Introduction
Primary intraosseous squamous cell carcinoma
(PIOSCC) is a rare lesion defined as an SCC that develops within
the jaw bones, with no initial connection to the oral mucosa, and
arises from remnants of odontogenic epithelium (1).
Loos (2) first defined
PIOSCC in 1913 as ‘central epidermoid carcinoma of the jaw’,
followed by Willis (3) in 1948 who
described the lesion as an ‘intra-alveolar epidermoid carcinoma’.
This description was modified by Shear (4) to ‘primary intra-alveolar epidermoid
carcinoma’ in 1969. In 1971, the term ‘primary intraosseous
carcinoma’ (PIOC) was introduced (5).
Elzay (6) reviewed the relevant
literature associated with PIOC of the jaw and suggested an
alteration to the World Health Organization (WHO) classification to
include ‘ameloblastic carcinoma’, which was later modified to
account for potential etiological factors (7). Intraosseous mucoepidermoid carcinoma was
also later included as an additional form of PIOC (8).
According to the 2005 WHO classification of tumors,
PIOSCC is subcategorized into three different types: i) PIOSCC
solid type (de novo); ii) PIOSCC originating from
keratocystic odontogenic tumors; and iii§§) PIOSCC originating from
odontogenic cysts (9).
PIOSCC is estimated to account for 1–2% of all cases
of oral cancer (1). The majority of
PIOSCCs arise from odontogenic cysts, including dentigerous cysts,
residual periapical cysts and keratocystic odontogenic tumors,
which were previously known as odontogenic keratocysts (1,10).
Commonly reported clinical features of PIOSCC
include jaw swelling, pain and sensory disturbances (1,11). Prior
to the diagnosis of PIOSCC, the existence of a primary tumor in
another site must be ruled out (11).
The histological diagnosis is complicated by the fact that the
histological features of PIOSCC are not pathognomonic (11).
With regard to patient survival, in 116 PIOSCC
cases, Bodner et al found the overall 2- and 5-year survival
rates to be 62 and 38%, respectively (1).
The present study describes the case of a PIOSCC
that originated from an ex-infected odontogenic cyst in the
mandible, in addition to a review of the relevant literature.
Case report
In January 2013, a 25-year-old male patient
presented to the Department of Oral and Maxillofacial Surgery,
University Hospitals of Leuven (Leuven, Belgium). In November 2011,
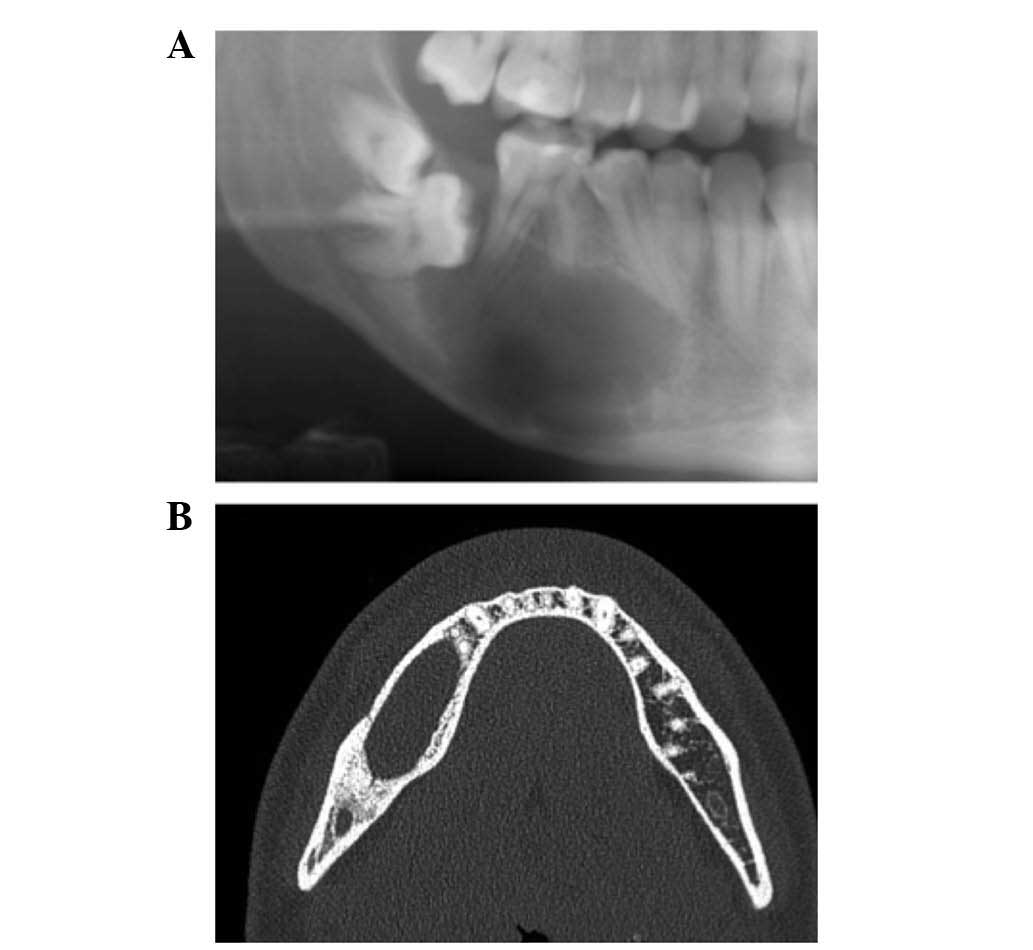
the patient was diagnosed with a benign odontogenic cyst of the
right mandible, which was supported by panoramic radiography and
computed tomography (CT) (Figs. 1 and
2). However, following initial
marsupialization, the cyst recurred and a second surgery was
performed in May 2012, which consisted of enucleation and
reconstruction of the bony defect using an iliac crest graft.
Nevertheless, the patient began to notice recurrence of the
swelling, and 6 months later, a further surgical intervention was
required. No improvement was noted, and the patient was
subsequently referred to the Department of Oral and Maxillofacial
Surgery, University Hospitals of Leuven.
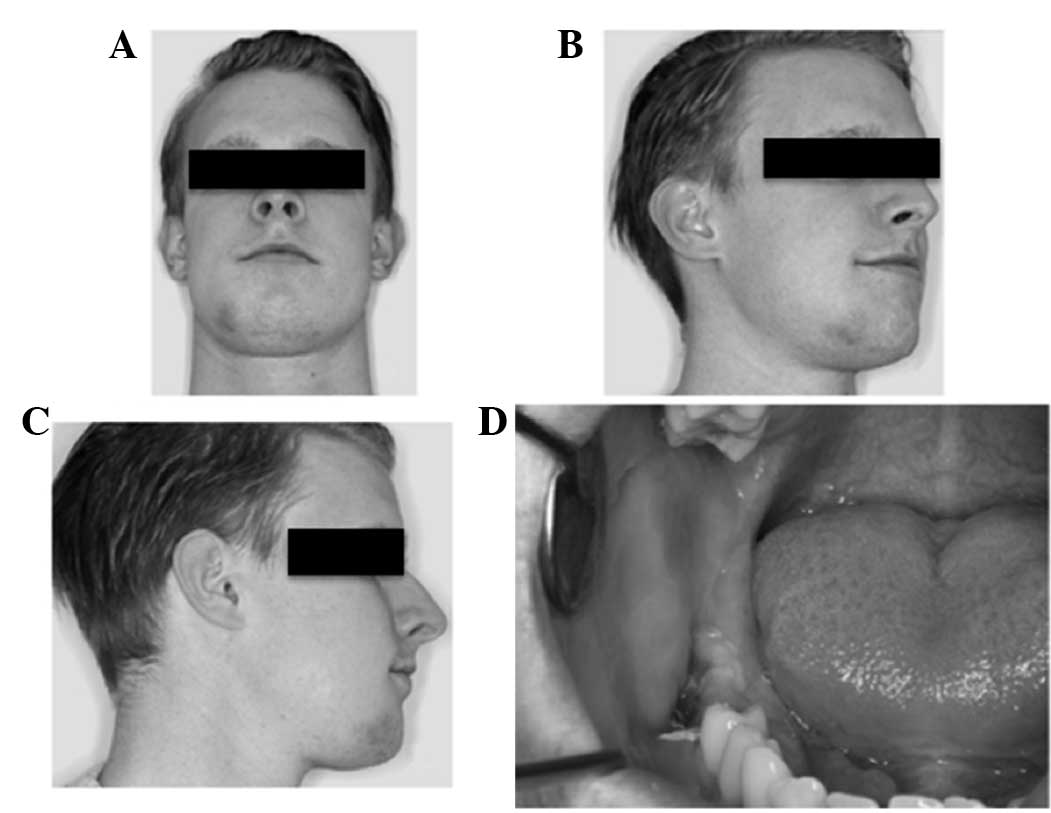
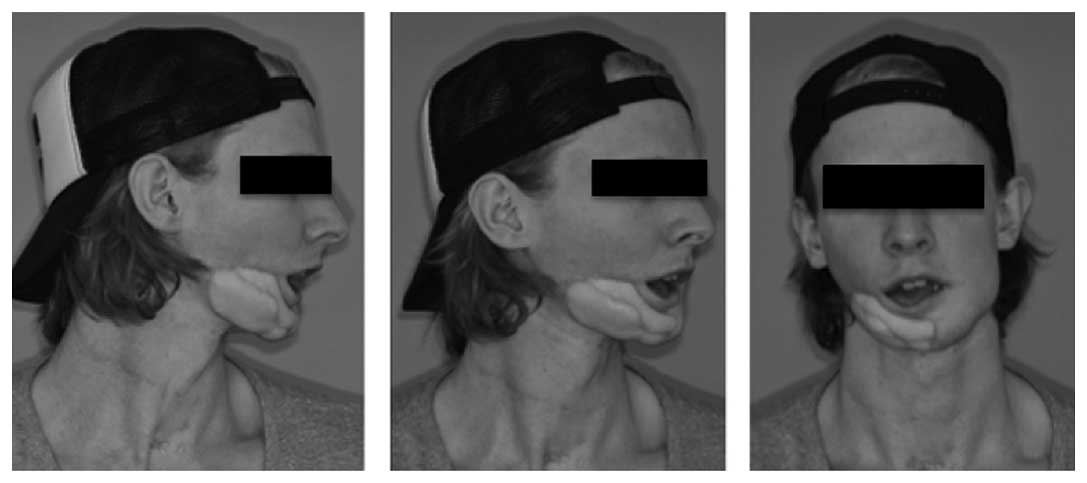
Initial observations revealed a right buccal
swelling, a paste-like aspect to the skin and hypoesthesia of the
right mental nerve. Apart from this, the patient reported no other
sensory disturbances. Sensibility in the area innervated by the
lingual nerve was intact. An intraoral examination identified
swelling in the vestibular region and discharge along the right
mandible in the molar region (Fig.
3). Given the clinical symptoms of inflammation, an initial
diagnosis of osteomyelitis of the mandible was made.
The additional medical history of the patient was
insignificant, while the dental history noted the extraction of
four third molars and the lower right second molar 2 years
previously. The patient's family and personal history was
non-contributory. It was noted that the individual was allergic to
penicillin.
Laboratory results, including routine blood work
with checking of infectious parameters and a CBC, revealed no
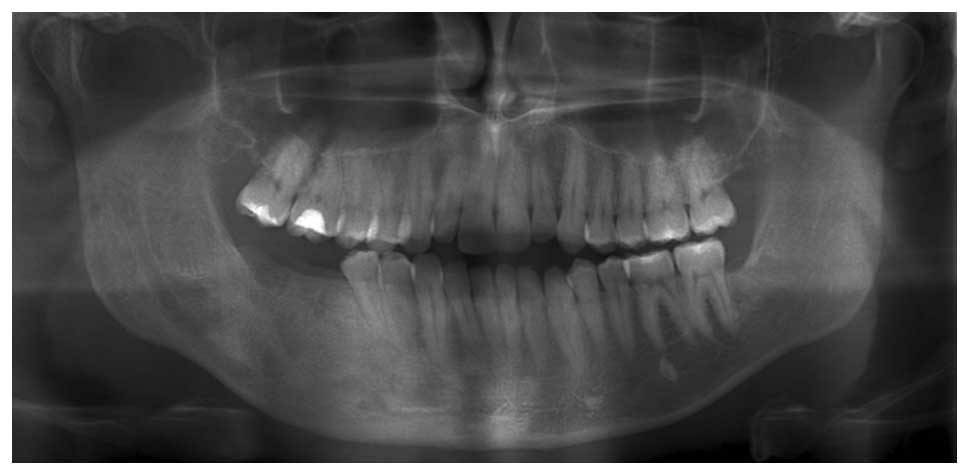
abnormalities. A panoramic radiograph detected irregular regions of
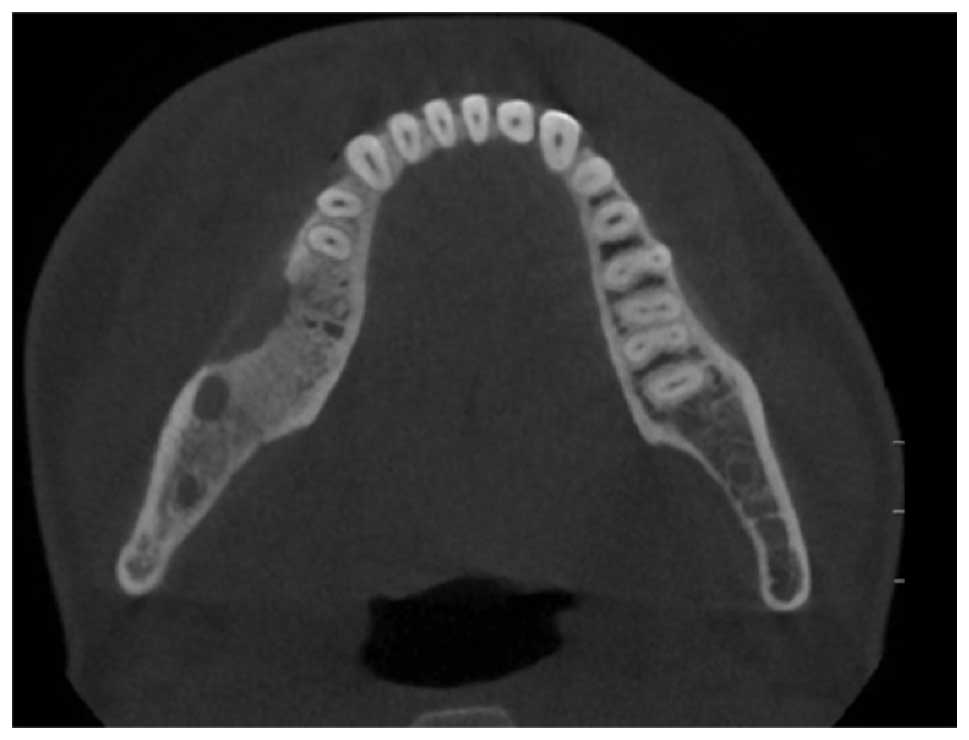
radiolucency along the right mandible in the molar region (Fig. 4). A cone beam CT scan supported the
clinical diagnosis of osteomyelitis of the mandible (Fig. 5). Wound debridement and buccal
decortication were performed.
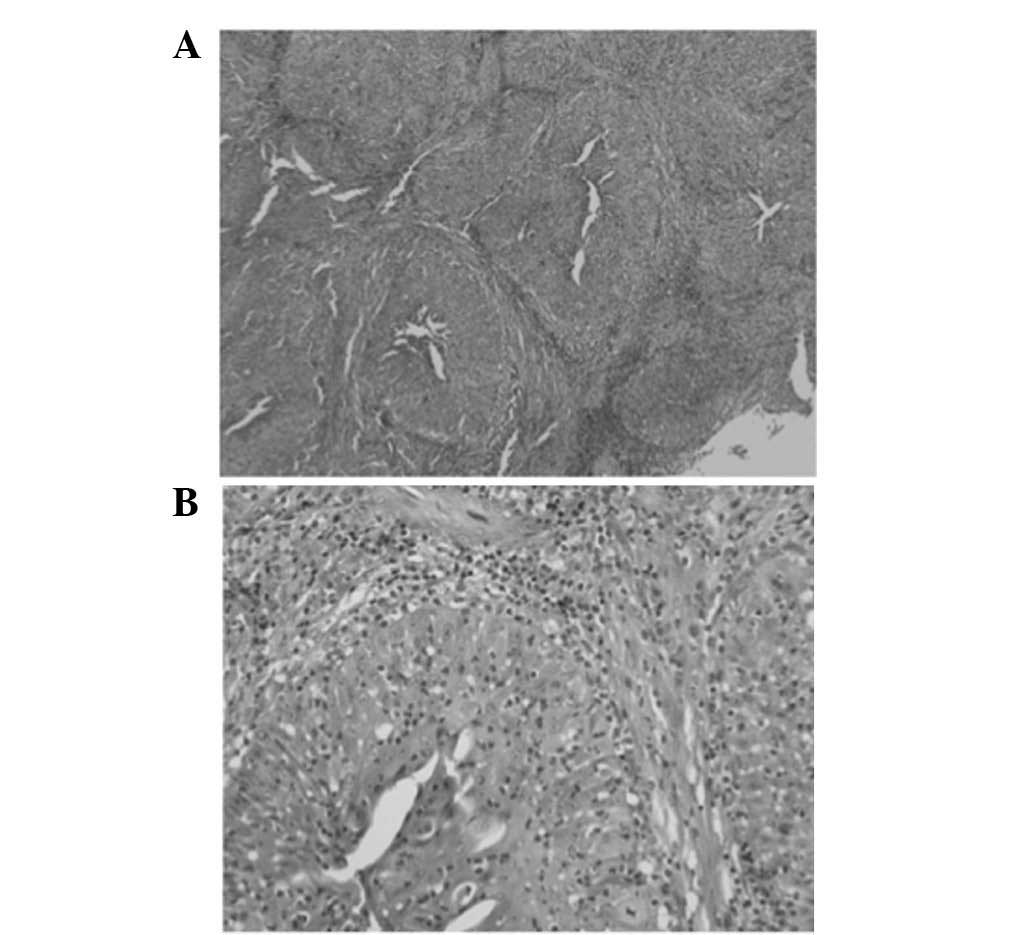
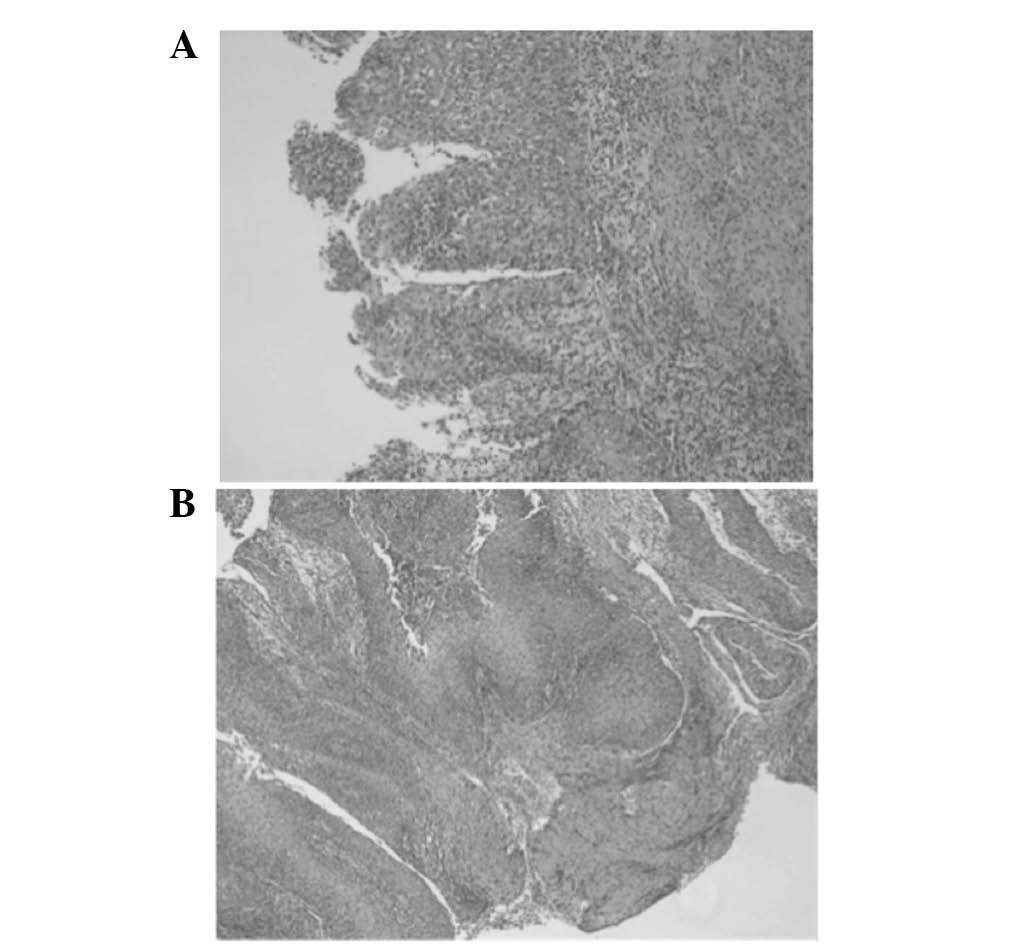
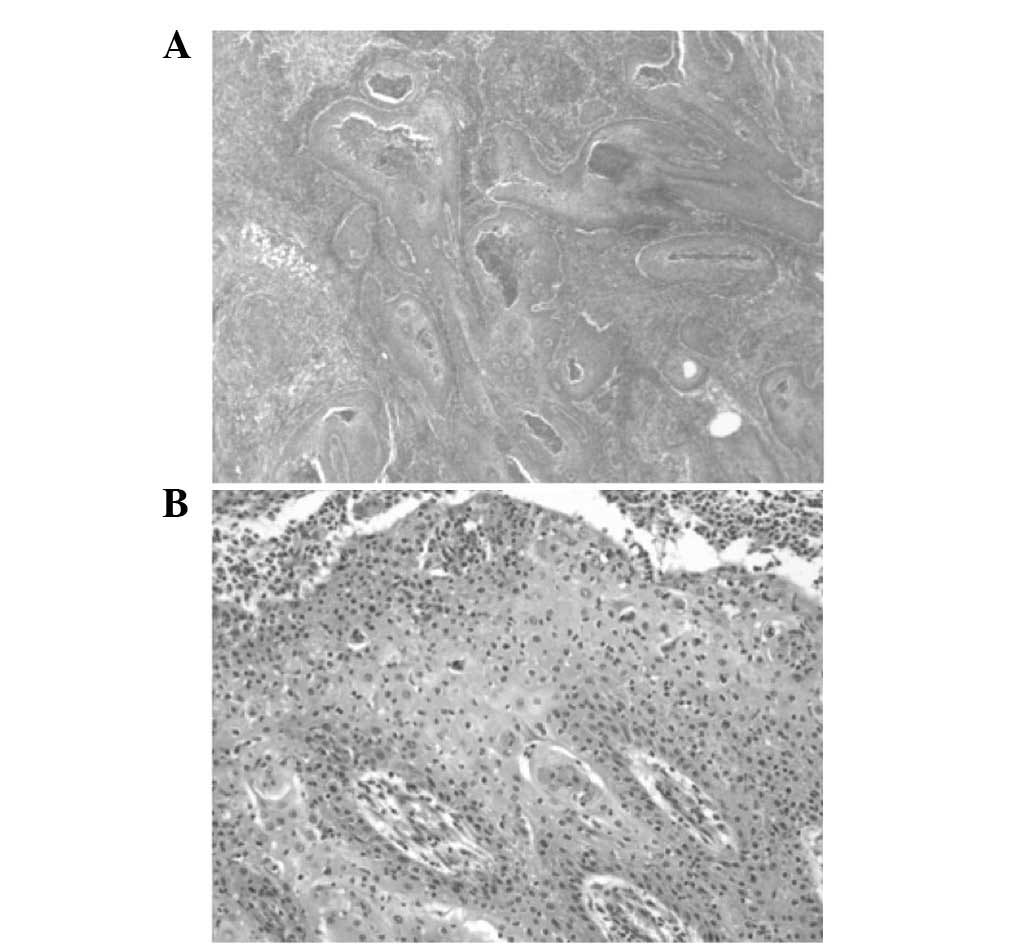
Histology of the resected specimen identified tissue
fragments lined with a multilayered squamous epithelium with focal
parakeratosis. The epithelium was densely infiltrated by
neutrophils (Fig. 6). No evidence of
malignancy was observed. Tissue cultures demonstrated the growth of
Actinomyces species. An infectious disease physician
(Department of Laboratory Medicine, University Hospitals of Leuven)
was consulted and the patient was administered intravenous
antibiotics, consisting of vancomycin (1,000 mg 2 times per day for
7 days) and levofloxacin (500 mg for 7 days). In addition,
adjunctive therapy consisting of 20 sessions of hyperbaric oxygen
over an interval of 4 weeks was initiated.
Despite multiple debridement procedures, frequent
follow-up and the administration of intravenous antibiotics, the
symptoms did not resolve. Repeated biopsies consistently
demonstrated no evidence of malignancy; however, it is possible
that the well-differentiated nature of the lesion, with little or
no atypia, and the background of long-term chronic inflammation
with repeated successive interventions disturbing the histological
picture prevented an early diagnosis of SCC.
In May 2013, 1 year after initial resection of the
recurrent lesion and reconstruction with an iliac crest bone graft,
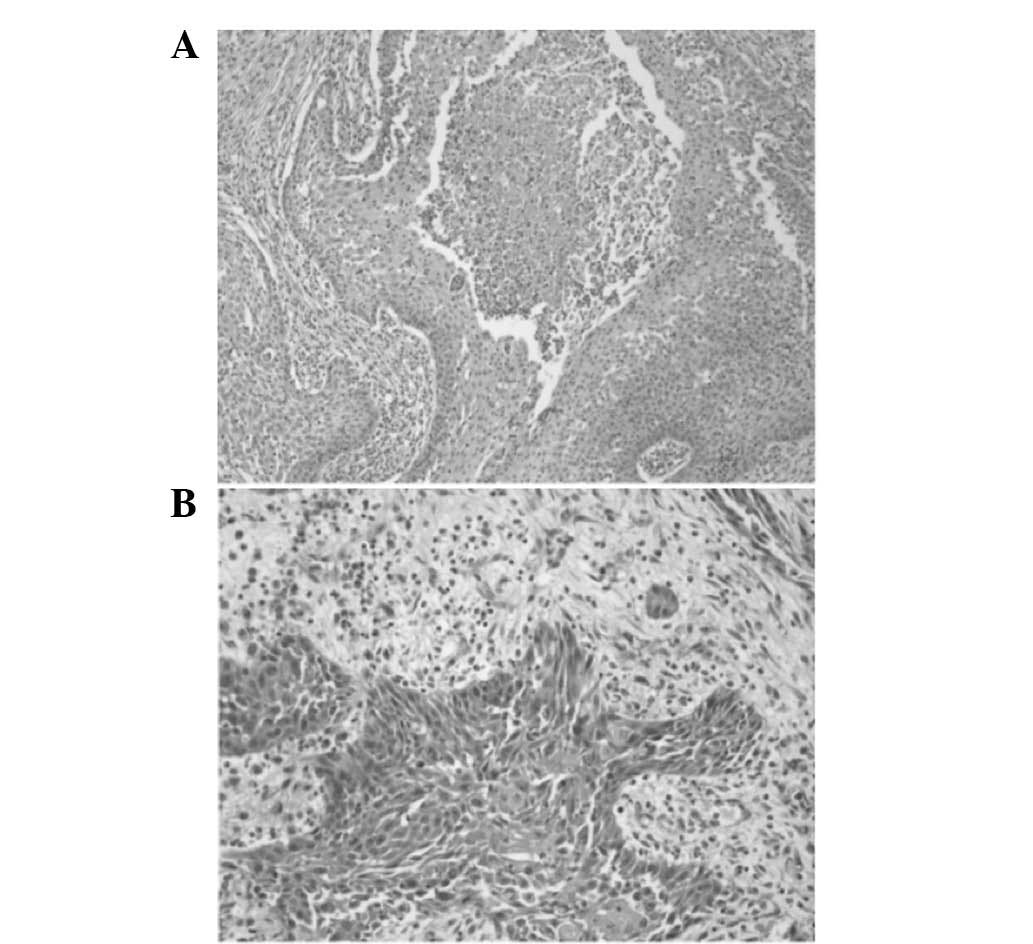
a new biopsy of the lesion was taken and histopathological
examination of the specimen identified central necrosis, nuclear
atypia and small epithelial strands infiltrating the surrounding
stroma (Fig. 7).
Following combination of the radiological,
histopathological and histochemical findings, the final diagnosis
was confirmed as a PIOSCC, which had developed from an ex-infected
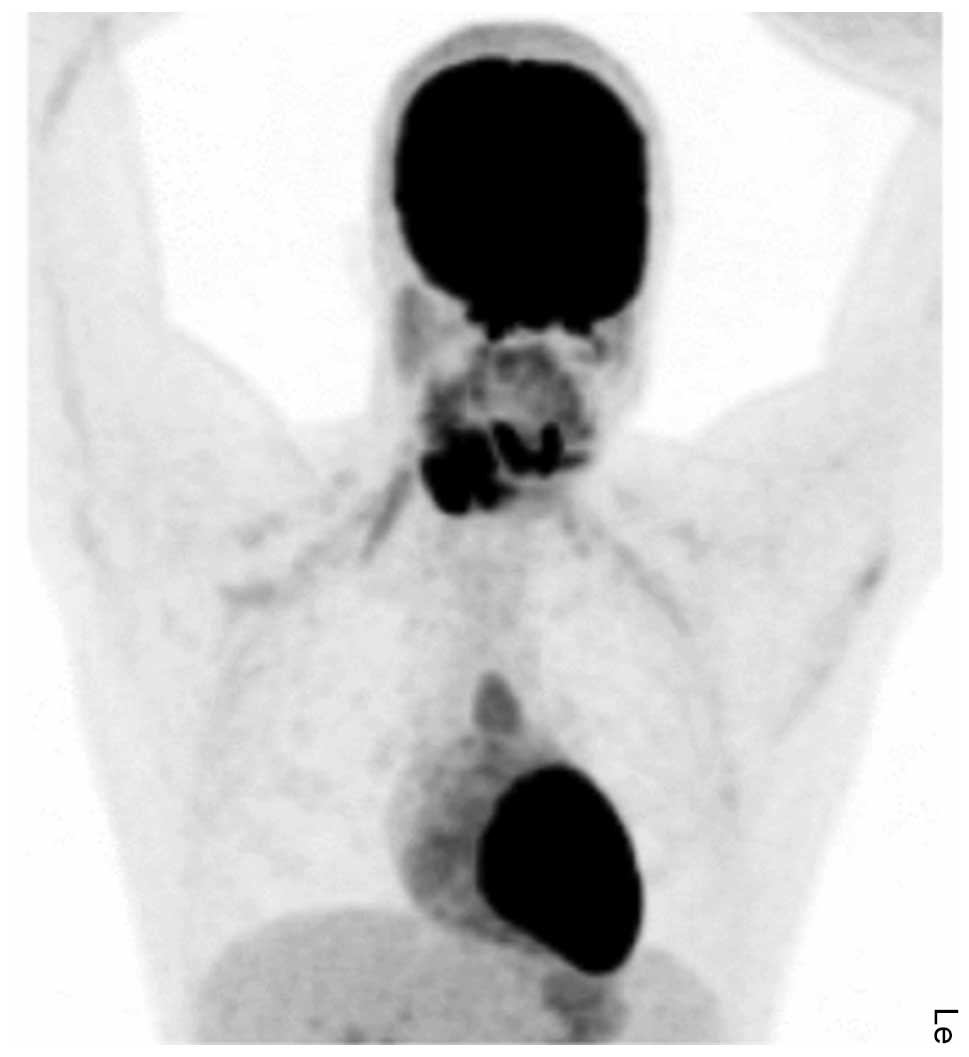
odontogenic cyst. In addition to the known lesion, positron
emission tomography revealed a limited hypermetabolic mass in the
anterior mediastinum (Fig. 8).
The case was categorized as pT4pN0 and a
multidisciplinary decision was made for the patient to undergo
complete surgical excision of the lesion, followed by postoperative
radiotherapy (60 Gy in 30 sessions of 2 Gy each).
Genetic sequencing did not identify any mutations in
the patched 1 gene; therefore, the presence of basal cell nevus
syndrome was highly improbable.
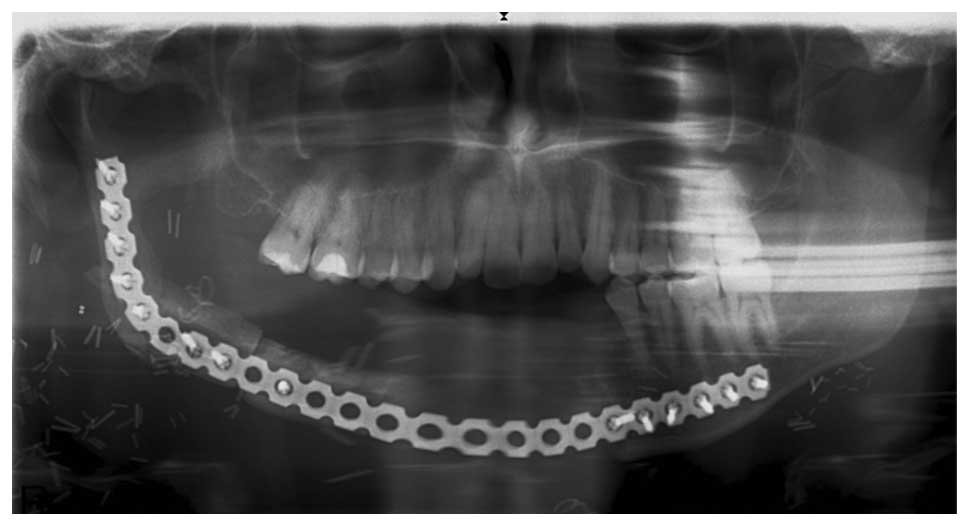
In June 2013, a right hemimandibulectomy was
performed from the left canine region to the right subcondylar
region, in addition to a supraomohyoid right neck dissection.
Resection margins were all clear, and no tumor was observed in the
lymph nodes. Reconstruction with an osteomyocutaneous fibular flap
was performed (Figs. 9 and 10).
During the postoperative final lesion biopsy, a
well-differentiated SCC was confirmed, with a 7.5-cm diameter,
expansive growth without lymphatic or perineural invasion, and free
surgical margins. No metastasis was observed in the level I, II and
III lymph nodes (Fig. 11).
The reconstruction with a free, vascularized,
fibular graft, for reasons not well understood, became infected and
after several phases of surgical debridement, it was decided that a
new reconstruction would be performed with a second free fibular
graft from the other leg. Ultimately, the situation in the mouth
healed, though with a severe amount of scarring (Fig. 10). All biopsies subsequent to the
first resection remained negative for malignancy recurrence.
At 18 months post-resection of the SCC, a
hypermetabolic mass in the anterior mediastinum was reported, which
was diagnosed as T-cell acute lymphoblastic leukemia and treated
accordingly with chemotherapy consisting of 1.2 g/m2
cyclofosfamide (days 1 and 17), 1.4 mg/m2 vincristine
(days 3, 10, 17, 24 and 31), 45 mg/m2 daunorubicine
(days 3 and 4) and 10,000 U/m2 asparaginase (days 3, 5,
7, 9, 11, 13 and 15).
At the 2-year follow-up, the patient was without
further evidence of recurrence. Due to the fact that the patient
preferred to continue treatment in a hospital closer to his
residence, there has not been follow-up of the patient in
University Hospitals Leuven since November 2015.
Discussion
PIOSCC is a form of SCC that arises within the jaw
bones from remnants of the odontogenic epithelium, without a
connection to the oral mucosa (1).
According to the 2005 WHO classification of tumors, PIOSCC is
subcategorized into three different types: i) PIOSCC solid type
(de novo); ii) PIOSCC derived from keratocystic odontogenic
tumors; and iii) PIOSCC derived from odontogenic cysts (9). Bodner et al (1) reported that the majority of PIOSCC cases
(85%) are associated with moderately- or well-differentiated SCCs.
In addition, the overall survival rate of patients with a PIOSCC at
2 and 5 years was 62 and 38%, respectively (1).
Despite classification of the disease improving
throughout the years, the etiology and pathogenesis remain to be
fully understood, and the factors underlying the malignant
transformation of the benign cystic lining of odontogenic cysts are
not known (12). It has been
suggested that chronic inflammation from infection of an
odontogenic cyst may serve as a key factor in carcinogenesis
(13–15). This is based on the observation that
chronic infiltration of plasma cells and lymphocytes in the
connective tissue of the cyst wall accompanied the malignant
transformation of cyst epithelium (1).
The current case presented with a persistent
infection that did not respond to treatment. It is likely that the
discharge and recurrent infections were caused by the underlying
malignancy. Since infected odontogenic cysts tend to respond to
treatment with intravenous or oral antibiotics, persistent
infections associated with odontogenic cysts should encourage the
clinician to consider the possibility of an underlying malignancy
(16). The possibility of chronic
infection leading to tumor formation cannot be excluded.
Radiological and clinical characteristics of PIOSCCs
are similar to those of benign odontogenic cysts and tumors. The
most commonly reported symptoms in patients with PIOSCC are
swelling and pain, and the mandible is more frequently involved
(79%) than the maxilla (21%) (1,17). Sensory
disturbances, including numbness and paresthesia, were also
experienced in the present case. A sensory disturbance of the jaw,
particularly one with no history of trauma, should always be
regarded as a potential symptom of malignancy, including PIOSCC
(18).
In certain cases, early-stage PIOSCC may mimic
routine dental disorders, such as periapical and periodontal
disease, which may lead to misdiagnosis or delayed diagnosis
(15,17). As malignant tumors that have
metastasized to the jaw from distant sites, tumors originating from
the maxillary sinus, and alveolar carcinomas that have invaded the
bone from the surface must be ruled out, the definitive diagnosis
of PIOSCC is often challenging (11).
The treatment of choice for PIOSCC is surgery and/or
radiation therapy (18,19). Surgery consists of en bloc resection
of the lesion followed by reconstruction and, if indicated, neck
dissection (16). Postoperatively,
chemoradiotherapy may be more effective than radiotherapy alone
(10).
Although genetic counseling in the present case
identified no familial factors, the conversion to a malignant tumor
and the subsequent emergence of a hematological tumor raise
suspicions to the existence of a malignant predisposition of an
unknown origin.
In conclusion, the current study described a case of
PIOSCC of the mandible, which, based on its clinical course, most
likely originated from an infected odontogenic cyst. Clinicians
must be aware that PIOSCC may initially present as a routine dental
disorder, and that unsuccessful treatment and recurrences may be a
sign of underlying malignancy. Although occurrence of the
hematological malignancy in the present case may have been
coincidental, the occurrence may also be based on a shared genetic
etiology.
References
|
1
|
Bodner L, Manor E, Shear M and van der
Waal I: Primary intraosseous squamous cell carcinoma arising in an
odontogenic cyst: A clinicopathologic analysis of 116 reported
cases. J Oral Pathol Med. 40:733–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loos D: Central epidermoid carcinoma of
the jaws. Dtsch Monatsschr Zahnheilk. 31:3081913.(In German).
|
|
3
|
Willis RA: Pathology of Tumors. Mosby; St.
Louis: pp. 310–316. 1948
|
|
4
|
Shear M: Primary intra-alveolar epidermoid
carcinoma of the jaw. J Pathol. 97:645–651. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pindborg JJ, Kramer IRH and Torloni H:
International Histological Classification of Tumours No. 5:
Histological Typing of Odontogenic TumoursJaw Cysts and Allied
Lesions. World Health Organization; pp. 35–36. 1972
|
|
6
|
Elzay RP: Primary intraosseous carcinoma
of the jaws: Review and update of odontogenic carcinoma. Oral Surg
Oral Med Oral Pathol. 54:299–303. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slootweg PJ and Müller H: Malignant
ameloblastoma or ameloblastic carcinoma. Oral Surg. 57:168–176.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Waldron CA and Mustoe TA: Primary
intraosseous carcinoma of mandible with probable origin in an
odontogenic cyst. Oral Surg Oral Med Oral Pathol. 67:716–724. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eversole LR, Siar CH and van der Waal I:
Primary intraosseous squamous cell carcinomasWorld Health
Organization Classification of Tumors. Pathology and Genetics Head
and Neck Tumors. Barnes L, Evson JW, Reichart P and Sidransky D:
Lyon: IARC Press; pp. 290–291. 2005
|
|
10
|
Woolgar JA, Triantafyllou A, Ferlito A,
Devaney KO, Lewis JS Jr, Rinaldo A, Slootweg PJ and Barnes L:
Intraosseous carcinoma of the jaws: A clinicopathologic review.
Part III: Primary intraosseous squamous cell carcinoma. Head Neck.
35:906–909. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suei Y, Tanimoto K, Taguchi A and Wada T:
Primary intraosseous carcinoma: Review of the literature and
diagnostic criteria. J Oral Maxillofac Surg. 52:580–583. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jain M, Mittal S and Gupta DK: Primary
intraosseous squamous cell carcinoma arising in odontogenic cysts:
An insight in pathogenesis. J Oral Maxillofac Surg. 71:e7–e14.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gardner AF: The odontogenic cyst as a
potential carcinoma: A clinicopathologic appraisal. J Am Dent
Assoc. 78:746–755. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: Implications for
therapy. J Dent Res. 87:14–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan B, Yan TS, Shermin L, Teck KC, Yoke
PC, Goh C and Balakrishnan A: Malignant transformation of
keratocystic odontogenic tumor: Two case reports. Am J Otolaryngol.
34:357–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Müller S and Waldron CA: Primary
intraosseous squamous carcinoma. Report of two cases. Int J Oral
Maxillofac Surg. 20:362–365. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thomas G, Pandey M, Mathew A, Abraham EK,
Francis A, Somanathan T, Iype M, Sebastian P and Nair MK: Primary
intraosseous carcinoma of the jaw: Pooled analysis of world
literature and report of two new cases. Int J Oral Maxillofac Surg.
30:349–355. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zwetyenga N, Pinsolle J, Rivel J,
Majoufre-Lefebvre C, Faucher A and Pinsolle V: Primary intraosseous
carcinoma of the jaws. Arch Otolaryngol Head Neck Surg.
127:794–797. 2001.PubMed/NCBI
|