Introduction
Lung cancer is the leading cause of
cancer-associated mortality in the USA. In 2015, there was an
estimated 221,200 novel cases of lung and bronchial cancer
diagnosed, and 158,040 mortalities were attributed to lung cancer
(1). In China, lung cancer-associated
mortality accounts for >20% of all cancer mortalities (2). Currently, chemotherapy regimens
containing platinum are the primary therapies for advanced
non-small cell lung cancer (NSCLC) (3). However, the efficacy of chemotherapy is
influenced by the resistance to platinum in the tumor tissue,
leading to variations in efficacy between individuals (4). Several mechanisms have been proposed to
explain the resistance of cancer cells to chemotherapy (5–7). It has
been demonstrated in previous studies that resistance to platinum
in lung cancer cells could be enhanced by P-glycoprotein (P-gP)
expression, which is upregulated by the multi-drug resistance-1
gene (8,9). In addition, other studies have
demonstrated that an imbalance in the expression of cell
apoptosis-related regulatory proteins, such as P53 and
Bcl2-associated X protein (Bax), can inhibit tumor cell apoptosis,
causing lung cancer cells to become resistant to platinum (10,11).
Radionuclide therapy is an important method of tumor
irradiation. The combination of 125I implantation in
tumor tissue and chemotherapy has an important role in the clinical
treatment of lung cancer, and this regimen has a significantly
positive efficacy compared with chemotherapy alone (12). However, to the best of our knowledge,
no studies have been performed regarding the therapeutic mechanism
of radionuclides in drug-resistant tumor cells. Highly concentrated
radioactivity also results in toxic side effects for patients, such
as myelosuppression and aplastic anemia (13), limiting the dosage of radionuclide
therapy that can be used in the clinic. Therefore, it is critical
to identify an effective radiosensitizer to reduce the side effects
of radionuclide therapy.
Gambogic acid (GA) is extracted from gamboge and the
monomers are purified. The molecular formula of GA is
C38H44O9 (molecular weight, 628.34
g/mol) and its chemical structure is known. GA is an effective
anti-tumor agent that has been shown to have multiple effects on
several types of solid human tumors in vitro and in
vivo (14,15). For example, studies have demonstrated
that GA can upregulate the expression of Bax and P53, and
downregulate the expression of B cell lymphoma-2 (Bcl-2),
inhibiting tumor cell apoptosis (16,17). GA
has also been implicated in several mechanisms of cisplatin
resistance (18). Furthermore, it has
exhibited detectable effects on patients with lung cancer,
colorectal cancer and renal cell carcinoma (19–21). Thus,
GA has been approved for evaluation in a phase II clinical trial
for NSCLC in China (approval no. 2004L00333) (22).
In vitro experiments were conducted in the
current study to determine whether NaI131 is able to
inhibit platinum resistance in cisplatin-resistant A549/DDP NSCLC
cells and whether GA is an effective NaI131
radiosensitizer during the treatment of A549/DDP cells. The present
study also aimed to determine the potential mechanism(s) of
GA-associated NaI131 radiosensitization in lung
cancer.
Materials and methods
Materials
Human cisplatin-resistant NSCLC cells were provided
by Dr Zhibo Hou (Department of Pneumology, Nanjing Chest Hospital,
Medical School of Southeast University, Nanjing, China). MTT and
mouse monoclonal anti-P-gP (catalog no. ab3366), anti-Bcl-2
(catalog no. ab692), anti-Bax (catalog no. ab77566), anti-P53
(catalog no. ab28), anti-β-actin (catalog no. ab8226) antibodies,
and goat anti-mouse IgG secondary antibody (catalog no. ab6789)
were acquired from Abcam (Cambridge, MA, USA). The secondary
antibody used for immunocytochemistry, goat anti-mouse
IgG/horseradish peroxidase-conjugated antibody, was purchased from
ZSGB-BIO (Beijing, China).
Cell culture
Cells were cultured in RPMI-l640 medium supplemented
10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Cells were digested with 0.25%
EDTA-phosphate-buffered saline (PBS) during the logarithmic
proliferation phase. Cell suspensions were transferred to frozen
vessels and stored at 4°C for 30 min, −20°C for 1 h, and −80°C
overnight in liquid nitrogen.
MTT assay
A549/DDP cells in the logarithmic proliferation
phase were randomly assigned to the NaI131 intervention
group, GA intervention group or control group. The A549/DDP cells
were seeded into 96-well plates (1×104-1×105
cells/well). The NaI131 group was treated with
NaI131 5.7, 11.4, 17.1, 22.8, 28.5 or 34.1 MBq; the GA
group was treated with GA 0.5, 1.0, 1.5, 2.0 or 3.0 µg/ml; and the
control group was treated with equal volumes of PBS. The cells were
incubated in a standard cell culture incubator at 37°C with 5%
CO2. After 48 h of cell culture, 5 mg/ml MTT (20
µl/well) was added to the media and the cells were additionally
incubated for 4 h. Dimethylsulfoxide (150 µl; Sigma-Aldrich, St.
Louis, MO, USA) was added to the cells in each of the wells after
the media was removed, and the cells were further incubated for 10
min. The optical density (OD) of each well was measured using a
microplate reader (Multiskan™ GO Microplate Spectrophotometer;
Thermo Fisher Scientific, Inc.) at 560 nm. All experiments were
performed in triplicate, and results were analyzed according to the
following formula: Cell inhibitory rate (%) = (1- OD test group /
OD control group) × 100.
Treatment with 5.7 MBq NaI131 and 0.2
µg/ml GA was also performed; these drug concentrations were
empirically determined based on the various doses of
NaI131 and GA evaluated, which were then used to measure
the half maximal inhibitory concentrations (IC50) of the
two drugs. Graphical representations of the isobolographic
analysis, performed as previously described (23,24), were
used to determine whether NaI131 and GA synergistically
inhibit the proliferation of A549/DDP cells.
Apoptosis and protein detection
Cell treatment
A549/DDP cells in the logarithmic proliferation
phase were assigned to the NaI131, GA, NaI131
combined with GA or control group. The A549/DDP cells were seeded
into 96-well plates (1×104-1×105 cells/well).
Based on IC50 values, the NaI131 group was
treated with 17.5 MBq NaI131; the GA group was treated
with 1.5 µg/ml GA; and the combined treatment group was treated
with 17.5 MBq NaI131 and 0.2 µg/ml GA. The control group
was treated with an equal volume of PBS.
Apoptosis analysis by flow cytometry
After 48 h of culture (at 37°C with 5%
CO2), cell apoptosis was analyzed using Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI)
iodide kit (Kaiji Bio, Co., Nanjing, China). Adherent and floating
cells were harvested and disaggregated to a single-cell suspension.
Staining was performed according to the manufacturer's protocols.
The data were analyzed by flow cytometry using CXP2.2 software
(Beckman Coulter, Inc., Brea, CA, USA).
Bcl-2, P-gP, Bax and P53 immunocytochemistry
After 48 h of culture (at 37°C with 5%
CO2), 5×105 cells/ml were placed on Culture
Slides (BD Biosciences, Bedford, MA, USA). The slides were washed
with PBS, and fixed using 4% paraformaldehyde, permeabilized with
0.5% Triton X-100, incubated with 3% hydrogen peroxide and then
maintained in a blocking solution composed of PBS with 3% albumin
from bovine serum (Sigma-Aldrich). The slides were subsequently
incubated overnight at 4°C with specific primary antibodies against
Bcl-2, P-gP, Bax and P53 (dilution, 1:200). Subsequently, the cells
were washed with PBS, and incubated with diluted biotinylated
secondary antibody and then with VECTASTAIN® ABC Reagent
(Dako Italia, Milan, Italy). Finally, the slides were washed,
incubated with peroxidase substrate solution until desired stain
intensity developed (Peroxidase/DAB; Dako Italia), rinsed in tap
water, counterstained with Mayer's hematoxylin and mounted with
BioMount. The results of immunocytochemical staining was negative
(−), weak positive (+) and positive (++) and strong positive (+++).
Analysis of the OD data was performed using ImageJ software
(version 1.37; National Institutes of Health, Bethesda, MD, USA).
The results were expressed as the mean ± standard deviation
(%).
Western blot analysis
Cell protein was extracted using 500 µl
radioimmunoprecipitation assay lysis buffer with 5 µl
phenylmethylsulfonyl fluoride and protease inhibitor (Abcam,
Cambridge, UK). Total proteins were quantified using the
Bicinchoninic Acid Assay (Beyotime Institute of Biotechnology,
Shanghai, China), according to the manufacturer's protocols. Total
protein (20 µg) was loaded onto an 8% sodium dodecyl
sulfate-polyacrylamide gel and transferred to a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA). The
membrane was incubated for 1 h at 25°C in 5% skimmed dried milk and
then washed three times for 5 min at 25°C using blocking buffer.
Subsequently, the membrane was incubated overnight at 4°C with
specific primary antibodies for P-gp, p53, Bax and Bcl-2. All
antibodies were diluted to 1:1,000. Subsequent to being washed
three times with TBST for 5 min each, the membranes were incubated
for 1 h with horseradish peroxidase-conjugated secondary antibodies
(dilution, 1:5,000). In order to evaluate protein expression
accurately, β-actin was used as an internal standard. Band
intensity was analyzed with ImageJ software, and protein expression
was presented as the ratio of the protein band intensity to β-actin
in the same blot.
Statistical analysis
Every experiment was performed in triplicate, and
each group was comprised of three duplicate wells. Statistics were
analyzed with SPSS (version 19.0; IBM SPSS, Armonk, NY, USA). All
data are presented as the mean ± standard deviation. Graphs were
plotted using Microsoft Office Excel 2007 (Microsoft Inc., Redmond,
WA, USA). Differences between groups were analyzed by one-way
analysis of variance or independent sample t-tests. The SNK test
are used at the second stage of the analysis of variance if the
null hypothesis was rejected. P<0.05 was considered to indicate
a statistically significant difference.
Results
Cell proliferation inhibition rates
per group
Inhibitory effect of NaI131 or GA alone on
A549/DDP cells
The inhibition of A549/DDP cell proliferation after
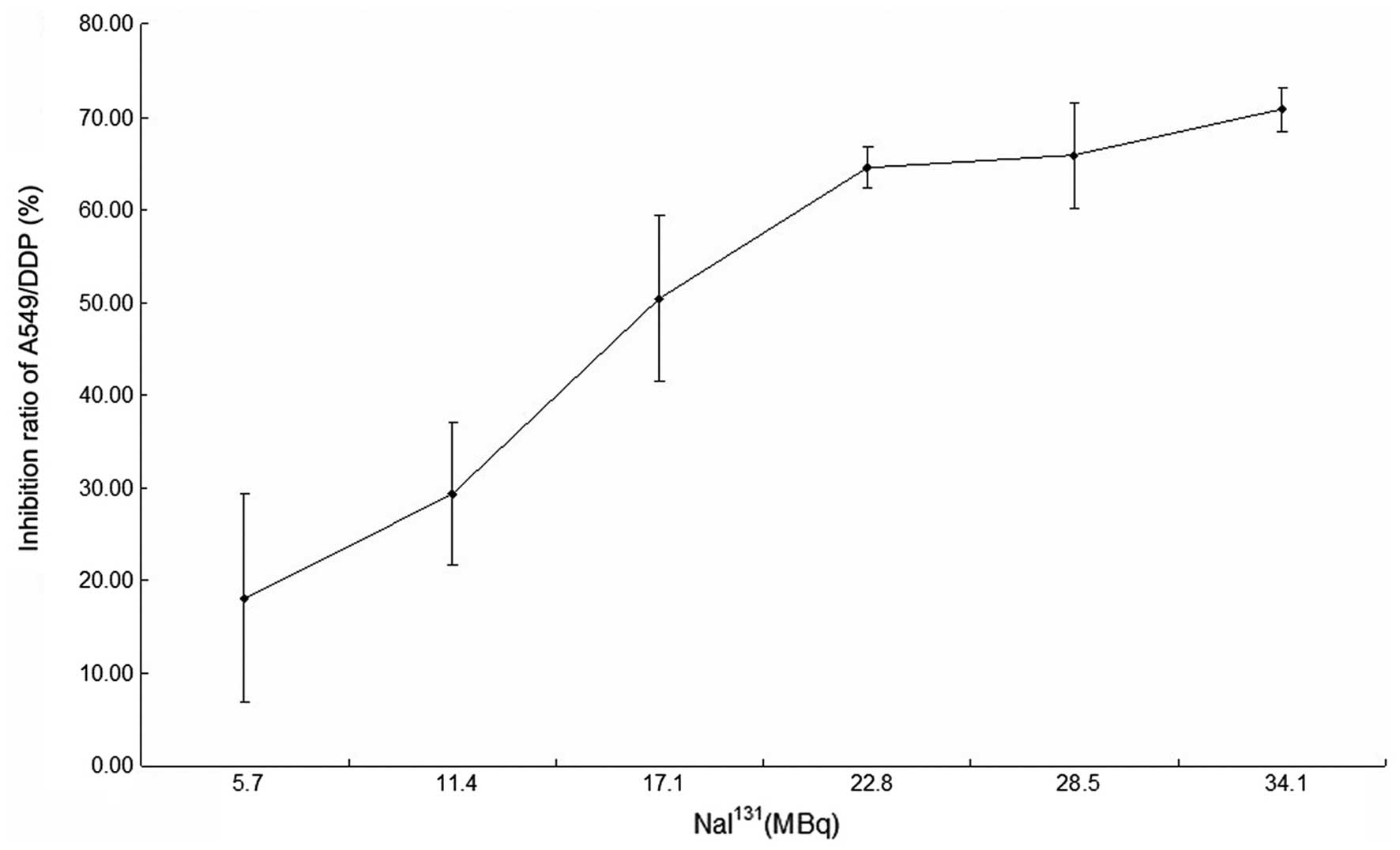
48 h of treatment with NaI131 is shown in Table I. The half maximal inhibitory
concentration (IC50) value was 17.54±1.86 MBq (Fig. 1).
 | Table I.Cell proliferation inhibition ratio
of A549/DDP cells following treatment with various doses of
NaI131. |
Table I.
Cell proliferation inhibition ratio
of A549/DDP cells following treatment with various doses of
NaI131.
| NaI131,
MBq | Inhibition ratio of
A549/DDP cells, a |
|---|
| 5.7 | 18.12±11.16 |
| 11.4 | 29.31±7.61 |
| 17.1 | 50.43±9.00 |
| 22.8 | 64.52±2.32 |
| 28.5 | 65.82±5.67 |
| 34.1 | 70.78±2.29 |
The cell proliferation inhibition ratio of A549/DDP
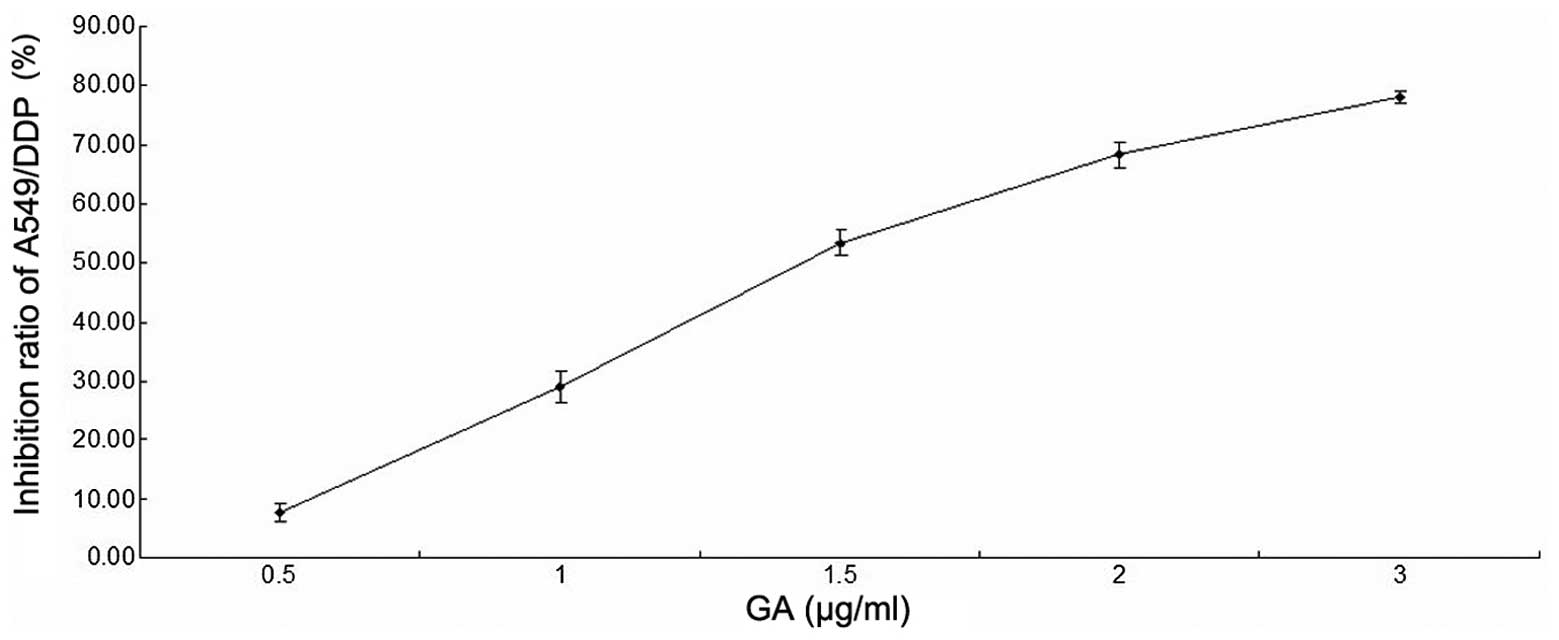
cells after 48 h of GA treatment is shown in Table II. The IC50 value was
1.46±0.07 µg/ml (Fig. 2).
 | Table II.Cell proliferation inhibition ratio
of A549/DDP cells following treatment with various doses of GA. |
Table II.
Cell proliferation inhibition ratio
of A549/DDP cells following treatment with various doses of GA.
| GA, µg/ml | Inhibition ratio of
A549/DDP cells, a |
|---|
| 0.5 | 7.67±1.53 |
| 1.0 | 29.00±2.65 |
| 1.5 | 53.33±2.08 |
| 2.0 | 68.33±2.08 |
| 3.0 | 78.00±1.00 |
Inhibitory effect of NaI131 combined
with GA on A549/DDP cells
NaI131 and GA administered singly would
kill a limited number of cells. Preincubation of A549/DDP cells
with the usual GA dose (1.46 µg/ml) followed by their exposure to
NaI131 (17.54 MBq) results in the rapid death of all
cells. Therefore, in principle, a combination consisting of a low
dose of GA and appropriately adjusted NaI131 dosage
would be effective. Consequently, doses of 5.7 MBq
NaI131 and 0.2 µg/ml GA were empirically determined for
the combined treatment. The effects of various doses of
NaI131 and GA on cell proliferation were determined by
the MTT assay, and the proliferation inhibition rates of the two
groups were calculated. The proliferation inhibition rates of
A549/DDP cells after treatment with NaI131 (5.7 MBq) and
GA (various doses) for 48 h are shown in Table III. The IC50 value of GA
was 0.22±0.03 µg/ml. The cell proliferation inhibition rates of
A549/DDP cells after 48 h of treatment with GA (0.2 µg/ml) and
NaI131 (various doses) are indicated in Table IV. The IC50 value of
NaI131 was 7.14±0.88 MBq.
 | Table III.Cell proliferation inhibition rate of
A549/DDP cells following treatment with NaI131 (5.7 MBq)
and GA (various doses). |
Table III.
Cell proliferation inhibition rate of
A549/DDP cells following treatment with NaI131 (5.7 MBq)
and GA (various doses).
| GA, µg/ml | Inhibition ratio of
A549/DDP cells, a |
|---|
| 0.1 | 33.10±4.30 |
| 0.2 | 40.30±6.00 |
| 0.3 | 52.83±8.56 |
| 0.4 | 75.16±3.47 |
 | Table IV.Cell proliferation inhibition rate of
A549/DDP cells following treatment with GA (0.2 µg/ml) and
NaI131 (various doses). |
Table IV.
Cell proliferation inhibition rate of
A549/DDP cells following treatment with GA (0.2 µg/ml) and
NaI131 (various doses).
| NaI131,
MBq | Inhibition ratio of
A549/DDP cells, a |
|---|
| 5.7 | 44.82±5.52 |
| 11.4 | 59.89±3.07 |
| 17.1 | 73.72±3.69 |
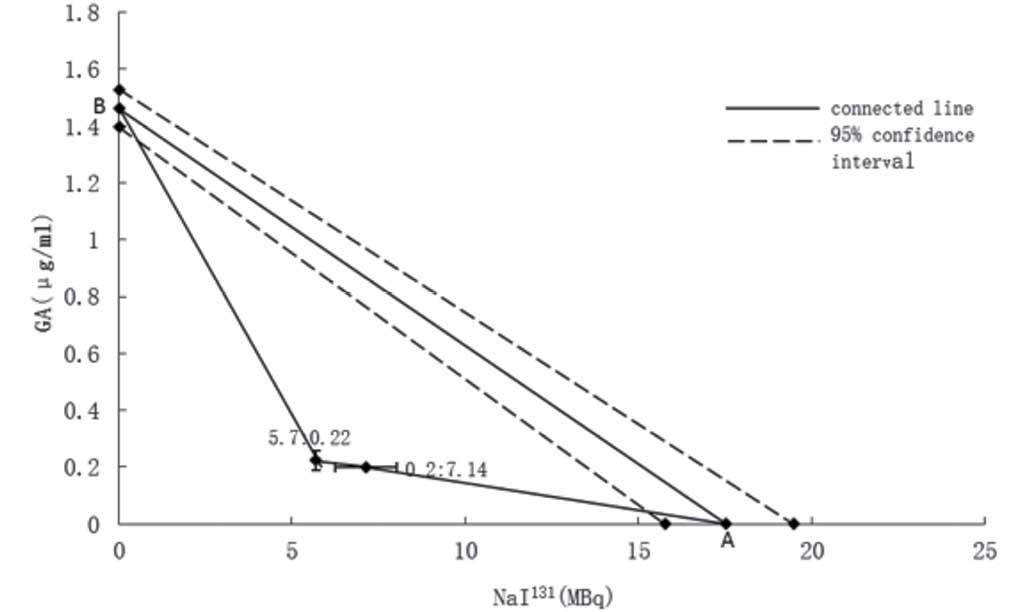
Based on the aforementioned data, two composite
points were plotted: i) 5.7 MBq NaI131 and 0.22±0.03
µg/ml GA; and ii) 0.2 µg/ml GA and 7.14±0.88 MBq NaI131.
In A549/DDP cells, the IC50 of NaI131 was
17.54±1.86 MBq (point A) and the IC50 of GA was
1.46±0.07 µg/ml (point B) (Fig. 3).
According to the isobolographic analysis, the straight line
connecting A and B is the locus of points (dose pairs) that will
produce this effect in a simply additive combination. Two composite
points are in the lower left from the hypotenuse, suggesting that
NaI131 and GA synergistically inhibit A549/DDP cell
proliferation.
Apoptosis detection analysis
Annexin V-FITC and PI flow cytometry revealed that
the cell count ratios in the Q4 quadrant were significantly
increased in the NaI131, GA and combined treatment
groups compared with the control group, indicating an increase in
the rate of early apoptosis. Furthermore, compared with the control
group, the cell count ratio in the Q2 quadrant (late apoptosis) was
significantly increased in the NaI131, GA and combined
treatment groups. In addition, the number of cells in early or late
apoptosis in the combined treatment group were increased
significantly compared with those in the NaI131 and GA
alone groups. Total apoptosis rates also showed the same patterns
(Table V; Fig. 4).
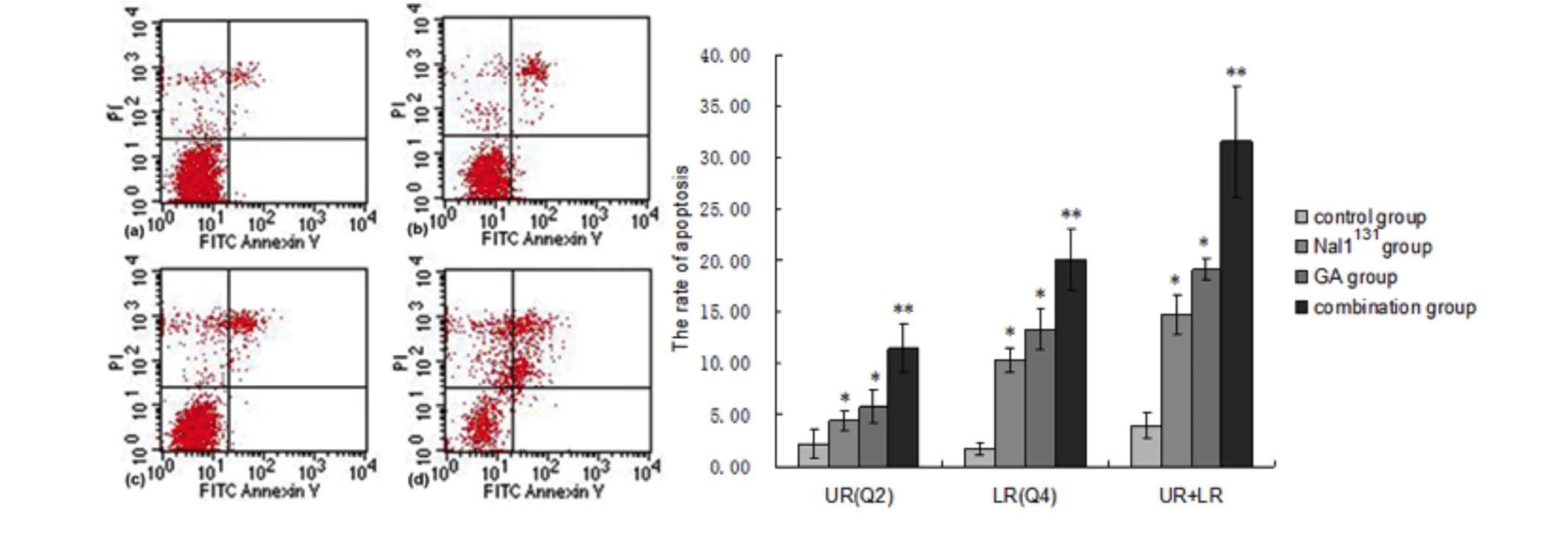 | Figure 4.Cell apoptosis was analyzed using
flow cytometry after 48 h of cell culture for the control,
NaI131, GA and combined treatment group. Data are
presented as representative graphs in the left panel. Q2 and Q4
flow cytometry data are quantified in the right panel. UL, dead
cells; UR, late apoptosis; LL, cell survival; LR, early apoptosis.
Data are presented as the mean ± standard deviation. *P<0.05 vs.
control group; **P<0.05 vs. control, NaI131 and GA
groups. PI, propidium iodide; FITC, fluorescein isothiocyanate; GA,
gambogic acid; UL, upper left; UR, upper right; LL, lower left; LR,
lower right. |
 | Table V.Cell apoptosis was analyzed using
flow cytometry following 48 h of cell culture. |
Table V.
Cell apoptosis was analyzed using
flow cytometry following 48 h of cell culture.
|
| Apoptosis, % |
|---|
|
|
|
|---|
| Treatment
group | Early | Late | Total |
|---|
| Control | 1.74±0.60 | 2.21±1.33 | 3.95±1.24 |
|
NaI131 |
10.39±1.17a |
4.39±0.96a |
14.78±1.91a |
| GA |
13.38±1.96a |
5.73±1.58a |
19.11±1.03a |
| Combined |
20.06±3.02b |
11.49±2.34b |
31.55±5.32b |
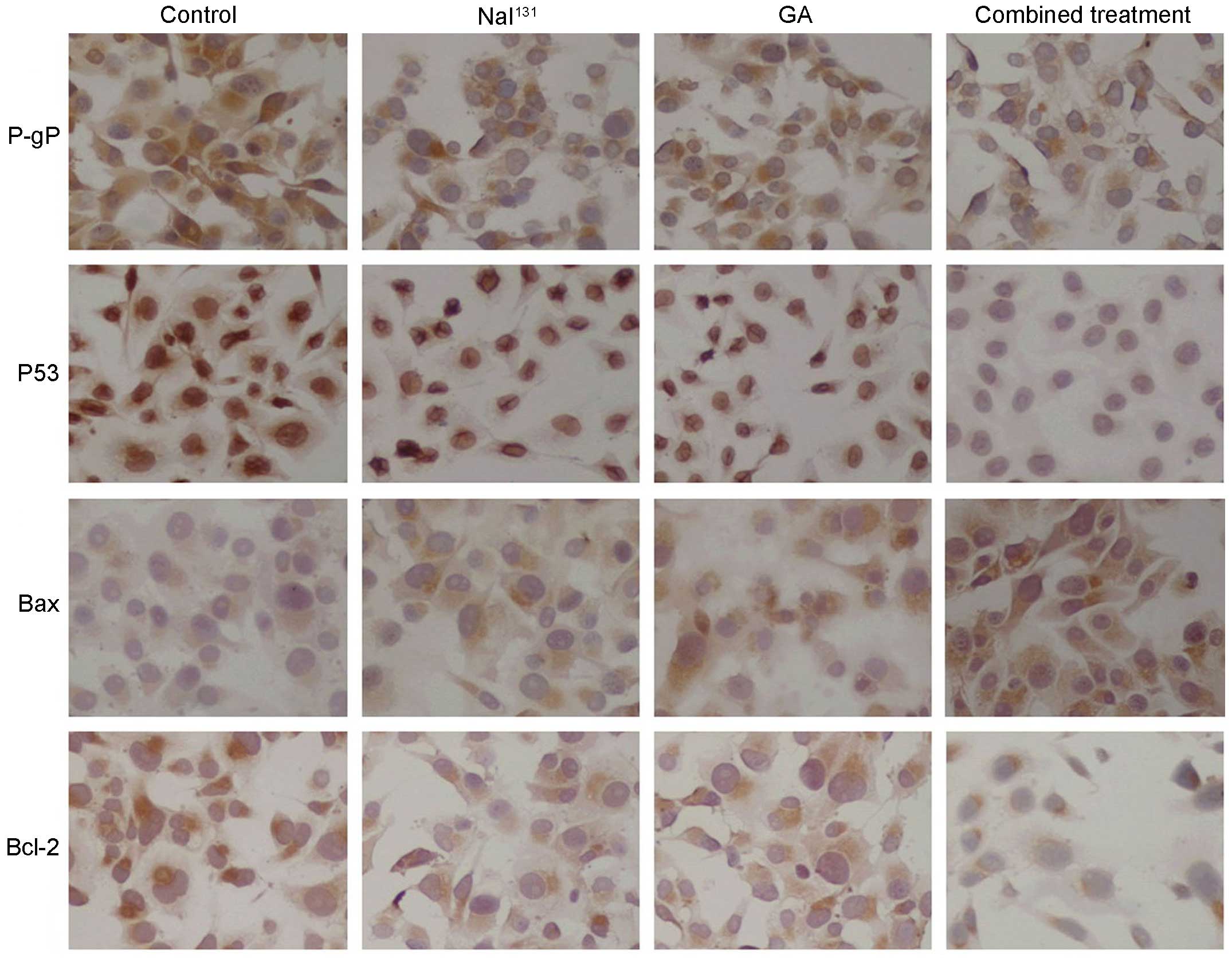
Intracellular P-gP, Bcl-2, Bax and P53 protein
expression detected by immunocytochemistry
The P-gP protein was detected by immunostaining with
granular coloring observed in the cell membrane and cytoplasm
surrounding the nucleus. Expression was strongly positive (+++) in
the control cells, positive (+) in the NaI131 and GA
cells, and moderate (±) in the combined treatment cells. P53
protein was primarily detected in the nucleus. Its expression was
positive (++) in the control group, weakly positive (+) in the
NaI131 and GA groups, and moderate (±) in the combined
treatment group. Bax protein was detected with cytoplasmic
staining. It showed moderate expression (±) in the control group,
weakly positive expression (+) in the NaI131 and GA
groups, and positive expression (++) in the combined treatment
group. Bcl-2 protein was detected by immunostaining with granular
coloring primarily observed in the cell membrane and cytoplasm. Its
expression was positive (++) in the control group, weakly positive
(+) in the NaI131 and GA groups, and moderate (±) in the
combined treatment group (Fig.
5).
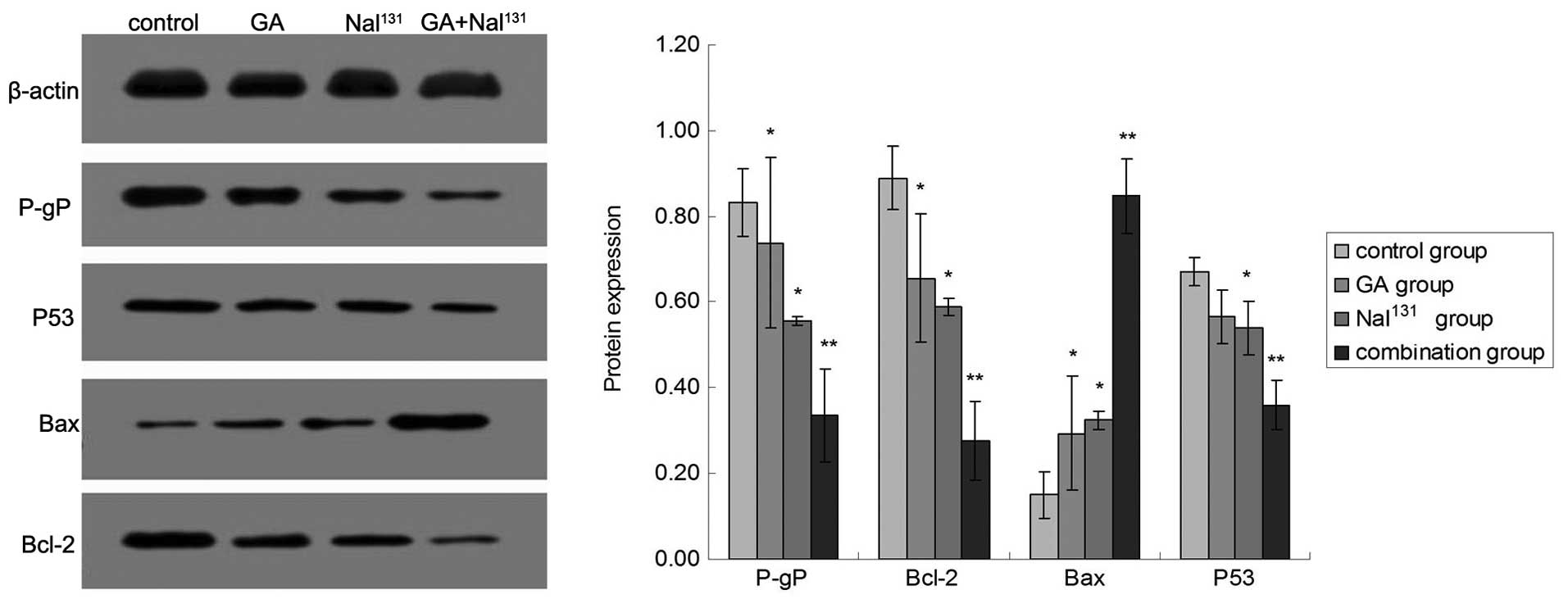
P-gP, Bcl-2, Bax and P53 protein expression
detected by western blot analysis
The protein expression levels of P-gP, Bcl-2 and P53
significantly decreased, while the expression level of Bax protein
significantly increased in the combined treatment group compared
with the control, NaI131 and GA groups. Similarly, P-gP,
Bcl-2 and P53 levels significantly decreased and Bax levels
significantly increased compared in the NaI131 and GA
alone groups compared with the control group (Table VI; Fig.
6).
 | Table VI.Expression of P-gP, Bcl-2, P53 and
Bax protein expression as detected by western blot assay. |
Table VI.
Expression of P-gP, Bcl-2, P53 and
Bax protein expression as detected by western blot assay.
|
| Protein expression
level |
|---|
|
|
|
|---|
| Treatment
group | P-gP | Bax | Bcl-2 | P53 |
|---|
| Control | 0.83±0.08 | 0.15±0.01 | 0.89±0.20 | 0.67±0.11 |
|
NaI131 |
0.55±0.05a |
0.32±0.02a |
0.59±0.13a | 0.54±0.09 |
| GA |
0.74±0.07a |
0.29±0.02a |
0.66±0.15a |
0.57±0.09a |
| Combined |
0.34±0.03b |
0.85±0.06b |
0.28±0.06b |
0.36±0.06b |
Discussion
Previous studies have demonstrated that cisplatin
resistance in NSCLC cells occurs via several mechanisms (8–11). These
mechanisms of resistance primarily involve the abnormal expression
of drug transporters and drug metabolizing enzymes in cells
(25), increased DNA damage repair
(26), abnormal regulation of cell
cycle and apoptosis-related protein expression (27).
In general, implanting radioactive particles into
tumor tissue is supplemented with chemotherapy (28,29).
Radiation can induce the apoptosis of tumor cells through
activation of the P53 and Bax genes, and inhibition of the Bcl-2
gene (30–33). The current results suggest that
NaI131 also induces apoptosis by regulating P53, Bax and
Bcl-2 expression in A549/DDP cells. However, due to their lack of
specificity and strong binding force, radionuclides can cause
extensive damage to non-targeted cells. The presence of nuclear
particles in the normal tissue surrounding the tumor lesion
commonly causes localized and systemic side effects during nuclide
particle therapy (13,34–36), which
limits their value in the clinic. Several studies have found that
GA can promote lung cancer cell apoptosis by multiple mechanisms
(37–40). Furthermore, GA has been reported to
act as a sensitizer for chemotherapy. A previous analysis of the
ability of GA to sensitize lung cancer for chemotherapy
demonstrated positive results (41).
According to the results of the isobolographic analysis performed
in the present study, we hypothesize that a combination of GA and
I131 may have a synergistic effect on the inhibition of
A549/DDP cells. Thus, only a low dose of GA combined with
I131 is required to significantly increase the rate of
apoptosis in A549/DDP cells.
P-gP is a membrane transport protein that can efflux
intracellular anticancer agents out of tumor cells, increasing a
patient's resistance to antitumor drugs (42). The current results suggest that GA and
I131 alone may decrease the expression of P-gP. In
addition, compared with GA or I131 treatment alone,
I131 combined with low doses of GA was better able to
inhibit the expression of P-gP. Application of this treatment
strategy may, therefore, further weaken the capacity of tumor cells
to efflux intracellular drugs, partially reversing the multidrug
resistance of cells.
It is widely accepted that the protein ratio of Bax
and Bcl-2 has an important role in apoptosis. Apoptosis is
considered to be promoted in cells with high Bax protein expression
and inhibited in cells with high Bcl-2 protein expression. Several
previous studies have also confirmed the role of both proteins in
lung cancer cells (43–45). The current results suggest that in
A549/DDP cells, I131 and GA alone are able to regulate
the protein expression levels of Bcl-2 and Bax. Compared with the
groups treated with each agent alone, the protein expression of
Bcl-2 was significantly decreased and that of Bax was significantly
increased in the I131 combined with GA group. Thus, we
hypothesize that a low dose of GA can enhance the ability of
I131 to regulate Bcl-2 and Bax expression, which may
promote apoptosis in A549/DDP cells.
Other previous studies of lung cancer have found
that wild-type P53 can promote tumor cell apoptosis (46,47),
increasing the chemosensitivity of tumor cells (48,49).
However, mutant P53 can promote chemotherapeutic tolerance in tumor
cells via several factors, such as decreased pro-apoptotic
capacity, increased DNA repair function (50,51) and
upregulated P-gP expression (9,52). It has
been confirmed that the wild-type P53 protein cannot be detected by
immunohistochemical techniques due to its instability, rapid
hydrolysis and short half-life, as well as for other unknown
reasons. However, mutant P53 protein can be detected by
immunohistochemical methods reasonably well due to its relative
stability and longer half-life (50,53). The
results of the current immunocytochemical analysis and western blot
assay suggest that A549/DDP cells exhibit high P53 protein
expression levels, and treatment with GA or I131 alone
was able to reduce this expression. In addition, compared with the
groups treated with each agent alone, P53 protein expression was
further decreased in the I131 combined with GA group,
suggesting that the combination of these two compounds may promote
apoptosis in A549/DDP cells by regulating the expression of mutant
P53.
In conclusion, the current results suggest that the
effect of NaI131 on A549/DDP cells may promote apoptosis
by increasing P53 and Bax protein expression, while reducing Bcl-2
protein expression. Treatment with NaI131 combined with
a low dose of GA was more capable of promoting apoptosis compared
with the two monotherapies. Additional protein detection suggested
that the protein expression levels of P-gP, P53 and Bcl-2 were
markedly decreased and the protein expression of Bax was
significantly increased in the combined treatment group compared
with each monotherapy group. These results suggest that low-dose GA
may enhance the pro-apoptotic role of NaI131
administered to A549/DDP cells in cisplatin-resistant NSCLC.
Therefore, low-dose GA may be a sensitizer to NaI131
radiotherapy, since reducing the dosage of NaI131 used
in the clinic would reduce the toxic side effects caused by
radiotherapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81372480 and
81202032).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen ZM, Peto R, Iona A, Guo Y, Chen YP,
Bian Z, Yang L, Zhang WY, Lu F, Chen JS, et al: Emerging
tobacco-related cancer risks in China: A nationwide, prospective
study of 0.5 million adults. Cancer 121 Suppl. 17:3097–3106. 2015.
View Article : Google Scholar
|
|
3
|
Wood DE, Kazerooni E, Baum SL, Dransfield
MT, Eapen GA, Ettinger DS, Hou L, Jackman DM, Klippenstein D, Kumar
R, et al: Lung cancer screening, version 1.2015: Featured updates
to the NCCN guidelines. J Natl Compr Canc Netw. 13:23–34.
2015.PubMed/NCBI
|
|
4
|
Chang A: Chemotherapy, chemoresistance and
the changing treatment landscape for NSCLC. Lung Cancer. 71:3–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bowden NA: Nucleotide excision repair: Why
is it not used to predict response to platinum-based chemotherapy.
Cancer Lett. 346:163–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamagishi T, Sahni S, Sharp DM, Arvind A,
Jansson PJ and Richardson DR: P-glycoprotein mediates drug
resistance via a novel mechanism involving lysosomal sequestration.
J Biol Chem. 288:31761–31771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Selivanova G: Wild type p53 reactivation:
From lab bench to clinic. FEBS Lett. 588:2628–2638. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Triller N, Korosec P, Kern I, Kosnik M and
Debeljak A: Multidrug resistance in small cell lung cancer:
Expression of P-glycoprotein, multidrug resistance protein 1 and
lung resistance protein in chemo-naive patients and in relapsed
disease. Lung Cancer. 54:235–240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Podolski-Renić A, Jadranin M, Stanković T,
Banković J, Stojković S, Chiourea M, Aljančić I, Vajs V, Tešević V,
Ruždijić S, et al: Molecular and cytogenetic changes in multi-drug
resistant cancer cells and their influence on new compounds
testing. Cancer Chemother Pharmacol. 72:683–697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chung SK, Zhu S, Xu Y and Fu X: Functional
analysis of the acetylation of human p53 in DNA damage responses.
Protein Cell. 5:544–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chakraborty S, Mazumdar M, Mukherjee S,
Bhattacharjee P, Adhikary A, Manna A, Chakraborty S, Khan P, Sen A
and Das T: Restoration of p53/miR-34a regulatory axis decreases
survival advantage and ensures Bax-dependent apoptosis of non-small
cell lung carcinoma cells. FEBS Lett. 588:549–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang YL, Meng N, Wang JJ, Jiang P, Yuan
HSh, Liu C, Qu A and Yang RJ: CT-guided iodine-125 seed permanent
implantation for recurrent head and neck cancers. Radiat Oncol.
5:682010.PubMed/NCBI
|
|
13
|
Schroeder T, Kuendgen A, Kayser S, Kröger
N, Braulke F, Platzbecker U, Klärner V, Zohren F, Haase D, Stadler
M, et al: Therapy-related myeloid neoplasms following treatment
with radioiodine. Haematologica. 97:206–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yi T, Yi Z, Cho SG, Luo J, Pandey MK,
Aggarwal BB and Liu M: Gambogic acid inhibits angiogenesis and
prostate tumor growth by suppressing vascular endothelial growth
factor receptor 2 signaling. Cancer Res. 68:1843–1850. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu H, You Q, Liu W, Yang Y, Zhao L, Qi Q,
Zhao J, Wang J, Lu N, Ling H, et al: Gambogic acid induced tumor
cell apoptosis by T lymphocyte activation in H22 transplanted mice.
Int Immunopharmacol. 8:1493–1502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang LH, Li Y, Yang SN, Wang FY, Hou Y,
Cui W, Chen K, Cao Q, Wang S, Zhang TY, et al: Gambogic acid
synergistically potentiates cisplatin-induced apoptosis in
non-small-cell lung cancer through suppressing NF-κB and MAPK/HO-1
signalling. Br J Cancer. 110:341–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Qi Q, Lu N, Dai Q, Li F, Wang X, You
Q and Guo Q: Gambogic acid promotes apoptosis and resistance to
metastatic potential in MDA-MB-231 human breast carcinoma cells.
Biochem Cell Biol. 90:718–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin Y, Meng L, Hu C, Duan W, Zuo Z, Lin L,
Zhang X and Ding J: Gambogic acid inhibits the catalytic activity
of human topoisomerase IIalpha by binding to its ATPase domain. Mol
Cancer Ther. 6:2429–2440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li D, Yang H, Li R, Wang Y, Wang W, Li D,
Ma S and Zhang X: Antitumor activity of gambogic acid on NCI-H1993
xenografts via MET signaling pathway downregulation. Oncol Lett.
10:2802–2806. 2015.PubMed/NCBI
|
|
20
|
Wen C, Huang L, Chen J, Lin M, Li W, Lu B,
Rutnam ZJ, Iwamoto A, Wang Z, Yang X and Liu H: Gambogic acid
inhibits growth, induces apoptosis, and overcomes drug resistance
in human colorectal cancer cells. Int J Oncol. 47:1663–1671.
2015.PubMed/NCBI
|
|
21
|
Jang JH, Kim JY, Sung EG, Kim EA and Lee
TJ: Gambogic acid induces apoptosis and sensitizes TRAIL-mediated
apoptosis through downregulation of cFLIPL in renal carcinoma Caki
cells. Int J Oncol. 48:376–384. 2016.PubMed/NCBI
|
|
22
|
Wang X, Deng R, Lu Y, Xu Q, Yan M, Ye D
and Chen W: Gambogic acid as a non-competitive inhibitor of
ATP-binding cassette transporter B1 reverses the multidrug
resistance of human epithelial cancers by promoting ATP-binding
cassette transporter B1 protein degradation. Basic Clin Pharmacol
Toxicol. 112:25–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wellman PJ, Tow S and McMahon L:
Isobolographic assessment of the effects of combinations of
phenylpropanolamine and fenfluramine on food intake in rats.
Pharmacol Biochem Behav. 50:287–291. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gessner PK: Isobolographic analysis of
interactions: An update on applications and utility. Toxicology.
105:161–179. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiou JF, Liang JA, Hsu WH, Wang JJ, Ho ST
and Kao A: Comparing the relationship of Taxol-based chemotherapy
response with P-glycoprotein and lung resistance-related protein
expression in non-small cell lung cancer. Lung. 181:267–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oliver TG, Mercer KL, Sayles LC, Burke JR,
Mendus D, Lovejoy KS, Cheng MH, Subramanian A, Mu D, Powers S, et
al: Chronic cisplatin treatment promotes enhanced damage repair and
tumor progression in a mouse model of lung cancer. Genes Dev.
24:837–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cetintas VB, Kucukaslan AS, Kosova B,
Tetik A, Selvi N, Cok G, Gunduz C and Eroglu Z: Cisplatin
resistance induced by decreased apoptotic activity in
non-small-cell lung cancer cell lines. Cell Biol Int. 36:261–265.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kroger LA, DeNardo GL, Gumerlock PH, Xiong
CY, Winthrop MD, Shi XB, Mack PC, Leshchinsky T and DeNardo SJ:
Apoptosis-related gene and protein expression in human lymphoma
xenografts (Raji) after low dose rate radiation using
67Cu-2IT-BAT-Lym-1 radioimmunotherapy. Cancer Biother Radiopharm.
16:213–225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kemény-Beke A, Berényi E, Facskó A,
Damjanovich J, Horváth A, Bodnár A, Berta A and Aradi J:
Antiproliferative effect of 4-thiouridylate on OCM-1 uveal melanoma
cells. Eur J Ophthalmol. 16:680–685. 2006.PubMed/NCBI
|
|
30
|
Szostak MJ, Kaur P, Amin P, Jacobs SC and
Kyprianou N: Apoptosis and bcl-2 expression in prostate cancer:
Significance in clinical outcome after brachytherapy. J Urol.
165:2126–2130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oka K, Suzuki Y, Iida H and Nakano T:
Radiation therapy induces the p53 (+) p21 (−) expression in
squamous cell carcinomas of the uterine cervix. Gynecol Oncol.
93:340–344. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brantley MA Jr, Worley L and Harbour JW:
Altered expression of Rb and p53 in uveal melanomas following
plaque radiotherapy. Am J Ophthalmol. 133:242–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harima Y, Nagata K, Harima K, Oka A,
Ostapenko VV, Shikata N, Ohnishi T and Tanaka Y: Bax and Bcl-2
protein expression following radiation therapy versus radiation
plus thermoradiotherapy in stage IIIB cervical carcinoma. Cancer.
88:132–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakada K, Ishibashi T, Takei T, Hirata K,
Shinohara K, Katoh S, Zhao S, Tamaki N, Noguchi Y and Noguchi S:
Does lemon candy decrease salivary gland damage after radioiodine
therapy for thyroid cancer? J Nucl Med. 46:261–266. 2005.PubMed/NCBI
|
|
35
|
Fallahi B, Adabi K, Majidi M,
Fard-Esfahani A, Heshmat R, Larijani B and Haghpanah V: Incidence
of second primary malignancies during a long-term surveillance of
patients with differentiated thyroid carcinoma in relation to
radioiodine treatment. Clin Nucl Med. 36:277–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iyer NG, Morris LG, Tuttle RM, Shaha AR
and Ganly I: Rising incidence of second cancers in patients with
low-risk (T1N0) thyroid cancer who receive radioactive iodine
therapy. Cancer. 117:4439–4446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Q, Cheng H, Zhu G, Yang L, Zhou A, Wang
X, Fang N, Xia L, Su J, Wang M, et al: Gambogenic acid inhibits
proliferation of A549 cells through apoptosis-inducing and cell
cycle arresting. Biol Pharm Bull. 33:415–420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qi Q, Lu N, Li C, Zhao J, Liu W, You Q and
Guo Q: Involvement of RECK in gambogic acid induced anti-invasive
effect in A549 human lung carcinoma cells. Mol Carcinog. 54:(Suppl
1). E13–E25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu X, Zhang H, Lin Y, Chen P, Min J, Wang
Z, Xiao W and Chen B: Mechanisms of gambogic acid-induced apoptosis
in non-small cell lung cancer cells in relation to transferrin
receptors. J Chemother. 21:666–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu ZQ, Guo QL, You QD, Zhao L and Gu HY:
Gambogic acid inhibits proliferation of human lung carcinoma SPC-A1
cells in vivo and in vitro and represses telomerase activity and
telomerase reverse transcriptase mRNA expression in the cells. Biol
Pharm Bull. 27:1769–1774. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang LH, Yang JY, Yang SN, Li Y, Ping GF,
Hou Y, Cui W, Wang ZZ, Xiao W and Wu CF: Suppression of NF-κB
signaling and P-glycoprotein function by gambogic acid
synergistically potentiates adriamycin-induced apoptosis in lung
cancer. Curr Cancer Drug Targets. 14:91–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sharom FJ: Complex interplay between the
P-glycoprotein multidrug efflux pump and the membrane: Its role in
modulating protein function. Front Oncol. 4:412014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bai L, Chen J, McEachern D, Liu L, Zhou H,
Aguilar A and Wang S: BM-1197: A novel and specific Bcl-2/Bcl-xL
inhibitor inducing complete and long-lasting tumor regression in
vivo. PLoS One. 9:e994042014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamaguchi M: The anti-apoptotic effect of
regucalcin is mediated through multisignaling pathways. Apoptosis.
18:1145–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mai CW, Yaeghoobi M, Abd-Rahman N, Kang YB
and Pichika MR: Chalcones with electron-withdrawing and
electron-donating substituents: Anticancer activity against TRAIL
resistant cancer cells, structure-activity relationship analysis
and regulation of apoptotic proteins. Eur J Med Chem. 77:378–387.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rohr UP, Wulf MA, Stahn S, Heyd F, Steidl
U, Fenk R, Opalka B, Pitschke G, Prisack HB, Bojar H, et al:
Non-small lung cancer cells are prime targets for p53 gene transfer
mediated by a recombinant adeno-associated virus type-2 vector.
Cancer Gene Ther. 10:898–906. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cuddihy AR, Jalali F, Coackley C and
Bristow RG: WTp53 induction does not override MTp53 chemoresistance
and radioresistance due to gain-of-function in lung cancer cells.
Mol Cancer Ther. 7:980–992. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lai SL, Perng RP and Hwang J: p53 gene
status modulates the chemosensitivity of non-small cell lung cancer
cells. J Biomed Sci. 7:64–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Osaki S, Nakanishi Y, Takayama K, Pei XH,
Ueno H and Hara N: Alteration of drug chemosensitivity caused by
the adenovirus-mediated transfer of the wild-type p53 gene in human
lung cancer cells. Cancer Gene Ther. 7:300–307. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Brattström D, Bergqvist M, Lamberg K,
Kraaz W, Scheibenflug L, Gustafsson G, Inganäs M, Wagenius G and
Brodin O: Complete sequence of p53 gene in 20 patients with lung
cancer: Comparison with chemosensitivity and immunohistochemistry.
Med Oncol. 15:255–261. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Blandino G, Levine AJ and Oren M: Mutant
p53 gain of function: Differential effects of different p53 mutants
on resistance of cultured cells to chemotherapy. Oncogene.
18:477–485. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gu J, Tang Y, Liu Y, Guo H, Wang Y, Cai L,
Li Y and Wang B: Murine double minute 2 siRNA and wild-type p53
gene therapy enhances sensitivity of the SKOV3/DDP ovarian cancer
cell line to cisplatin chemotherapy in vitro and in vivo. Cancer
Lett. 343:200–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Levine AJ, Momand J and Finlay CA: The p53
tumour suppressor gene. Nature. 351:453–456. 1991. View Article : Google Scholar : PubMed/NCBI
|