Introduction
Pancreatic cancer has a mortality rate of >95%,
which has not improved for 3 decades (1). The current treatment of pancreatic
carcinoma consists of a combination of chemotherapy (including
gemcitabine), radiotherapy and surgery, although the treatment may
vary (2). The majority of patients
with pancreatic cancer are diagnosed at a late and inoperable stage
and the 5-year survival rate is poor (3). In investigating novel therapeutic and
preventative strategies, a number of studies have focused on
developing novel chemotherapeutic agents. For example, Lewis et
al (4) have demonstrated that the
sphingosine kinase-2 inhibitor ABC294640 acts as a chemotherapeutic
agent for the treatment of pancreatic cancer through suppression of
c-Myc and RRM2 expression. In addition, Mandhare et al
(5) reviewed the effect of
Azaepothilone B on chemotherapeutic medication for pancreatic
cancer. Pourmorteza et al (6)
demonstrated that evofosfamide targets tumor hypoxia, and may be a
potential agent against pancreatic cancer. Although numerous novel
chemotherapeutic agents have been identified, their antitumor
mechanisms, and how these may be manipulated to promote antitumor
efficacy, are not clear at present.
Wogonin (5,7-dihydroxy-8-methoxyflavone) is a potent
inhibitor of myeloid cell leukemia 1 (Mcl-1) and B-cell lymphoma 2
(Bcl-2), which are anti-apoptotic proteins that are expressed in
various tumors, including pancreatic cancer (7,8). Previous
studies (9,10) have demonstrated the antitumor
potential of wogonin, particularly its ability to induce apoptosis
and its high maximum-tolerated dose. Wogonin has also been observed
to be a potential anti-pancreatic cancer drug through its ability
to inhibit the Mcl-1 and nuclear factor-κB (NF-κB) pathways
(11,12).
Autophagy has become a novel target for the
treatment of certain types of cancer, and may prevent cell death
(13). Few studies have previously
reported wogonin-induced autophagy (14,15). In
present study, wogonin was added into the medium of HPCCs, and the
influence of wogonin on autophagy and its mechanism were
investigated. Furthermore, the antioxidant N-acetyl-L-cysteine
(NAC) was used as a chemosensitizer of wogonin to detect whether it
can enhance cancer cell death, and its mechanism was
investigated.
Materials and methods
Cell culture
Panc-1 and Colo-357 HPCCs were obtained from the
American Type Culture Collection (Manassas, VA, USA). All cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine,
100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in an
atmosphere containing 5% CO2. HPCCs were treated with 40
µM wogonin, 40 µM wogonin combined with 40 µM chloroquine (CQ), or
50 µM rapamycin for 24 h; in addition, HPCCs were pretreated with
sterile water or 10 mM NAC for 2 h and then treated with 0.1% DMSO
or 40 µM wogonin for 24 h. All cell culture reagents were purchased
from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Western blotting
The cells (5×106) were lysed for 30 min
in radioimmunoprecipitation assay lysis buffer [50 mM Tris (pH
7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1%
SDS; Beyotime Institute of Biotechnology, Haimen, China] on ice
then centrifuged at 12,000 × g for 12 min at 4°C. A
bicinchoninic acid assay (Beyotime Institute of Biotechnology) was
used to determine the protein concentration. The proteins were
separated by 8–15% sodium dodecyl sulfate polyacrylamide gel
electrophoresis, and blotted onto a polyvinylidene difluoride
membrane (PVDF) following blocking with 5% nonfat dry milk in
phosphate buffered saline (PBS) with 0.05% Tween 20 (PBST). The
membranes were incubated with the primary antibodies (dilution,
1:1,000) for 2 h at 37°C, then the PVDF membranes were washed three
times for 10 min in PBST. Secondary antibodies (dilution, 1:10,000)
were incubated for 1 h at 37°C and the PVDF membranes were then
washed three times for 10 min in PBST. The antibodies used included
rabbit polyclonal IgG primary antibodies against LC3α (#sc-134226),
Beclin-1 (#sc-11427), phosphatidylinositol 3-kinase (PI3K;
#sc-134766), total mammalian target of rapamycin (mTOR; #sc-8319),
phospho-mTOR (#sc-101738), Unc-51-like autophagy-activating kinase
1 (ULK1; #sc-33182) and cylindromatosis (CYLD; #sc-28211), mouse
monoclonal IgG1 primary antibodies against protein
kinase B (AKT; #sc-5298), eukaryotic initiation factor 4E-binding
protein 1 (4E-BP1; #sc-9977) and GAPDH (#sc-365062), and anti-mouse
IgG (#sc-2380) and anti-rabbit IgG (#sc-2385) secondary antibodies.
All antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Subsequently, the PVDF membranes were exposed to
BeyoECL Star (#P0018A; Beyotime Institute of Biotechnology) and
ECL-sensitive films, and developed by OPTIMAX X-Ray film processor
(Optimax 2010; Protec GmbH & Co. KG, Oberstenfeld,
Germany).
Acridine orange (AO) staining
The degradation of autophagosomes is mediated by
lysosomes; autolysis lowers the pH value and allows AO dye to
penetrate into the acidic organelles and exhibit a red
fluorescence; therefore, the intensity of red fluorescence
following AO staining can be used to indicate the levels of
autolysosomes (16). Cells were
treated with dimethyl sulfoxide (DMSO) or 40 µM wogonin for 24 h in
DMEM supplemented with 10% FBS at 37°C in an atmosphere containing
5% CO2, then washed three times with PBS, fixed with 4%
paraformaldehyde for 15 min, stained with 1 mg/l AO dye solution
for 10 min at 37°C and then imaged under a fluorescence microscope
(Nikon TE2000; Nikon Corporation, Tokyo, Japan).
Green fluorescent protein
(GFP)-conjugated microtubule-associated protein 1A/1B-light chain 3
(LC3) analysis
When autophagy is induced, cytosolic LC3 is cleaved
by hydrolysis to a shorter peptide (LC3 II) that is located on the
membrane of the autophagosome; therefore, the expression levels of
LC3 II may be used to estimate the level of autophagy. In order to
estimate the location and processing of LC3-II, assessment of
LC3-GFP puncta is an effective method (16). Panc-1 and Colo-357 cells were
incubated in 6-well plates with DMEM supplemented with 10% FBS at
37°C in an atmosphere containing 5% CO2. When they had
reached 80% confluence, the cells were incubated with Opti-MEM
medium (Thermo Fisher Scientific, Inc.), and transfected with a
GFP-LC3 vector (GeneChem Co., Ltd., Shanghai, China) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 24 h. Subsequently, the cells were treated
with DMSO or 40 µM wogonin for 24 h, then fixed with 4%
paraformaldehyde for 15 min, and washed three times with PBS. The
cells were imaged under a fluorescence microscope (Nikon TE2000)
and LC3-GFP puncta-positive cells were counted (≥5 puncta was
considered positive).
Co-immunoprecipitation
experiments
Panc-1 cells (5×106) were plated onto
15-cm dishes and attached overnight. The cells were treated with
DMSO or 40 µM wogonin for 12 h at 37°C then lysed for 30 min in
hypotonic lysis buffer (Beyotime Institute of Biotechnology) on ice
and centrifuged (1,000 × g, 4°C, 15 min) as a whole-cell
lysate. Whole-cell lysates were precleared with protein A-agarose
(50% in PBS), and then incubated with antibodies against Beclin-1
or PI3K (#sc-11427 and #sc-134766; Santa Cruz Biotechnology, Inc.;
dilution, 1:300) for 1 h at 4°C. The immunoprecipitates were
captured on a protein-A agarose gel and then detected by
immnuoblotting; whole-cell lysates and immunoprecipitates were
separated by 12% SDS-PAGE and transferred to PVDF membranes for
detection, as described above (17).
Cell viability assay
Cell Counting kit-8 (CCK8) was used to assess cell
viability. Cells (1×104) were seeded into a 96-well
plate and incubated overnight in the previously described
conditions. The cells were pretreated with sterile water or 10 mM
NAC for 2 h and then with 0.1% DMSO or 40 µM wogonin for 24 h.
Following this, the medium was removed and the cells were washed
three times with PBS. DMEM (90 µl) and CCK8 (10 µl) were
subsequently added to each well and incubated for 1.5 h at 37°C; a
microplate reader was used to measure the optical density (OD) at
450 nm.
ROS detection
The levels of intracellular ROS generation were
detected through measuring the conversion of cell-permeable
2,7-dichlorofluorescein diacetate (DCFH-DA; Beyotime Institute of
Biotechnology) to fluorescent dichlorofluorescein (DCF). Cells
(1×104) were seeded into 96-well plates and incubated
overnight in the standard conditions. The cells were pretreated
with sterile water or 10 mM NAC for 2 h and then treated with 0.1%
DMSO or 40 µM wogonin for 24 h. The medium was removed and the
cells were washed three times with PBS prior to incubation with
DCFH-DA at 37°C for 20 min. A fluorescence microplate reader, with
488 nm excitation wavelength and 525 nm emission wavelength, was
used to determine the levels of ROS in the cells.
Detection of glutathione (GSH) and
superoxide anions (O2−)
The intracellular levels of GSH were measured by GSH
Assay kit (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. The OD value of GSH was detected with a
fluorescence microplate reader (Tecan, Männedorf, Switzerland) at
420 nm. In addition, the intracellular levels of
O2− were detected by Superoxide Assay kit
(Beyotime Institute of Biotechnology), and the fluorescence value
of O2− was measured at a wavelength of 550
nm.
Trypan blue assay
Following pretreatment with sterile water or 10 mM
NAC for 2 h and treatment with 0.1% DMSO or 40 µM wogonin for 24 h,
the cells were suspended in 0.25% Trypsin-EDTA (Thermo Fisher
Scientific, Inc.), and 0.4% (w/v) trypan blue solution was added to
each 2-µl suspension; the ratio of the cell suspension to the
trypan blue solution was 9:1. The cells were counted under a light
microscope. As the dead cells fail to exclude the dye, the cell
death rate was calculated using the following equation: Total cell
death rate = (no. dyed cells / total no. cells) × 100%.
Transmission electron microscopy
(TEM)
TEM is the gold standard for the assessment of
autophagosomes (16). Samples were
prepared using a previously described method (18). Briefly, the treated cells were washed
three times with PBS times, suspended in 0.25% Trypsin-EDTA, rinsed
three times in the distilled water and dehydrated in the different
concentrations of ethanol (50, 70, 80, 90, 95 and 100%; 8 min
each). Subsequently, the cells were placed into propylene oxide for
10 min, and then into Embed 812 Resin medium and propylene oxide
mixture (1:1) overnight at room temperature. Samples were then
cured in BEEM capsules overnight at 60°C and the sections were cut
at 50 nm by use of an ultramicrotome (EM UC7/FC7; Leica Biosystems,
Nussloch, Germany). The cut sections were stained with lead citrate
and 2% (w/v) uranyl acetate for TEM detection. TEM was performed on
a JEOL 1230 TEM (JEOL, Tokyo, Japan) at an accelerating voltage of
80 kV. Images were acquired with an AMT Advantage Plus 2K × 2K
digital camera connected to the TEM (18).
Small interfering RNA (siRNA)
transfection experiment
Control siRNA and siRNA specifically targeted to
Beclin-1 (GAG AUC UUA GAG CAA AUG ATT) were purchased from RiboBio
Co., Ltd. (Guangzhou, China). Cells were plated in 6-well plates
and grown to ~80% confluence before transient transfections with
siRNAs (100 pmol per well) were performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific), according to the manufacturer's instructions. After a
36-h transfection, 0.1% DMSO or 40 µM wogonin were added for an
additional 24 h prior to collection of the cells for western
blotting.
Apoptosis detection
The cells were pretreated with sterile water or 10
mM NAC for 2 h and then treated with 0.1% DMSO or 40 µM wogonin for
24 h, prior to collection and washing three times in PBS. The cells
were then resuspended in 100 µl 1X binding buffer (10 mM HEPES/NaOH
pH 7.4, 140 mM NaCl and 2.5 mM CaCl2), and 5 µl of
Annexin V-fluorescein isothiocyanate (FITC) (Beyotime Institute of
Biotechnology) and 5 µl of propidium iodide (PI; Beyotime Institute
of Biotechnology) were added into the suspension, which was gently
vortexed and incubated at room temperature for 15 min in the dark.
The samples were assessed using a FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA), and the data were analyzed
by CellQuest Pro software (version 3.3; BD Biosciences) to
determine the ratios of apoptotic cells.
Statistical analysis
Data are presented as the mean ± standard deviation
from triplicated experiments and SPSS 19.0 software (IBM SPSS,
Armonk, NY, USA) was used to analyze the data. Two-way analysis of
variance was used to analyze the differences between the groups.
P<0.05 was considered to indicate a statistically significant
result.
Results
Wogonin induces autophagy in HPCC
During autophagy, LC3-I is converted to LC3-II by
lipidation through a ubiquitin-like system which involves Atg3 and
Atg7; therefore, LC3 is associated with autophagic vesicles, and
the presence of LC3 in autophagosomes and the presence LC3-II are
the indicators of autophagy (16). In
HPCCs, wogonin was observed to increase the expression levels of
LC3-II; the quantified expression levels (wogonin/DMSO treatment)
were 4.33 in Panc-1 cells and 1.58 in Colo-357 cells. In the
rapamycin positive control group, the corresponding levels were
2.17 (Panc-1) and 3.49 (Colo-357). When wogonin was combined with
CQ, an autophagy inhibitor that blocks the fusion of autophagosomes
and lysosomes, an accumulation of LC3-II was observed
[wogonin+CQ/DMSO levels: 4.61 (Panc-1) and 3.75 (Colo-357)]
(Fig. 1A). These results indicated
that wogonin could significantly increase the expression levels of
LC3-II protein as compared with DMSO (P=0.038). The effect of
wogonin on LC3-II activation was not significantly different
compared with the positive control rapamycin (P=0.105).
Furthermore, accumulation of LC3-II was observed when the autophagy
inhibitor CQ combined with wogonin was used, which suggests that
wogonin could activate autophagic flux at prophase.
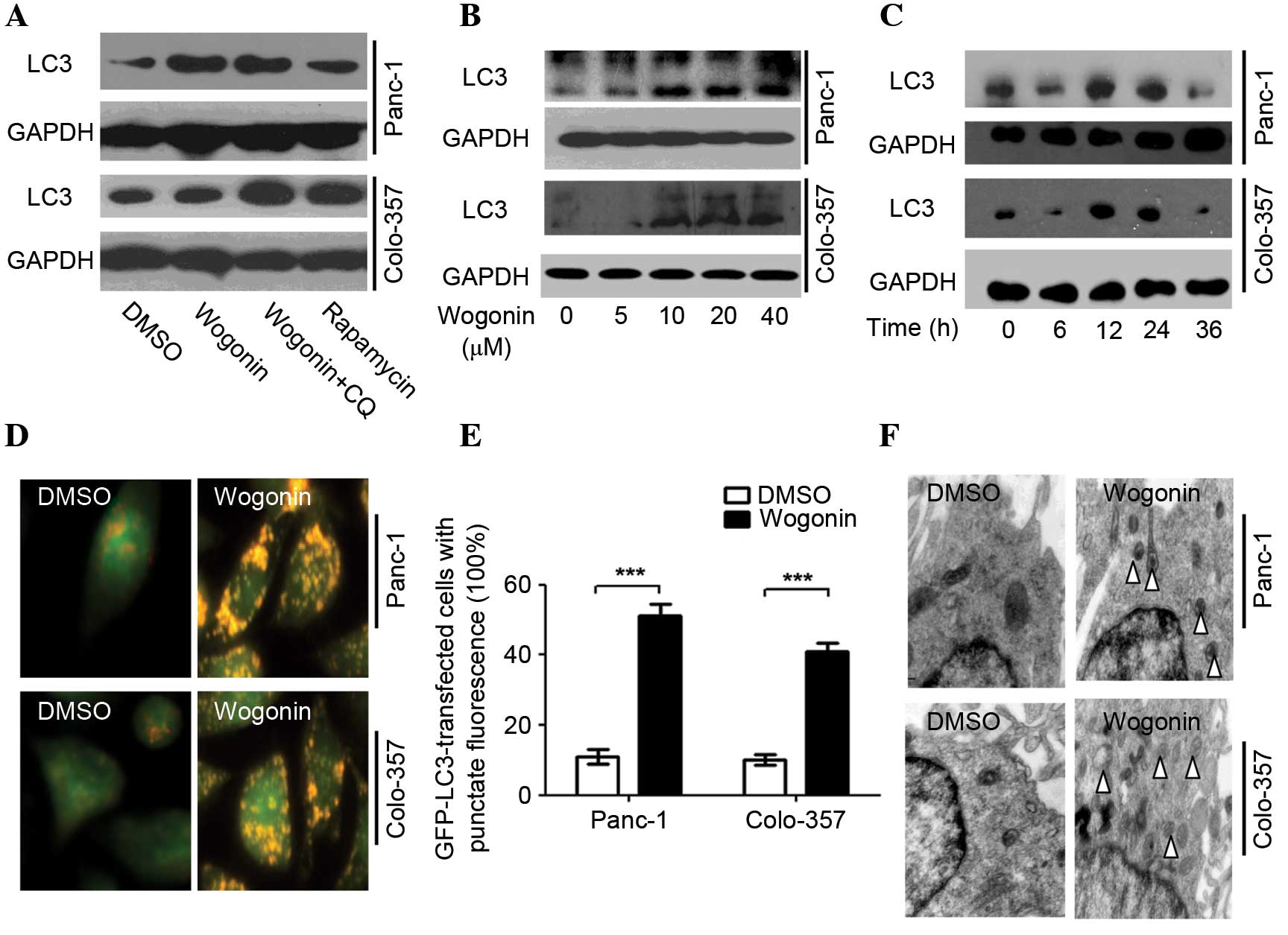 | Figure 1.Wogonin induces autophagy in HPCC.
(A) HPCCs were treated with 40 µM wogonin, wogonin combined with 40
µM CQ or 50 µM rapamycin for 24 h; LC3 expression was induced by
wogonin, indicating its ability to induce autophagy. (B) HPCCs were
treated with various doses of wogonin for 24 h, and wogonin-induced
autophagy was found to be dose-dependent, with an optimal dose of
40 µM. (C) HPCCs were treated with 40 µM wogonin and incubated for
various times; wogonin-induced autophagy was time-dependent
(optimal time, 24 h). (D) HPCCs were treated with 40 µM wogonin and
processed for fluorescent microscopy for 24 h; the level of
autolysosomes markedly increased following treatment with wogonin.
(E) Following GFP-LC3 transfection, cells were treated with 0.1%
DMSO or 40 µM wogonin for 24 h, and the LC3-GFP puncta-positive
cells were counted (≥5 puncta was considered positive); the number
of LC3-GFP puncta-positive cells was increased with wogonin
treatment (***P<0.005). Data are presented as the mean ±
standard deviation from triplicated experiments. (F) TEM was used
to detect autophagic vacuoles (arrows), indicating that wogonin
treatment induced autophagy. HPCC, human pancreatic cancer cell;
CQ, chloroquine, LC3, microtubule-associated protein 1A/1B-light
chain 3; GFP, green fluorescent protein; TEM, transmission electron
microscopy; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; DMSO,
dimethyl sulfoxide. |
Wogonin-induced autophagy was revealed to be time-
and dose-dependent (Fig. 1B and C)
with an optimal time of 24 h and an optimal dose of 40 µM. Notably,
when cells were treated with 40 µM wogonin for 36 h, the LC3-II
protein expression levels were decreased.
AO staining demonstrated that wogonin markedly
enhanced the expression of autolysosomes, and the red fluorescence
intensity was greater than for DMSO (Panc-1, P=0.003; Colo-357,
P=0.007; Fig. 1D). Wogonin also
significantly increased the quantity of LC3-GFP puncta (Panc-1,
P=0.001; Colo-357, P=0.002) (Fig.
1E). The TEM results demonstrated that wogonin significantly
increased the expression of autophagosomes (Fig. 1F) and, therefore, may significantly
induce autophagy in HPCC.
Wogonin induces autophagy through the
Beclin-1/PI3K signaling pathway
A co-immunoprecipitation pull-down assay was used to
investigate the mechanisms underlying the effects of wogonin in
HPCCs. Immunoprecipitation of Beclin-1 was able to pull down PI3K,
and immunoprecipitation of PI3K was able to pull down Beclin-1,
indicating that Beclin-1 and PI3K are bound to each other in HPCC
(Fig. 2A and B).
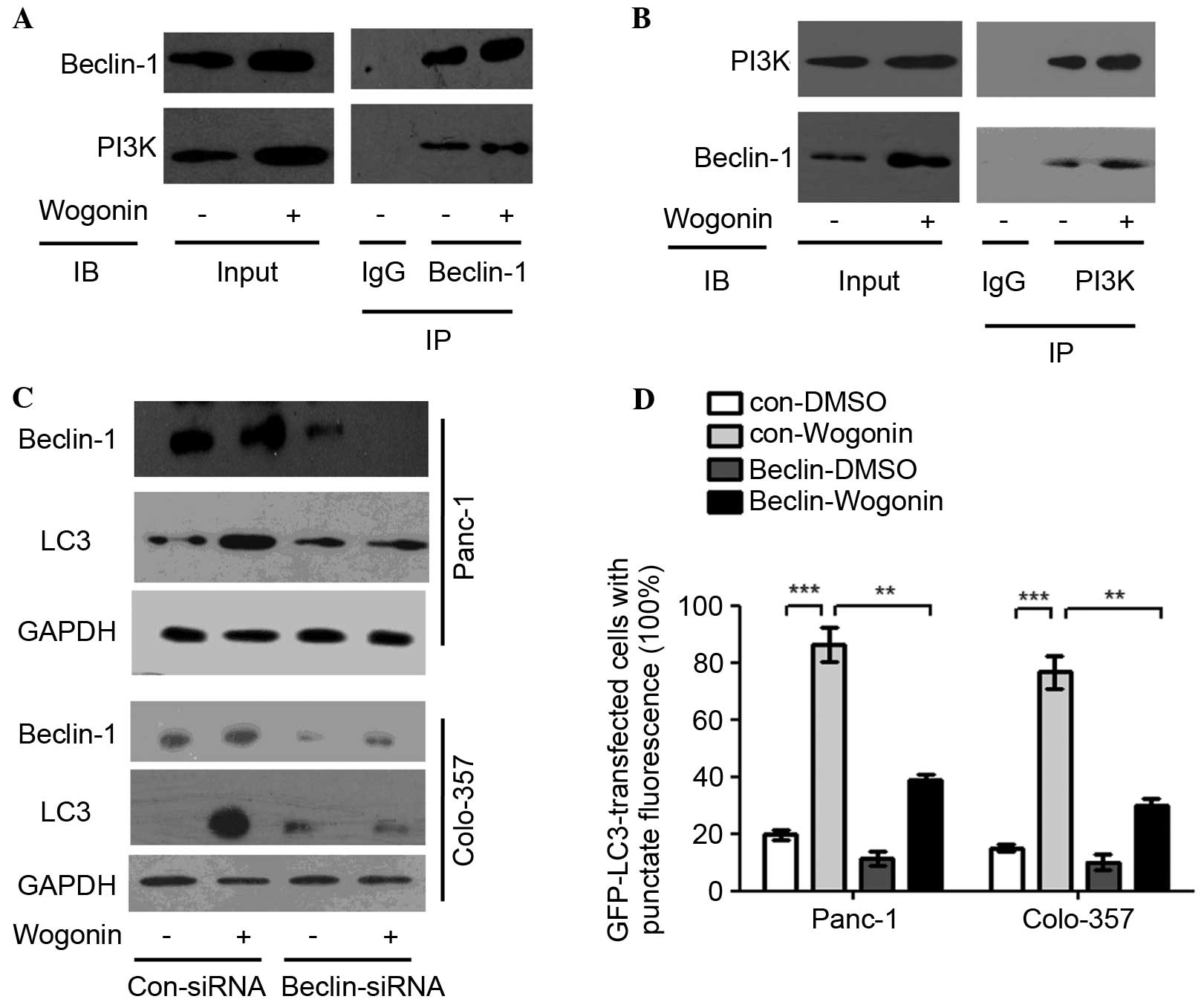 | Figure 2.Wogonin activates the Beclin-1/PI3K
signaling pathway. (A and B) HPCCs were treated with 40 µm wogonin
or 0.1% DMSO for 12 h. Antibodies targeting (A) Beclin-1 or (B)
PI3K were added to immunoprecipitate the Beclin-1- or
PI3K-containing complexes and then IB for PI3K or Beclin-1 was
performed. The results revealed that Beclin-1 and PI3K were pulled
down after PI3K and Beclin-1 were downregulated, respectively, and
wogonin could upregulate Beclin-1 and PI3K, which indicated that
Beclin-1 and PI3K were bound to one another, and wogonin could
promote integration to form the Beclin-1/PI3K complex. (C) HPCCs
were transfected with control siRNA or siRNA-Beclin-1; at 36 h
post-transfection, HPCCs were treated with 0.1% DMSO or 40 µM
wogonin for 24 h and then immunoblotted for LC3 and Beclin-1,
revealing that LC3 expression was decreased following Beclin-1
knowckdown. (D) HPCCs expressing LC3-GFP were transfected with
control siRNA or siRNA-Beclin-1. At 36 h post-transfection, HPCCs
were treated with 0.1% DMSO or 40 µM wogonin for 24 h, and the
LC3-GFP puncta-positive cells were counted (≥5 puncta was
considered positive). After Beclin-1 was knocked down, the number
of positive cells was significantly decreased, but not reduced to
zero, which indicated that the Beclin-1/PI3K complex was involved
in, but not solely responsible for, wogonin-induced autophagy. Data
are presented as the mean ± standard deviation from triplicated
experiments. **P<0.01; ***P<0.005. HPCC, human pancreatic
cancer cell; CQ, chloroquine, LC3, microtubule-associated protein
1A/1B-light chain 3; GFP, green fluorescent protein; TEM,
transmission electron microscopy; DMSO, dimethyl sulfoxide; siRNA,
small interfering RNA; PI3K, phosphatidylinositol 3-kinase; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; Con, control; IgG,
immunoglobulin G; IB, immunoblotting; IP, immunoprecipitation. |
Beclin-1 and PI3K expression may be induced by
wogonin; when Beclin-1 was knocked down with a specific siRNA, the
LC3-II conversion was lower as compared with the control, although
it was not absent (Fig. 2C).
Similarly to wogonin treatment, the number of cells with positive
GFP-LC3 puncta when Beclin-1 was knocked down was significantly
decreased compared with cells in which Beclin-1 was not knocked
down (Panc-1, P=0.009; Colo-357, P=0.008; Fig. 2D). The results suggest that wogonin
may induce autophagy by modulating the Beclin-1/PI3K signaling
pathway.
Wogonin may promote ROS generation in
HPCC
ROS was observed to mediate the survival and
proliferation of cancer cells; therefore, the current study
investigated the variations in ROS levels induced by wogonin in
HPCCs. The results demonstrated that wogonin decreases the levels
of glutathione (GSH; Fig. 3A),
increases the levels of superoxide (O2−) and
ROS (Fig. 3B-D). In addition, when
the cells were co-treated with 10 mM of the antioxidant NAC, the
effect of wogonin on GSH inhibition was reversed, and the level of
GSH was significantly increased (Panc-1, P=0.003; Colo-357,
P=0.001). Similarly, the effects of wogonin on
O2− and ROS generation promotion were
reversed; the levels of O2− (Panc-1, P=0.027;
Colo-357, P=0.031) and ROS generation (Panc-1, P<0.001;
Colo-357, P<0.001) were significantly decreased (Fig. 3A-D). Therefore, the results indicate
that wogonin promotes ROS generation in HPCC, and the antioxidant
NAC can attenuate the activation of ROS generation by wogonin.
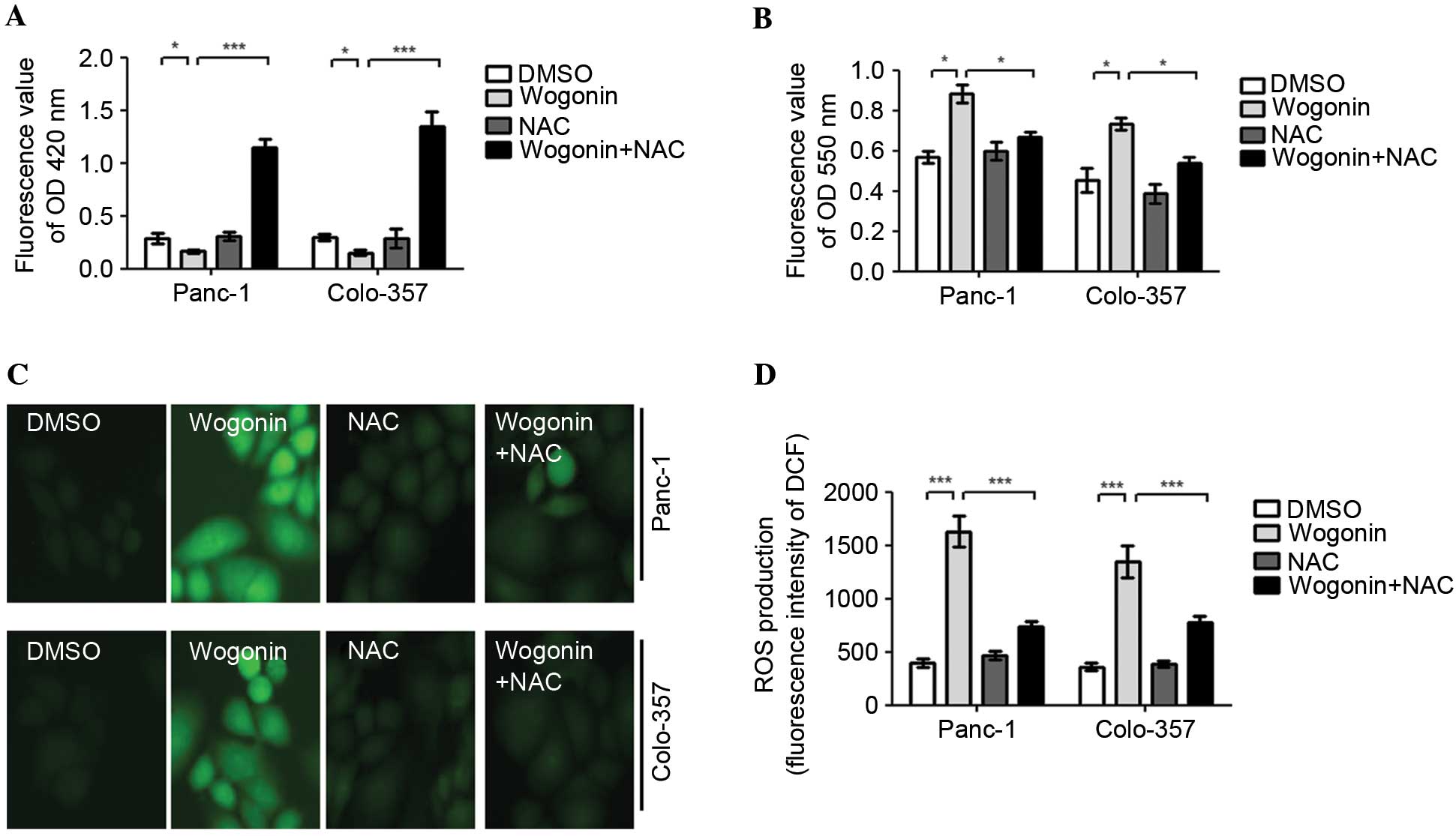 | Figure 3.Wogonin may promote ROS generation in
HPCCs. HPCCs were pretreated with sterile water or NAC for 2 h and
then treated with 0.1% DMSO or 40 µM wogonin for 24 h. (A) The
levels of GSH were determined using an ELISA kit at 420 nm. Wogonin
reduced the intracellular levels of GSH, and wogonin combined with
NAC could significantly increase the intracellular levels of GSH.
(B) The levels of O2− were determined using
an ELISA kit at 550 nm. The intracellular levels of
O2− were promoted by wogonin treatment and
significantly inhibited by wogonin and NAC co-treatment. The levels
of ROS were evaluated by DCFH-DA using (C) a fluorescence
microscope or (D) a fluorescence microplate reader. ROS generation
was promoted by wogonin treatment alone, but was inhibited by
wogonin and NAC co-treatment. These results indicate that wogonin
promotes ROS generation in HPCCs, and the antioxidant NAC can
attenuate the effect of wogonin on ROS generation. Data are
presented as the mean ± standard deviation from triplicated
experiments. *P<0.05; ***P<0.005. HPCC, human pancreatic
cancer cell; GSH, glutathione; DMSO, dimethyl sulfoxide; ROS,
reactive oxygen species; NAC, N-acetyl-cysteine; DCFH-DA,
2,7-dichlorofluorescein diacetate; OD, optical density; DCF,
2,7-dichlorofluorescein. |
Wogonin induces autophagy through the
ROS signaling pathway in HPCC
As an antioxidant, NAC is considered as inhibitor of
ROS. In order to negate the role of ROS in
wogonin-induced-autophagy, HPCCs were co-treated with 10 mM NAC and
40 µM wogonin. The results demonstrated that the quantity of
LC3-GFP puncta in cells co-treated with NAC and wogonin was lower,
as compared with the cells treated with wogonin alone (Panc-1,
P=0.007; Colo-357, P=0.006; Fig. 4A).
AO staining also indicated that when HPCCs were co-treated with NAC
and wogonin, wogonin-induced autophagy was inhibited by NAC
(Panc-1, P<0.001; Colo-357, P<0.001; Fig. 4B).
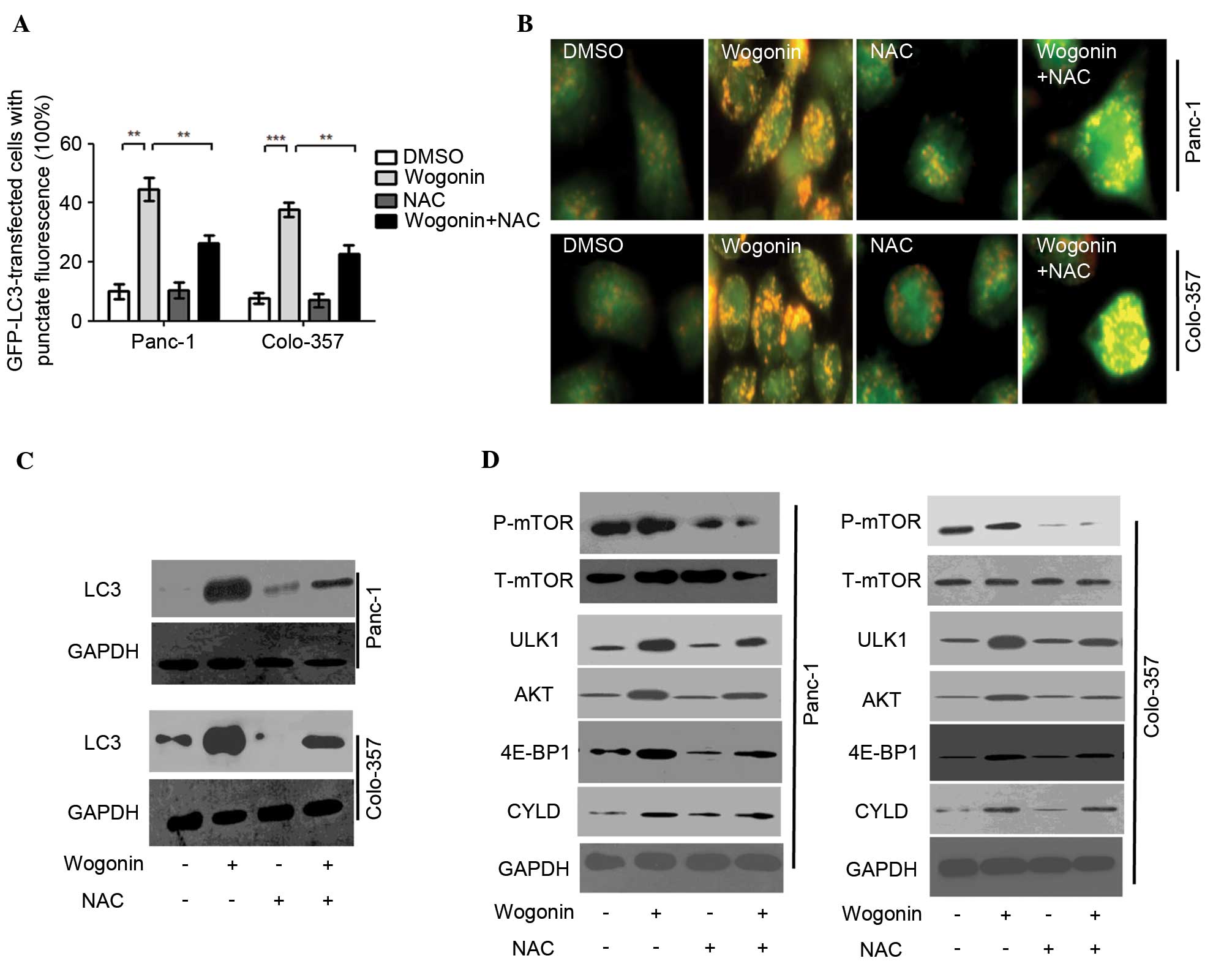 | Figure 4.Wogonin induces autophagy through the
ROS signaling pathway in HPCC. HPCC were pretreated with sterile
water or NAC for 2 h, then treated with 0.1% DMSO or 40 µM wogonin
for 24 h. (A) LC3-GFP puncta-positive cells were counted (≥5 puncta
was considered positive) and the results indicated that wogonin
could increase the number of LC3-GFP puncta-positive cells, whereas
co-treatment with NAC and wogonin attenuated this increase. Data
are presented as the mean ± standard deviation from triplicated
experiments. **P<0.01; ***P<0.005. (B) AO staining for
autolysosomes indicated that autolysosomes were activated by
wogonin treatment, but inhibited by NAC and wogonin co-treatment.
(C) Immunoblotting for LC3 revealed that the expression levels of
LC3 were reduced by NAC and wogonin co-treatment compared with
wogonin single treatment. (D) The levels of p-mTOR, t-mTOR, ULK1,
AKT, 4E-BP1 and CYLD were evaluated by immunoblot. Wogonin could
downregulate the expression of mTOR, and upregulate the expression
levels of ULK1, AKT, 4E-BP1 and CYLD; these effects were
significantly inhibited by NAC and wogonin co-treatment. AO,
acridine orange; HPCC, human pancreatic cancer cell; DMSO, dimethyl
sulfoxide; ROS, reactive oxygen species; NAC, N-acetyl-cysteine;
LC3, microtubule-associated protein 1A/1B-light chain 3; GFP, green
fluorescent protein; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; mTOR, mammalian target of rapamycin; P-,
phosphorylated; T-, total; ULK1, Unc-51 like autophagy activating
kinase 1; AKT, protein kinase B; 4E-BP1, 4E-binding protein-1;
CYLD, cylindromatosis. |
Similarly, LC3 expression was inhibited by NAC, as
demonstrated when HPCCs were co-treated with NAC and wogonin
(Fig. 4C). The results suggest that
ROS may serve as an activator of wogonin-induced autophagy. The
antioxidant NAC can inhibit the effect of ROS on autophagy
activation. Further investigations are required to determine
whether ROS are involved in additional pro-autophagy pathways in
wogonin-induced autophagy.
Additional pro-autophagy pathways were detected to
confirm whether they are involved in ROS-mediated autophagy
following wogonin treatment. Previous reports (19–21)
demonstrated that mTOR, ULK1, AKT, 4E-BP1 and CYLD participate in
the mediation of autophagy. The present results revealed that
wogonin could downregulate the expression of mTOR, and upregulate
the expression of ULK1, AKT, 4E-BP1 and CYLD in HPCCs. When HPCCs
were co-treated with wogonin and NAC, the effects of wogonin on the
inhibition of mTOR, and the activation of ULK1, AKT, 4E-BP1 and
CYLD were significantly attenuated. As the aforementioned results
suggested that NAC could inhibit the generation of ROS in HPCCs, it
is possible that mTOR, ULK1, AKT, 4E-BP1 and CYLD participate in
wogonin-induced autophagy, acting as upstream signals of autophagy.
In addition, these pro-autophagy molecules were mediated by ROS,
acting as the downstream signals of ROS. Therefore, the
pro-autophagy molecules mTOR, ULK1, AKT, 4E-BP1 and CYLD are
involved in the ROS-mediated autophagy following wogonin
treatment.
NAC may enhance wogonin-induced
apoptotic cell death
To investigate the role of ROS-mediated autophagy in
wogonin-induced-apoptotic cell death, HPCCs were treated with
wogonin or co-treated with NAC and wogonin. The results
demonstrated that co-treatment with NAC and wogonin was able to
significantly enhance the wogonin-induced cell death ratio (Panc-1,
P=0.024; Colo-357, P=0.037; Fig. 5A),
and the ratio of cell viability was lower than in HPCCs treated
with wogonin alone (Panc-1, P=0.009; Colo-357, P=0.008; Fig. 5B). Furthermore, when HPCCs were
co-treated with NAC and wogonin, the apoptotic signaling molecule
caspase-3 was activated (Fig. 5C).
The results of Annexin V-FITC and PI staining revealed that NAC
could enhance the ratio of apoptotic cells induced by wogonin
(Panc-1, P=0.022; Colo-357, P=0.039). Furthermore, when HPCCs were
co-treated with NAC and wogonin, the apoptosis ratio of HPCCs was
higher than with wogonin treatment alone (Panc-1, P=0.034;
Colo-357, P=0.026; Fig. 5D). The
results suggest that the suppression of ROS-mediated autophagy may
enhance the antitumor potential of wogonin by increasing apoptosis
in HPCCs.
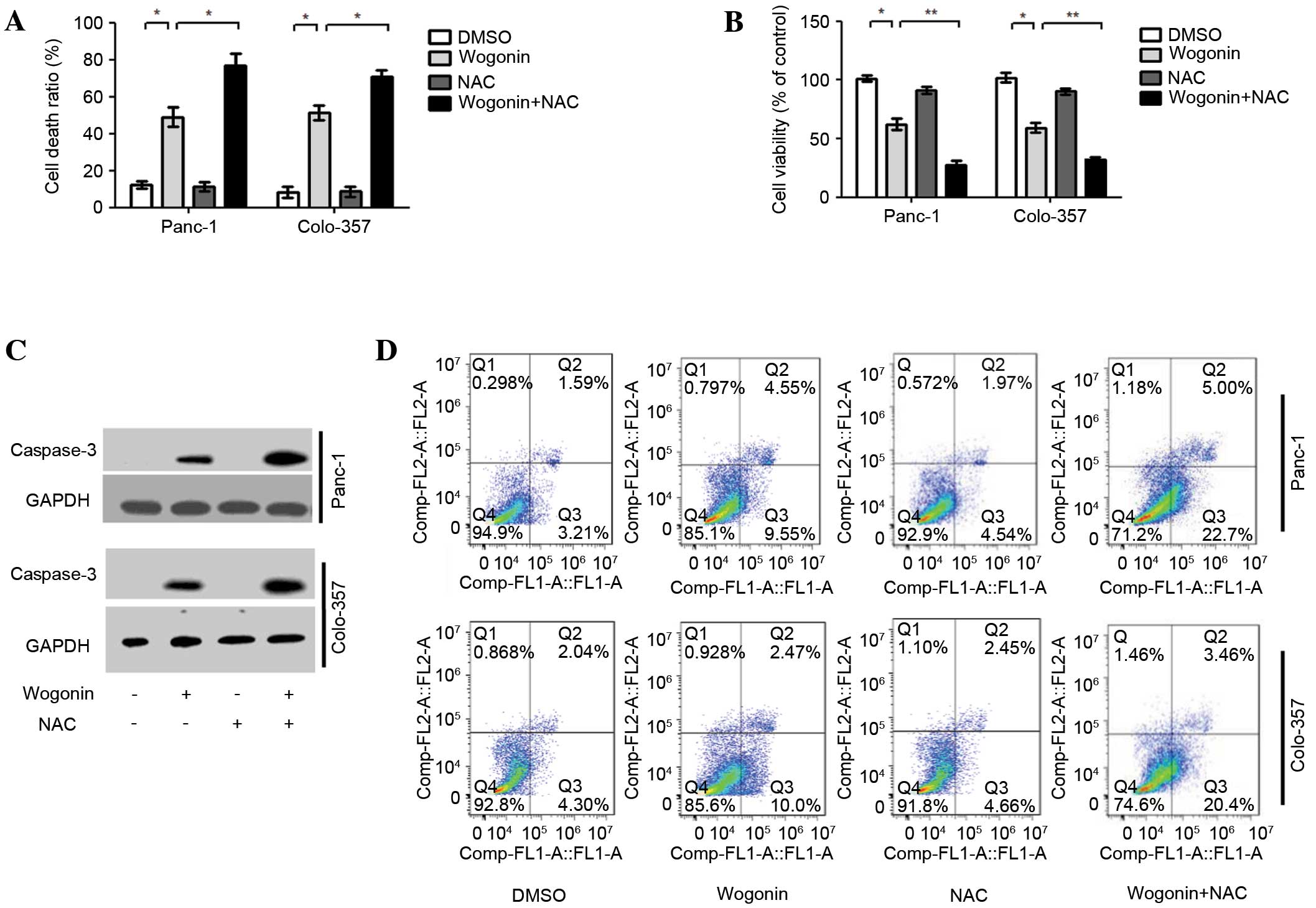 | Figure 5.NAC enhances wogonin-induced
apoptotic cell death. HPCCs were pre-treated with sterile water or
NAC for 2 h, then treated with 0.1% DMSO or 40 µM wogonin for 24 h.
(A) Analysis of the cell death ratio by trypan blue revealed that
wogonin could enhance the cell death ratio, and this effect could
be further promoted by co-treatment with NAC. (B) Cell viability
was determined by CCK8; cell viability was reduced by wogonin and
was further inhibited by co-treatment with NAC. (C) Caspase-3
levels were assessed by immunoblotting, revaling that the
expression level of caspase-3 could be upregulated by wogonin, and
was further promoted by co-treatment with NAC. (D) Flow cytometry
revealed that wogonin could increase the cell apoptosis ratio, and
the increased effect of co-treatment with NAC was significant. Data
are presented as the mean ± standard deviation from triplicated
experiments. *P<0.05; **P<0.01. HPCC, human pancreatic cancer
cell; DMSO, dimethyl sulfoxide; NAC, N-acetyl-cysteine; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; CCK-8, cell counting
kit-8. |
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that wogonin-induced autophagy is
regulated by the Beclin-1/PI3K and ROS signaling pathways. Wogonin
is able to activate Beclin-1 and PI3K and promote ROS generation,
inducing ROS-mediated autophagy, through activating ULK1, AKT,
4E-BP1 and CYLD, and inhibiting the mTOR signaling pathway
(Fig. 6). The antitumor mechanisms
underlying the role of wogonin in HPCC have been demonstrated to be
an increase in apoptosis via the NF-κB, signal transducer and
activator of transcription 3 and PI3K/AKT signaling pathways, and
induction of cell cycle arrest through modulation of the
AKT/glycogen synthase kinase 3β/β-catenin signaling pathways
(12,22–24).
Additionally, wogonin has also exhibited anti-inflammatory effects;
the mechanisms underlying the anti-inflammatory effects were
demonstrated to be through the regulation of the NF-κB signaling
pathway that is mediated by Toll-like receptor 4/myeloid
differentiation primary response gene 88/transforming growth
factor-β-activated kinase 1 (25–27). The
effects of wogonin are not isolated: The anti-inflammatory function
promotes apoptosis and cell cycle inhibition, although the
underlying mechanisms of these three functions require further
study.
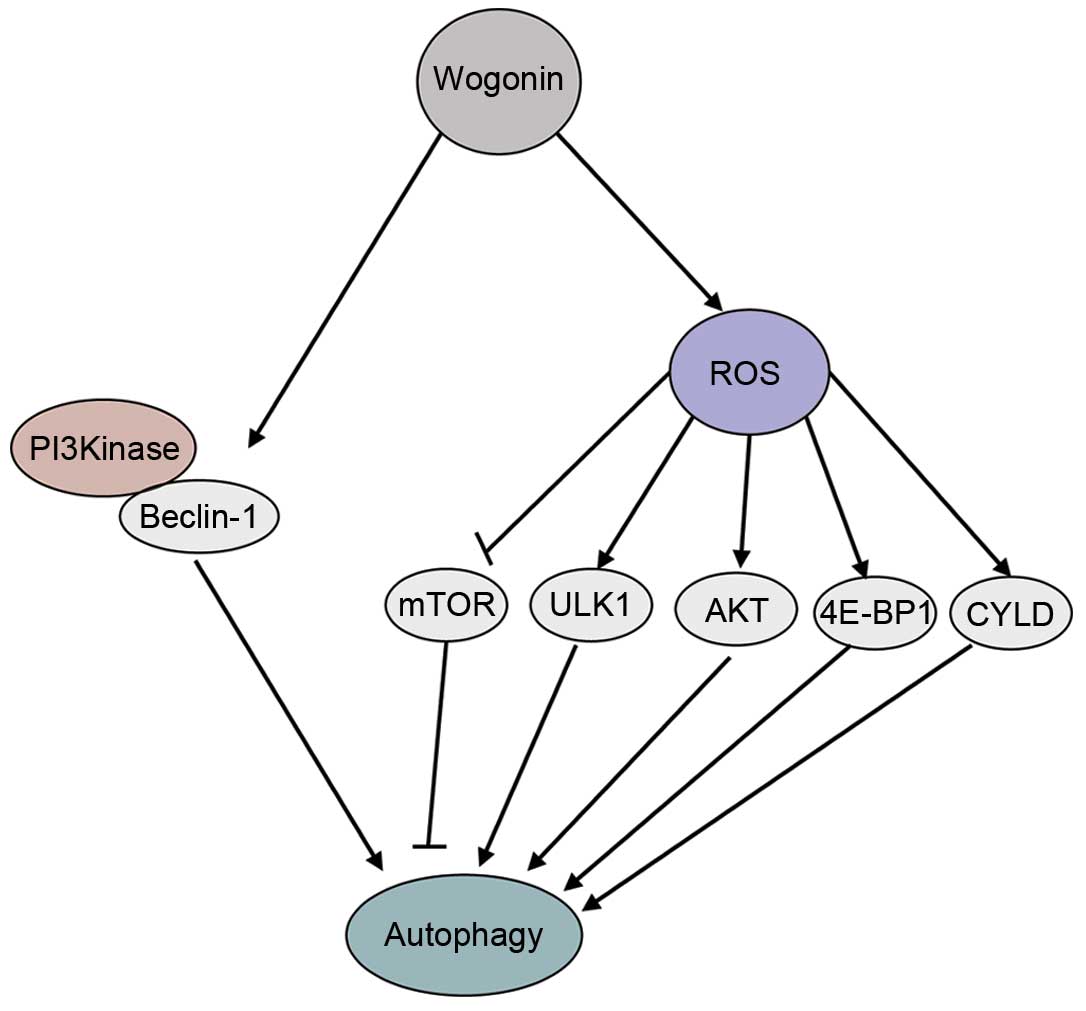 | Figure 6.The signaling pathways involved in
wogonin-induced autophagy. Wogonin activates Beclin-1 and PI3K.
Wogonin also promotes ROS generation, inducing ROS-mediated
autophagy by activating the ULK1, AKT, 4E-BP1 and CYLD signaling
pathways, and inhibiting the mTOR signaling pathway. GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; mTOR, mammalian target of
rapamycin; P-, phosphorylated; T-, total; ULK1, Unc-51 like
autophagy activating kinase 1; AKT, protein kinase B; 4E-BP1,
4E-binding protein-1; PI3K, phosphatidylinositol-3-kinase; ROS,
reactive oxygen species; CYLD, cylindromatosis. |
Autophagy contributes to the survival of cells under
various stresses, including starvation, drug stimulation and
radiation, and is therefore a protective mechanism (16). The majority of chemotherapy drugs
(including sorafenib, gemcitabine and paclitaxel) can induce
autophagy in cancer cells, and autophagy acts a protective
mechanism for cell. Drug-induced autophagy can make cells resistant
to the therapeutic effect of the drug (28–30).
Therefore, it is necessary to detect whether chemotherapy drugs can
induce autophagy in cancer cells, and to investigate the mechanism
of chemotherapy drug-induced autophagy in order to design more
appropriate treatments for patients.
In mammalian cells, PI3K can bind to Beclin-1
through special ECD and CCD domains to form the Beclin-1/PI3K core
complex, and then participate in the mediation of phosphorylation
and ubiquitination, eventually inducing autophagy (31). In the present study it was shown that
Beclin-1 interacts with PI3K to form the Beclin-1/PI3K complex, and
that wogonin promoted the interaction of Beclin-1 and PI3K,
inducing autophagy in HPCCs. These results indicate that
Beclin-1/PI3K is important in wogonin-induced autophagy.
ROS can aggravate cell injury by oxidative stress.
As the cell reacts to the injury, autophagy is activated to promote
cell survival (32). With the
promotion of the ROS-enhanced oxidative stress response, induced
ROS can activate the transcription factors P53, NFE2-related factor
2 and hypoxia inducible factor 1, followed by the activation of
LC3, P62 and NIP3-like protein X. Finally, the corresponding
protein products can increase autophagic flux in the cytoplasm
(32–35). In the present study, an increased
level of ROS generation was observed in cells undergoing
wogonin-induced autophagy, with upregulated
O2− and downregulated GSH levels.
Furthermore, the antioxidant NAC could block the activation of ROS
generation and inhibit autophagic flux following wogonin treatment
in HPCCs. These results indicate that ROS may serve as an activator
in wogonin-induced autophagy in HPCCs. As previously reported,
wogonin is an inhibitor of the mTOR pathway (16), and ULK1 may induce autophagy through
regulation of the Beclin-1/VPS34 signaling pathway (19). Chow et al (14) reported that wogonin is able to induce
autophagy and apoptosis by regulating the AKT signaling pathway.
Lee et al (20) indicated that
wogonin actives 4E-BP1, which is upstream of autophagy (21). The importance of CYLD in triggering
autophagy has also been demonstrated (36). In the present study, wogonin was
demonstrated to act as an inhibitor of mTOR and an activator of
ULK1, AKT, 4E-BP1 and CYLD in HPCCs. In addition, the antioxidant
NAC could attenuate the effect of wogonin on mTOR, ULK1, AKT,
4E-BP1 and CYLD, and inhibit wogonin-induced autophagy. These
results indicate that the pro-autophagy molecules mTOR, ULK1, AKT,
4E-BP1 and CYLD are involved in the ROS-mediated autophagy
following wogonin treatment in HPCCs. As ROS are involved in
wogonin-induced autophagy, leading to inhibition of the
cytotoxicity of wogonin for HPCCs, our results indicate that
co-treatment with NAC and wogonin may be able to enhance
wogonin-induced cell death through enhancement of apoptosis due to
the ROS inhibition followed by autophagy inhibition. Thus, NAC may
be a potential co-treatment with wogonin in HPCC, contributing to
the antitumor effect of wogonin.
In summary, the findings of the present study
indicate that wogonin can induce autophagy through activation of
the Beclin-1/PI3K complex and triggering the ROS-mediated autophagy
pathway. The pro-autophagy molecules mTOR, ULK1, AKT, 4E-BP1 and
CYLD are involved in the ROS-mediated autophagy following wogonin
treatment. Furthermore, the antioxidant NAC can inhibit
wogonin-induced autophagy through inhibition of ROS generation, and
enhance the ratio of wogonin-induced apoptotic cell death. In the
future, wogonin combined with NAC may be a novel combination
therapy for clinical pancreatic cancer therapy trials.
Glossary
Abbreviations
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
NAC
|
N-acetyl-L-cysteine
|
|
HPCCs
|
human pancreatic cancer cells
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Diener MK, Combs SE and Büchler MW:
Chemoradiotherapy for locally advanced pancreatic cancer. Lancet
Oncol. 14:269–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akimoto M, Iizuka M, Kanematsu R, Yoshida
M and Takenaga K: Anticancer effect of ginger extract against
pancreatic cancer cells mainly through reactive oxygen
species-mediated autotic cell death. PLoS One. 10:e01266052015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lewis CS, Voelkel-Johnson C and Smith CD:
Suppression of c-Myc and RRM2 expression in pancreatic cancer cells
by the sphingosine kinase-2 inhibitor ABC294640. Oncotarget.
2016.[Epub ahead of print]. View Article : Google Scholar
|
|
5
|
Mandhare A, Biradar S and Gurule A:
Azaepothilone B and its derivatives: A patent review. Expert Opin
Ther Pat. 26:891–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pourmorteza M, Rahman ZU and Young M:
Evofosfamide, a new horizon in the treatment of pancreatic cancer.
Anticancer Drugs. 27:723–725. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Polier G, Ding J, Konkimalla BV, Eick D,
Ribeiro N, Köhler R, Giaisi M, Efferth T, Desaubry L, Krammer PH
and Li-Weber M: Wogonin and related natural flavones are inhibitors
of CDK9 that induce apoptosis in cancer cells by transcriptional
suppression of Mcl-1. Cell Death Dis. 2:e1822011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polier G, Giaisi M, Köhler R, Müller WW,
Lutz C, Buss EC, Krammer PH and Li-Weber M: Targeting CDK9 by
wogonin and related natural flavones potentiates the anti-cancer
efficacy of the Bcl-2 family inhibitor ABT-263. Int J Cancer.
136:688–698. 2015.PubMed/NCBI
|
|
9
|
Baumann S, Fas SC, Giaisi M, Müller WW,
Merling A, Gülow K, Edler L, Krammer PH and Li-Weber M: Wogonin
preferentially kills malignant lymphocytes and suppresses T-cell
tumor growth by inducing PLCgamma1-and Ca2+-dependent
apoptosis. Blood. 111:2354–2363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang H, Hui H, Wang Q, Li H, Zhao K, Zhou
Y, Zhu Y, Wang X, You Q, Guo Q and Lu N: Wogonin induces cell cycle
arrest and erythroid differentiation in imatinib-resistant K562
cells and primary CML cells. Oncotarget. 5:8188–8201. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi H, Chen MC, Pham H, Angst E,
King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, et
al: Baicalein, a component of Scutellaria baicalensis, induces
apoptosis by Mcl-1 down-regulation in human pancreatic cancer
cells. Biochim Biophys Acta. 1813:1465–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kallifatidis G, Rausch V, Baumann B, Apel
A, Beckermann BM, Groth A, Mattern J, Li Z, Kolb A, Moldenhauer G,
et al: Sulforaphane targets pancreatic tumour-initiating cells by
NF-kappaB-induced antiapoptotic signalling. Gut. 58:949–963. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen N and Karantza V: Autophagy as a
therapeutic target in cancer. Cancer Biol Ther. 11:157–168. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chow SE, Chen YW, Liang CA, Huang YK and
Wang JS: Wogonin induces cross-regulation between autophagy and
apoptosis via a variety of Akt pathway in human nasopharyngeal
carcinoma cells. J Cell Biochem. 113:3476–3485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu Y and Wang J: Wogonin increases
β-amyloid clearance and inhibits tau phosphorylation via inhibition
of mammalian target of rapamycin: Potential drug to treat
Alzheimer's disease. Neurol Sci. 36:1181–1188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Arozena A Acevedo, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steinbrenner J, Eldridge M, Tomé DF and
Beynon JL: A simple and fast protocol for the protein complex
immunoprecipitation (Co-IP) of effector: Host protein complexes.
Methods Mol Biol. 1127:195–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mikhaylova O, Stratton Y, Hall D, Kellner
E, Ehmer B, Drew AF, Gallo CA, Plas DR, Biesiada J, Meller J and
Czyzyk-Krzeska MF: VHL-regulated MiR-204 suppresses tumor growth
through inhibition of LC3B-mediated autophagy in renal clear cell
carcinoma. Cancer Cell. 21:532–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Russell RC, Tian Y, Yuan H, Park HW, Chang
YY, Kim J, Kim H, Neufeld TP, Dillin A and Guan KL: ULK1 induces
autophagy by phosphorylating Beclin-1 and activating VPS34 lipid
kinase. Nat Cell Biol. 15:741–750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee DH, Lee TH, Jung CH and Kim YH:
Wogonin induces apoptosis by activating the AMPK and p53 signaling
pathways in human glioblastoma cells. Cell Signal. 24:2216–25.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thomas HE, Mercer CA, Carnevalli LS, Park
J, Andersen JB, Conner EA, Tanaka K, Matsutani T, Iwanami A, Aronow
BJ, et al: mTOR inhibitors synergize on regression, reversal of
gene expression, and autophagy in hepatocellular carcinoma. Sci
Transl Med. 4:139ra842012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao W, Wu K, Yin M, Han S, Ding Y, Qiao
A, Lu G, Deng B, Bo P and Gong W: Wogonin Inhibits Tumor-derived
Regulatory Molecules by Suppressing STAT3 Signaling to Promote
Tumor Immunity. J Immunother. 38:167–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu C, Xu M, Qin R, Chen W and Xu X:
Wogonin induces apoptosis and endoplasmic reticulum stress in HL-60
leukemia cells through inhibition of the PI3K-AKT signaling
pathway. Oncol Rep. 33:3146–3154. 2015.PubMed/NCBI
|
|
24
|
Zhao L, Miao HC, Li WJ, Sun Y, Huang SL,
Li ZY and Guo QL: LW-213 induces G2/M cell cycle arrest through
AKT/GSK3β/β-catenin signaling pathway in human breast cancer cells.
Mol Carcinog. 55:778–792. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang W, Xia T and Yu X: Wogonin suppresses
inflammatory response and maintains intestinal barrier function via
TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm Res.
64:423–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JY and Park W: Anti-inflammatory
effect of wogonin on RAW 264.7 mouse macrophages induced with
polyinosinic-polycytidylic acid. Molecules. 20:6888–6900. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lucas CD, Dorward DA, Sharma S, Rennie J,
Felton JM, Alessandri AL, Duffin R, Schwarze J, Haslett C and Rossi
AG: Wogonin induces eosinophil apoptosis and attenuates allergic
airway inflammation. Am J Respir Crit Care Med. 191:626–636. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prieto-Domínguez N, Ordóñez R, Fernández
A, García-Palomo A, Muntané J, González-Gallego J and Mauriz JL:
Modulation of autophagy by sorafenib: Effects on Treatment
Response. Front Pharmacol. 7:1512016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kleger A, Perkhofer L and Seufferlein T:
Smarter drugs emerging in pancreatic cancer therapy. Ann Oncol.
25:1260–1270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xi G, Hu X, Wu B, Jiang H, Young CY, Pang
Y and Yuan H: Autophagy inhibition promotes paclitaxel-induced
apoptosis in cancer cells. Cancer Lett. 307:141–148. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maiuri MC, Tasdemir E, Criollo A, Morselli
E, Vicencio JM, Carnuccio R and Kroemer G: Control of autophagy by
oncogenes and tumor suppressor genes. Cell Death Differ. 16:87–93.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Scherz-Shouval R and Elazar Z: Regulation
of autophagy by ROS: Physiology and pathology. Trends Biochem Sci.
36:30–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang J, Kim J, Alexander A, Cai S,
Tripathi DN, Dere R, Tee AR, Tait-Mulder J, Di Nardo A, Han JM, et
al: A tuberous sclerosis complex signalling node at the peroxisome
regulates mTORC1 and autophagyin response to ROS. Nat Cell Biol.
15:1186–1196. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim SJ, Jung HJ and Lim CJ: Reactive
oxygen species-dependent down-regulation of tumor suppressor genes
PTEN, USP28, DRAM, TIGAR, and CYLD under oxidative stress. Biochem
Genet. 51:901–915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen C, Liu Y and Zheng D: An agonistic
monoclonal antibody against DR5 induces ROS production, sustained
JNK activation and Endo G release in Jurkat leukemia cells. Cell
Res. 19:984–995. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bonapace L, Bornhauser BC, Schmitz M,
Cario G, Ziegler U, Niggli FK, Schäfer BW, Schrappe M, Stanulla M
and Bourquin JP: Induction of autophagy-dependent necroptosis is
required for childhood acute lymphoblastic leukemia cells to
overcome glucocorticoid resistance. J Clin Invest. 120:1310–1323.
2010. View Article : Google Scholar : PubMed/NCBI
|




















