these conditions. Embolism or complete
thrombosis in microvessels can cause local hypoxia, which has been
proven to induce EMT in various cancers, including hepatocellular
carcinoma, gastric cancer, pancreatic cancer, breast cancer and
colon cancer (37–41). EMT of CTCs may contribute to tumor
cell extravasation from vessels into tissues. This model does not
require CTCs to permanently sustain mesenchymal characteristics in
the circulatory system, and fits well with the fact that the
majority of CTCs detected show epithelial characteristics. In
addition, the marked change in the inflammation-immune-cytokine
local environment induced by platelet aggregation and subsequent
cascades can also upregulate certain EMT-triggering factors, such
as TGF-β (21). These changes provide
an initial impetus for CTCs to extravasate from vessels into
tissues.
CTC clusters may have different characteristics
according to their origins. For example, CTC clusters directly from
the primary tumor may contain evidently more epithelial traits (if
the primary tumor is an epithelial malignancy), while those
aggregated by single CTCs may tend to be mesenchymal. In addition,
heterogeneity may exist within a CTC cluster if it contains a large
number of cells. Little is known about the physical or biochemical
details of these CTC clusters, including the oxygen and nutrient
supply, and the distribution (42).
Single CTC model
To date, the majority of detected CTCs have been
single cells and their existence has been reported to be associated
with tumor metastasis, treatment response and the long-term
prognosis of patients (43–45). However, only certain patients with
malignancies exhibited CTCs in their peripheral blood (46). Even in patients with detectable
metastases, CTCs could only be detected in half of them (47). The threshold of positive and negative
results of CTC detection by successfully detecting one or two CTCs
in a 7.5-ml blood sample is also non-robust. Considering the
discrepancy of detection of single CTCs (early stage) and tumor
metastasis (late stage) regarding the stage of the disease, certain
single CTCs are likely to survive in the circulatory system for a
long time or alternatively undergo dormancy in tissues or organs
(6). Although few CTCs can stay for a
long time in the blood (6), the
survival and proliferation of CTCs in ectopic organs demands
certain necessary prerequisites. Based on the present point of
view, abundant cells are prerequisites of tumor inoculation in
ectopic organs. Recently, it was demonstrated that the CTC clusters
had 23- to 50-fold increased metastatic potential compared with
single CTC cells (48).
Cancer stem cells (CSCs), which have a potent
capacity for tumor initiation, require a certain number of cells to
form tumors subcutaneously in nude mice (49). Although CTCs have a number of the
characteristics of CSCs, a certain number of CTCs is also necessary
for the formation of tumor metastases (50). From this point of view, single CTCs
can barely effectively generate tumor metastases due to a lack of
enough cells. A small number of tumor cells in ectopic organs are
easily removed by the local immune system or die due to
insufficient self-support in the early stage of metastasis
(51). While it is impossible that
hundreds or thousands of single CTCs independently extravasate from
the same anatomical site of vessels and aggregate together in
ectopic organs, the role of CTC clusters, rather than single CTCs,
provides a more logical and rational explanation for the initial
formation of tumor metastasis.
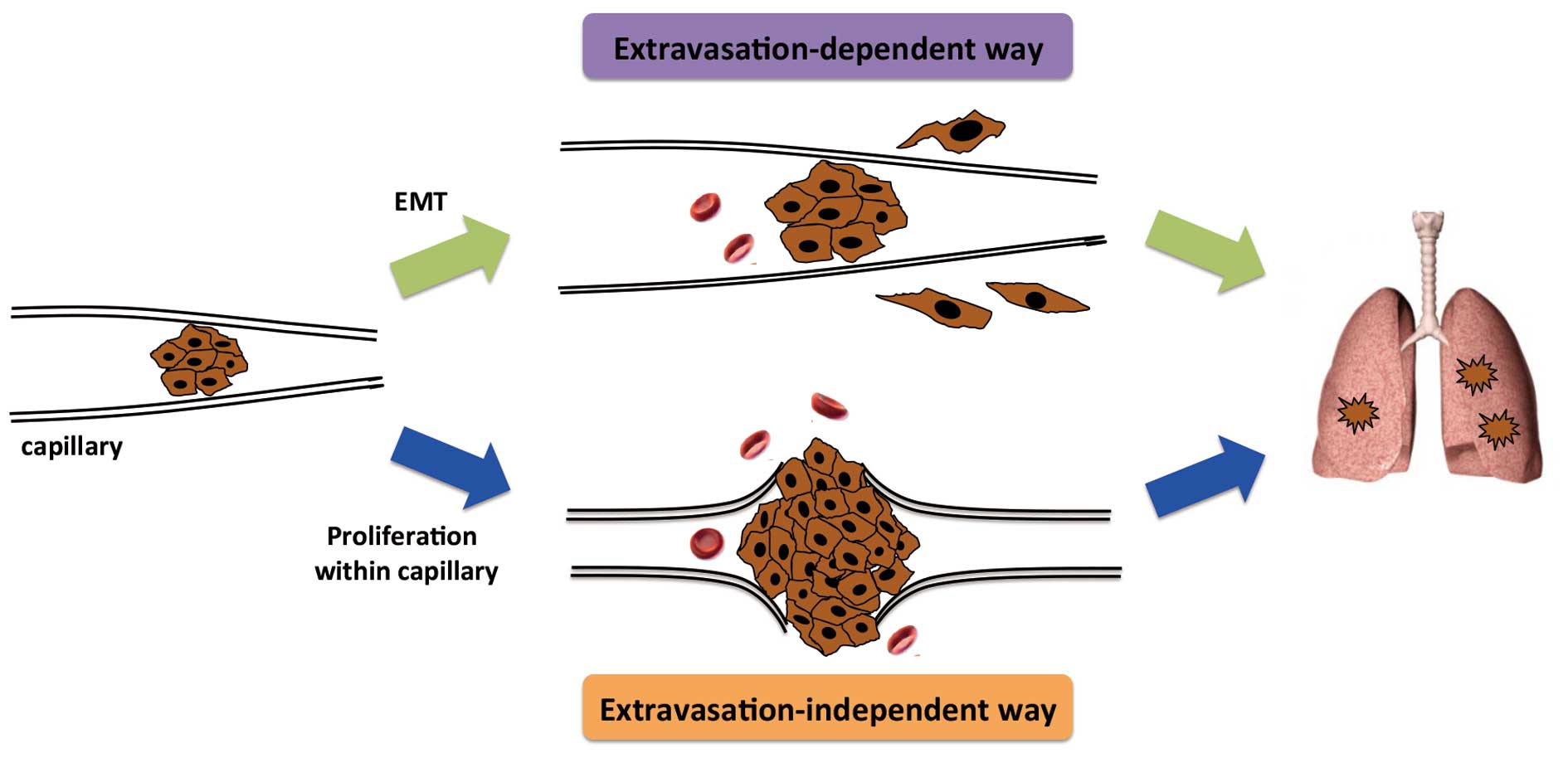
Indispensability of dose
extravasation
Following interception of CTC clusters, the local
microenvironment is relatively stable. Removing the bloodstream and
re-attaching to the vascular endothelial cells makes proliferation
possible. Expansion of CTC clusters due to cancer cell
proliferation can result in the rupture of fragile microvessels,
allowing tumor cells to invade into the tissues (Fig. 2). This extravasation-independent
manner of micrometastasis formation may be more likely to be
associated with the previously mentioned CTC cluster-based
metastasis.
Test of hypothesis
To test our hypothesis, three points are
indispensable, namely, histological evidence of micro-cancer
emboli, extravasation of the cancer cells from the cancer emboli or
rupture of vessels at the site of the cancer emboli, and formation
of metastases from these cancer cells.
Since the majority of distant metastasis involves
the lungs, liver, bone marrow and brain, lung metastasis can be an
ideal model due to convenient sampling and easy observation.
Spontaneous tumor models in animals are required, such as
metastatic melanoma inoculation in immunodeficient mice. Specimens
of lung with hematoxylin and eosin staining, and immunohistological
staining with suitable markers should be checked with great care
and patience at different times post-inoculation. Genetic or
specific protein information can be useful for the identification
of cells in a micro-tumor embolus. Alternatively, caudal vein
injection of tumor cells is another model for CTC study; however,
this model differs from the previously mentioned spontaneous tumor
models in that it cannot mimic intravasation of the tumor cells and
it has a much simpler intercellular cooperation. Furthermore,
single cells or small cell clusters near narrow vessels have to be
found. To accomplish this, cell lines expressing green fluorescent
protein or other fluorescent proteins are of use to increase the
likelihood of identification. Finally, simultaneous evidence of
lung metastases and micro-cancer emboli with an identical cell
origin are required to verify our hypothesis.
Summary and perspectives
Based on current evidence, we hypothesize that CTC
clusters, whatever their origin, are directly responsible for tumor
metastasis, with the stipulation that CTCs can be found in
circulatory system. Novel detection and sorting methods for CTC
clusters are urgently required to manifest the actual role of CTC
clusters in tumor metastasis. Physical techniques based on size,
adhesion and electrophysiological characteristics of CTCs may be
useful in developing the next generation CTC detection methods, and
certain studies have shown promising techniques with significantly
increased efficiency of CTC capture (52–54).
Furthermore, cell cluster filtration and fractal structure capture
systems may improve the efficiency of CTC capture. These strategies
cannot only independently increase yield or/and purity of captured
CTCs, but also improve the efficiency and specificity of CTC
capture synergistically together with novel biomarkers of CTCs.
However, there are numerous questions that require answering, such
as: i) whether CTC clusters grow/decade or are dynamically
self-retained when they moves in the circulatory system; ii)
whether the number, proportion, size or nature of CTC clusters is
associated with tumor metastasis; iii) whether tumor cells within
CTC clusters evolve or undergo EMT; iv) whether there are any
specific characteristics of CTC clusters regarding the interaction
with the immune system; whether there are any differences in tumor
cell extravasation induced by CTC clusters or single CTCs; and vi)
what the time-spectrum is of CTC clusters during primary tumor
progression according to next-generation detection methods;
particularly, whether CTC clusters can be found in the early stages
of disease.
By resolving these questions, a further
understanding of the characteristics of CTC clusters and their role
in tumor metastasis, as well as the integrated mechanism of tumor
metastasis, should be gained.
Clinical implications
The test of these models will provide a novel
perspective for cancer metastasis, and assist in improving
CTC-based cancer diagnostics and monitoring. Novel strategies for
the prevention of cancer metastasis may be also expected once proof
has been obtained for these models.
Acknowledgements
This study was financially supported by the National
Natural Science Foundation of China (grant no. 81401954) and the
Medical Science and Technology Program of Zhejiang Province, China
(grant no. 2015KYA114).
Glossary
Abbreviations
Abbreviations:
|
CTC
|
circulating tumor cell
|
|
EMT
|
epithelial-mesenchymal transition
|
|
EpCAM
|
epithelial cell adhesion molecule
|
|
CSC
|
cancer stem cell
|
References
|
1
|
Plaks V, Koopman CD and Werb Z: Cancer.
Circulating tumor cells. Science. 341:1186–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maheswaran S and Haber DA: Circulating
tumor cells: A window into cancer biology and metastasis. Curr Opin
Genet Dev. 20:96–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baccelli I, Schneeweiss A, Riethdorf S,
Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bäuerle T,
Wallwiener M, et al: Identification of a population of blood
circulating tumor cells from breast cancer patients that initiates
metastasis in a xenograft assay. Nat Biotechnol. 31:539–544. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giancotti FG: Mechanisms governing
metastatic dormancy and reactivation. Cell. 155:750–764. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramis-Conde I, Chaplain MA, Anderson AR
and Drasdo D: Multi-scale modelling of cancer cell intravasation:
The role of cadherins in metastasis. Phys Biol. 6:0160082009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gorges TM, Tinhofer I, Drosch M, Röse L,
Zollner TM, Krahn T and von Ahsen O: Circulating tumour cells
escape from EpCAM-based detection due to epithelial-to-mesenchymal
transition. BMC Cancer. 12:1782012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Königsberg R, Obermayr E, Bises G, Pfeiler
G, Gneist M, Wrba F, de Santis M, Zeillinger R, Hudec M and
Dittrich C: Detection of EpCAM positive and negative circulating
tumor cells in metastatic breast cancer patients. Acta Oncol.
50:700–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barrière G, Tartary M and Rigaud M:
Epithelial mesenchymal transition: A new insight into the detection
of circulating tumor cells. ISRN Oncol. 2012:3820102012.PubMed/NCBI
|
|
11
|
Ocaña OH, Córcoles R, Fabra A, et al:
Metastatic colonization requires the repression of the
epithelial-mesenchymal transition inducer Prrx1. Cancer Cell.
22:709–724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsai JH, Donaher JL, Murphy DA, Chau S and
Yang J: Spatiotemporal regulation of epithelial-mesenchymal
transition is essential for squamous cell carcinoma metastasis.
Cancer Cell. 22:725–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kallergi G, Konstantinidis G,
Markomanolaki H, Papadaki MA, Mavroudis D, Stournaras C,
Georgoulias V and Agelaki S: Apoptotic circulating tumor cells in
early and metastatic breast cancer patients. Mol Cancer Ther.
12:1886–1895. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakagawa T, Martinez SR, Goto Y, et al:
Detection of circulating tumor cells in early-stage breast cancer
metastasis to axillary lymph nodes. Clin Cancer Res. 13:4105–4110.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagrath S, Sequist LV, Maheswaran S, Bell
DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky
A, et al: Isolation of rare circulating tumour cells in cancer
patients by microchip technology. Nature. 450:1235–1239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y and Zhou BP: New insights of
epithelial-mesenchymal transition in cancer metastasis. Acta
Biochim Biophys Sin (Shanghai). 40:643–650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu M, Stott S, Toner M, Maheswaran S and
Haber DA: Circulating tumor cells: Approaches to isolation and
characterization. J Cell Biol. 192:373–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schlüter K, Gassmann P, Enns A, et al:
Organ-specific metastatic tumor cell adhesion and extravasation of
colon carcinoma cells with different metastatic potential. Am J
Pathol. 169:1064–1073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo
RA, Tan DS, Lim WT, Han J, Bhagat AA and Lim CT: Isolation and
retrieval of circulating tumor cells using centrifugal forces. Sci
Rep. 3:12592013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Molnar B, Ladanyi A, Tanko L, Sréter L and
Tulassay Z: Circulating tumor cell clusters in the peripheral blood
of colorectal cancer patients. Clin Cancer Res. 7:4080–4085.
2001.PubMed/NCBI
|
|
23
|
Cianfrocca M and Goldstein LJ: Prognostic
and predictive factors in early-stage breast cancer. Oncologist.
9:606–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jonas S, Bechstein WO, Steinmüller T,
Herrmann M, Radke C, Berg T, Settmacher U and Neuhaus P: Vascular
invasion and histopathologic grading determine outcome after liver
transplantation for hepatocellular carcinoma in cirrhosis.
Hepatology. 33:1080–1086. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Falvo L, Catania A, D'Andrea V, Marzullo
A, Giustiniani MC and De Antoni E: Prognostic importance of
histologic vascular invasion in papillary thyroid carcinoma. Ann
Surg. 241:640–646. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakagohri T, Kinoshita T, Konishi M, Inoue
K and Takahashi S: Survival benefits of portal vein resection for
pancreatic cancer. Am J Surg. 186:149–153. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Adham M, Mirza DF, Chapuis F, Mayer AD,
Bramhall SR, Coldham C, Baulieux J and Buckels J: Results of
vascular resections during pancreatectomy from two European
centres: An analysis of survival and disease-free survival
explicative factors. HPB (Oxford). 8:465–473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Green TL, Cruse JM, Lewis RE and Craft BS:
Circulating tumor cells (CTCs) from metastatic breast cancer
patients linked to decreased immune function and response to
treatment. Exp Mol Pathol. 95:174–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gruber I, Landenberger N, Staebler A, Hahn
M, Wallwiener D and Fehm T: Relationship between circulating tumor
cells and peripheral T-cells in patients with primary breast
cancer. Anticancer Res. 33:2233–2238. 2013.PubMed/NCBI
|
|
30
|
Williams SC: Circulating tumor cells. Proc
Natl Acad Sci USA. 110:48612013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Frisch SM and Screaton RA: Anoikis
mechanisms. Curr Opin Cell Biol. 13:555–562. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liotta LA and Kohn E: Anoikis: Cancer and
the homeless cell. Nature. 430:973–974. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Xu LH and Yu Q: Cell aggregation
induces phosphorylation of PECAM-1 and Pyk2 and promotes tumor cell
anchorage-independent growth. Mol Cancer. 9:72010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumar S, Park SH, Cieply B, et al: A
pathway for the control of anoikis sensitivity by E-cadherin and
epithelial-to-mesenchymal transition. Mol Cell Biol. 31:4036–4051.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao Q, Qu K, Wang C, Kong Y, Liu C, Jiang
D, Saiyin H, Jia F, Ni C, Chen T, et al: HDGF-related protein-3 is
required for anchorage-independent survival and chemoresistance in
hepatocellular carcinomas. Gut. 62:440–451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tormoen GW, Haley KM, Levine RL and
McCarty OJ: Do circulating tumor cells play a role in coagulation
and thrombosis? Front Oncol. 2:1152012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang
C, Xie S, Chen C, Hu L, Xu S and Liang T: Wnt/β-catenin signaling
enhances hypoxia-induced epithelial-mesenchymal transition in
hepatocellular carcinoma via crosstalk with hif-1α signaling.
Carcinogenesis. 34:962–973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Misra A, Pandey C, Sze SK and Thanabalu T:
Hypoxia activated EGFR signaling induces epithelial to mesenchymal
transition (EMT). PLoS One. 7:e497662012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y,
Kato Y, Shinto O, Noda S, Kashiwagi S, Aomatsu N, Hirakawa T, et
al: Hypoxia stimulates the EMT of gastric cancer cells through
autocrine TGFβ signaling. PLoS One. 8:e623102013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Salnikov AV, Liu L, Platen M, Gladkich J,
Salnikova O, Ryschich E, Mattern J, Moldenhauer G, Werner J,
Schemmer P, et al: Hypoxia induces EMT in low and highly aggressive
pancreatic tumor cells but only cells with cancer stem cell
characteristics acquire pronounced migratory potential. PLoS One.
7:e463912012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cannito S, Novo E, Compagnone A, Valfrè di
Bonzo L, Busletta C, Zamara E, Paternostro C, Povero D, Bandino A,
Bozzo F, et al: Redox mechanisms switch on hypoxia-dependent
epithelial-mesenchymal transition in cancer cells. Carcinogenesis.
29:2267–2278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hong Y and Zhang Q: Phenotype of
circulating tumor cells: Face-off between epithelial and
mesenchymal masks. Tumor Biol. 37:5663–5674. 2016. View Article : Google Scholar
|
|
43
|
Pierga JY, Hajage D, Bachelot T, et al:
High independent prognostic and predictive value of circulating
tumor cells compared with serum tumor markers in a large
prospective trial in first-line chemotherapy for metastatic breast
cancer patients. Ann Oncol. 23:618–624. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Giuliano M, Giordano A, Jackson S, et al:
Circulating tumor cells as prognostic and predictive markers in
metastatic breast cancer patients receiving first-line systemic
treatment. Breast Cancer Res. 13:R672011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schulze K, Gasch C, Staufer K, Nashan B,
Lohse AW, Pantel K, Riethdorf S and Wege H: Presence of
EpCAM-positive circulating tumor cells as biomarker for systemic
disease strongly correlates to survival in patients with
hepatocellular carcinoma. Int J Cancer. 133:2165–2171. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Allard WJ, Matera J, Miller MC, Repollet
M, Connelly MC, Rao C, Tibbe AG, Uhr JW and Terstappen LW: Tumor
cells circulate in the peripheral blood of all major carcinomas but
not in healthy subjects or patients with nonmalignant diseases.
Clin Cancer Res. 10:6897–6904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Aceto N, Bardia A, Miyamoto DT, Donaldson
MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al:
Circulating tumor cell clusters are oligoclonal precursors of
breast cancer metastasis. Cell. 158:1110–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tinhofer I, Saki M, Niehr F, Keilholz U
and Budach V: Cancer stem cell characteristics of circulating tumor
cells. Int J Radiat Biol. 90:622–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Swann JB and Smyth MJ: Immune surveillance
of tumors. J Clin Invest. 117:1137–1146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stott SL, Hsu CH, Tsukrov DI, Yu M,
Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK,
et al: Isolation of circulating tumor cells using a
microvortex-generating herringbone-chip. Proc Natl Acad Sci USA.
107:18392–18397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hosokawa M, Yoshikawa T, Negishi R,
Yoshino T, Koh Y, Kenmotsu H, Naito T, Takahashi T, Yamamoto N,
Kikuhara Y, et al: Microcavity array system for size-based
enrichment of circulating tumor cells from the blood of patients
with small-cell lung cancer. Anal Chem. 85:5692–5698. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sollier E, Go DE, Che J, et al:
Size-selective collection of circulating tumor cells using Vortex
technology. Lab Chip. 14:63–77. 2014. View Article : Google Scholar : PubMed/NCBI
|