Introduction
Primary breast lymphoma (PBL), a rare lymphoma
subtype, was first described in 1959 (1), and accounts for <3% of extranodal
lymphomas, ~1% of all non-Hodgkin lymphoma (NHL) and 0.5% of breast
malignancies (2–7). Female patients account for >95% of
PBL cases (3–13) and the most frequently occurring
histological subtype is diffuse large B-cell lymphoma (DLBCL)
(14). The definition of PBL, as
proposed by Wiseman and Liao (15),
and modified by Hugh et al (16), is the presence of breast tissue in
close proximity to lymphoma, with no antecedent diagnosis of
lymphoma and no extramammary disease other than ipsilateral
axillary nodes (15,16). In addition, it has been suggested to
include patients presenting with lymphoma of regional
(supraclavicular and internal mammary) nodes and bilateral breast
lymphoma (14).
Previously, the International Extranodal Lymphoma
Study Group reported the largest retrospective series of 204
patients with PBL and concluded that the combination of limited
surgery, anthracycline-containing chemotherapy, and involved-field
radiotherapy produced the best outcome for PBL (5). For patients with primary breast DLBCL,
rituximab was recommended (14).
However, due to the limited number of patients, prolonged time
span, combined primary and secondary breast involvement, and low-
and high-grade malignant lymphomas, PBL prognosis remains poorly
defined. The purpose of the present study was to summarize the
clinical characteristics of PBL and evaluate its management
approaches.
Materials and methods
Patients and patient workup
Ethical approval was obtained from the Independent
Ethics Committee of Zhejiang Cancer Hospital (Hangzhou, China). A
total of 29 patients (1 male and 28 female) newly diagnosed with
PBL and treated between April 2006 and May 2013 were
retrospectively evaluated. All records were considered valuable if
there was available data on patient demographics, pathological
diagnoses, tumor details, therapeutic outcomes and follow-ups.
The pretreatment workup included obtaining a
complete patient history and conducting a physical examination,
liver and renal biochemical analysis, complete blood cell count,
bone marrow biopsy, and computed tomography of the chest, abdomen
and pelvis. Staging classification was performed according to the
Ann Arbor classification (17) and
histopathological diagnosis was based on the World Health
Organization nomenclature (18).
When data were available, the stage-modified
international prognostic index (IPI) score was defined for each
patient included in the study. This score was established by Miller
et al (19) and gives one
point each for age, increased serum lactate dehydrogenase (LDH) and
Eastern Cooperative Oncology Group (ECOG) performance status 2 or
higher.
Treatment protocol
Following diagnosis of DLBCL using a core needle or
surgery, chemotherapy alone or in combination with radiotherapy was
administered. The chemotherapy consisted of between 4 and 6 cycles
of treatment with
cyclophosphamide-doxorubicin-vincristine-prednisone (CHOP) or a
CHOP-like regimen. Chemotherapy was administered with or without
central nervous system (CNS) prophylaxis, consisting of intrathecal
methotrexate or cytarabine. The radiotherapy consisted of treatment
with between 15 and 25 site-directed radiotherapy sessions, of
between 1.8 and 2.0 Gy/session (total, 30–46 Gy), in the month
following the completion of the chemotherapy program. Rituximab was
recommended for patients with primary breast DLBCL. For other PBL
histological subtypes, treatment was confirmed by the
multidisciplinary lymphoma team of Zhejiang Cancer Hospital. The
efficacy of treatment was assessed according to the International
Workshop to standardize response criteria for NHLs (20).
Follow-up and statistical
analysis
Follow-up was performed by the oncologic outpatient
clinic, and patients or relatives were contacted by telephone. The
final follow-up was in June 2015. SPSS (version 17.0; SPSS, Inc.,
Chicago, IL, USA) software was used for statistical analysis.
Kaplan-Meier estimators were used to calculate the overall survival
(OS) and progression-free survival (PFS) rates. OS was measured
from the date of diagnosis to the date of death or final follow-up.
PFS was defined as the length of time from the date of diagnosis to
the date of initial disease progression or death. Survival curves
were plotted using the Kaplan-Meier estimator and compared using
the log-rank test. Univariate analysis was performed to determine
prognostic factors. P<0.05 was considered to indicate a
statistically significant difference and all P-values were
two-tailed.
Results
Baseline characteristics
A total of 29 patients were analyzed
retrospectively. The baseline characteristics are listed in
Table I. In total, 28 patients were
female (96.6%) and 1 patient was male (3.4%). The median age was 50
years (range, 24–69). None of the patients had a previous history
of benign or malignant breast disease, or breast implantation. The
most frequent presentation was with a palpable mass (96.6%) and
3.4% presented with palpable axillary lymph nodes. Left breast
involvement was similar to right (44.8 vs. 41.4%, respectively) and
4 (13.8%) patients presented with bilateral breast involvement. The
median tumor size was 4 cm (range, 1–10 cm). A total of 16 (55.2%)
patients presented with stage IE disease and 13 (44.8%) with stage
IIE. A total of 2 (6.9%) patients presented with B-symptoms. The
majority of patients (72.4%) presented with a low stage-modified
IPI score of between 0 and 1. The most frequent histopathological
types were as follows: DLBCL, 82.8%; marginal zone lymphoma (MZL),
6.9%; anaplastic large cell lymphoma (ALCL), 6.9%; and mantle cell
lymphoma (MCL), 3.4%. Germinal center (GC) or non-germinal center
(non-GC) phenotypic information based on immunohistochemistry using
the Hans method (21) were available
in 14/24 patients with DLBCL: GC, 6 patients; non-GCB, 8 patients;
and undefined, 10 patients.
 | Table I.Clinical characteristics of the 29
patients evaluated. |
Table I.
Clinical characteristics of the 29
patients evaluated.
| Characteristic | Patient no., % |
|---|
| Gender |
| Male | 1 (3.4) |
|
Female | 28 (96.6) |
| Age, years |
|
|
Median | 50 |
|
Range | 24–69 |
| ECOG performance
status at presentation |
|
| 0 | 14 (48.3) |
| 1 | 15 (51.7) |
| Laterality |
|
|
Right | 12 (41.4) |
| Left | 13 (44.8) |
|
Bilateral | 4 (13.8) |
| Tumor
sizea, cm |
|
|
Median | 4 |
|
Range | 1–10 |
| Nodal site
involvement at diagnosis |
|
| None | 16 (55.2) |
|
Axillary | 11 (37.9) |
|
Supraclavicular +
axillary | 2 (6.9) |
| Pregnant at
diagnosis |
|
| Yes | 0 (0.0) |
| Lactating at
diagnosis |
|
| Yes | 1 (3.4) |
| No | 28 (96.6) |
| Lactate dehydrogenase
levels |
|
|
Elevated | 8 (27.6) |
|
Wild-type | 21 (72.4) |
| Presence of
B-symptoms |
|
|
Absent | 27 (93.1) |
|
Present | 2 (6.9) |
| Ann Arbor stage |
|
| IE | 16 (55.2) |
| IIE | 13 (44.8) |
| Adjusted IPI |
|
| 0 | 10 (34.5) |
| 1 | 11 (37.9) |
| 2 | 7
(24.1) |
| 3 | 1 (3.4) |
| Pathological
classification |
|
|
DLBCL | 24 (82.8) |
| ALCL | 2 (6.9) |
| MZL | 2 (6.9) |
| MCL | 1 (3.4) |
Treatment and response
The first-line therapy administered is summarized in
Table II. The majority of patients
(93.1%) received chemotherapy, of which four patients received CNS
prophylaxis consisting of intrathecal methotrexate (n=3), or
cytarabine (n=1). The chemotherapeutic treatment regime was
supplemented with rituximab in 11 patients. Radiation therapy was
administered in 13 (44.8%) patients to give a median total dose of
36 Gy (range, 30–46 Gy). Among the 27 patients treated with
chemotherapy: 21 (77.8%) exhibited a complete response; 5 (18.5%)
exhibited a partial response; and 1 (3.7%) exhibited disease
progression.
 | Table II.Summary of the first-line treatment
administered. |
Table II.
Summary of the first-line treatment
administered.
| Treatment type | Patient no., n
(%) |
|---|
| Regime |
| Surgery
alone | 2 (6.9) |
|
Chemotherapy alone | 3 (10.3) |
|
Radiation and
chemotherapy | 5 (17.2) |
| Surgery
and chemotherapy | 11 (37.9) |
|
Surgery, chemotherapy, and
radiation | 8 (27.6) |
| Surgery (n=21) |
|
Lumpectomy | 16 (76.2) |
|
Modified
mastectomya | 5 (23.8) |
|
Chemotherapyb (n=27) |
|
Anthracycline | 27 (100.0) |
|
Rituximab | 11 (40.7) |
| Cycle no. |
|
<4 | 1 (3.7) |
|
4–6 | 23 (85.2) |
|
>6 | 3 (11.1) |
| Radiation |
|
| Fields (n=13) |
|
| Breast
only | 4 (30.8) |
| Breast
and regional lymph nodes | 9 (69.2) |
| Radiation dose
(Gy) |
|
|
Median | 36 |
|
Range | 30–46 |
The median follow-up time for all patients was 66.8
(range, 25.4–110.0) months. By the final follow-up session, 22
patients were alive without lymphoma and 7 patients had succumbed
to PBL. A total of 6 patients succumbed to lymphoma-associated
mortality, including 1 patient who developed progressive disease
during chemotherapy, and 1 patient succumbed to
chemotherapy-associated hepatic failure. Among the 5 patients who
relapsed, 4 (80.0%) relapsed within the first two years. One
patient who presented with bilateral breast involvement developed
left breast relapse following lumpectomy and chemotherapy, 2
patients developed lymphoma of the bone marrow, 1 patient developed
relapses of the lung and mediastinal lymph nodes, and 1 patient
developed lymphoma of the skin. No patients developed relapses of
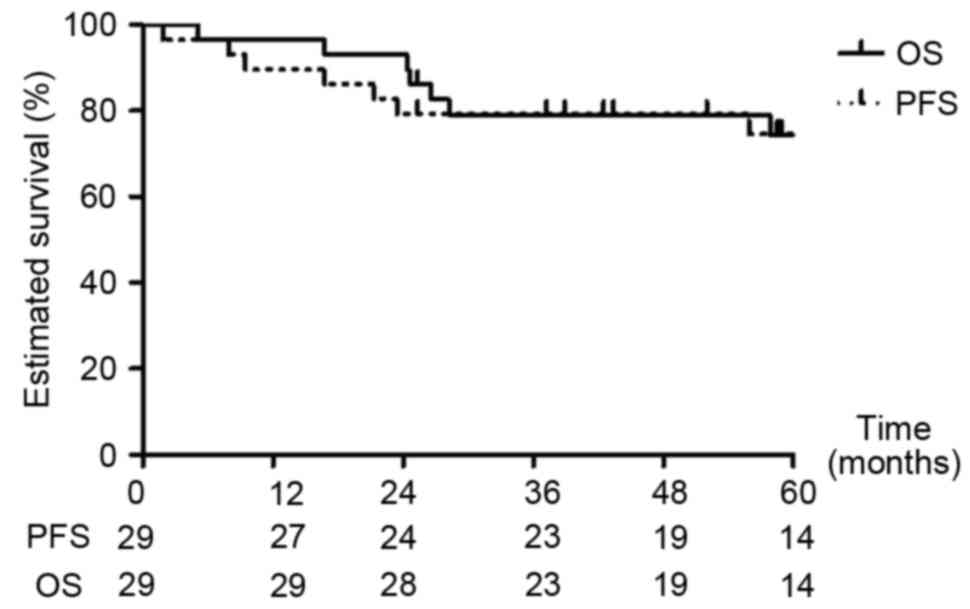
the CNS. Kaplan-Meier estimator analysis predicted the 1-, 3- and
5-year PFS rates of all patients to be 89.7, 79.3 and 74.6%,
respectively (Fig. 1). Kaplan-Meier
estimator analysis predicted the 1-, 3- and 5-year OS rates to be
96.6, 79.0 and 74.4%, respectively (Fig.
1).
Outcome in patients with MZL, ALCL and
MCL
The patient with MZL, who received a lumpectomy and
five cycles of treatment with CHOP, was alive and disease-free by
the final follow-up session. Of the 2 patients with ALCL, the
patient who received a lumpectomy, five cycles of treatment with
CHOP and 36 Gy of radiotherapy (18 sessions/day at 2.0 Gy/session),
succumbed to lung and mediastinal lymph node relapse after 26.6
months. The other patient, who received hyperfractionated
cyclophosphamide, vincristine, Adriamycin and dexamethasone/1A
alternating with high-dose methotrexate and cytarabine/1B was alive
and disease-free by the final follow-up. Of the 2 patients with
MCL, the patient who received a lumpectomy and six cycles of
treatment with R-CHOP [rituximab (375 mg/m2),
cyclophosphamide (750 mg/m2), doxorubicin (50
mg/m2) and vincristine (1.4 mg/m2, to a
maximum of 2 mg), administered intravenously on day 1 and 100 mg
oral prednisone on days 1–5] succumbed to a relapse of the bone
marrow after 57.9 months. The other patient, who received a
lumpectomy alone was alive and disease-free by the final
follow-up.
Prognostic factors
The value of various potential prognostic factors,
including age, ECOG performance status at presentation, tumor size,
laterality, LDH levels, Ann Arbor stage, adjusted IPI value,
surgery, cycles of chemotherapy received (>4), administration of
rituximab and administration of radiotherapy, in predicting PFS and
OS were evaluated. The impact of the prognostic factors is listed
in Table III. The 5-year PFS rates
for patients with bilateral and unilateral breast involvement were
50.0 and 78.4%, respectively (P=0.146 bilateral vs. unilateral).
The 5-year OS for patients with bilateral and unilateral breast
involvement was 50.0 and 78.1%, respectively (P=0.129 bilateral vs.
unilateral). No statistically significant difference was observed
in PFS and OS rates between the patients treated with rituximab and
those without.
 | Table III.Univariate analysis of the impact of
various prognostic factors on the results of treatment. |
Table III.
Univariate analysis of the impact of
various prognostic factors on the results of treatment.
|
| 5-year PFS | 5-year OS |
|---|
|
|
|
|
|---|
| Prognostic
factor | Rate, % | P-value | Rate, % | P-value |
|---|
| Age |
| 0.257 |
| 0.273 |
| ≥50
years | 65.2 |
| 85.1 |
|
| <50
years | 85.7 |
| 65.2 |
|
| ECOG performance
status at presentation |
| 0.666 |
| 0.617 |
| 0 | 77.1 |
| 77.1 |
|
| 1 | 73.3 |
| 72.7 |
|
| Tumor size |
| 0.812 |
| 0.886 |
| ≥4
cm | 72.0 |
| 72.0 |
|
| <4
cm | 78.6 |
| 78.6 |
|
| Laterality |
| 0.146 |
| 0.129 |
|
Bilateral | 50.0 |
| 50.0 |
|
|
Unilateral | 78.4 |
| 78.1 |
|
| Lactate
dehydrogenase levels |
| 0.281 |
| 0.309 |
|
Elevated | 60.0 |
| 60.0 |
|
|
Normal | 81.0 |
| 80.4 |
|
| Ann Arbor
stage |
| 0.084 |
| 0.071 |
| IE | 85.9 |
| 85.6 |
|
|
IIE | 61.5 |
| 61.5 |
|
| Adjusted IPI |
| 0.281 |
| 0.309 |
|
0–1 | 81.0 |
| 80.4 |
|
|
2–3 | 60.0 |
| 60.0 |
|
| Surgery |
| 0.848 |
| 0.809 |
|
Yes | 74.7 |
| 74.2 |
|
| No | 75.0 |
| 75.0 |
|
| Cycles of
chemotherapy |
| 0.398 |
| 0.437 |
|
>4 | 77.1 |
| 77.1 |
|
| ≤4 | 66.7 |
| 62.5 |
|
| Rituximab
administered |
| 0.426 |
| 0.354 |
|
Yes | 77.9 |
| 77.9 |
|
| No | 68.8 |
| 68.2 |
|
| Radiotherapy
received |
| 0.379 |
| 0.397 |
|
Yes | 83.3 |
| 83.3 |
|
| No | 68.0 |
| 67.6 |
|
Discussion
Several collaborative investigations have been
conducted to define the clinical characteristics of PBL and
evaluate its management approaches (4,5,14). The criteria of PBL defined by Wiseman
and Liao (15) were used in the
majority of these studies. This definition has been challenged as
it relies on an anatomic definition of the disease more appropriate
when assessing solid tumors compared with lymphoma (11). In addition, the definition was based
on a limited number of patients (11). However, there is insufficient data to
revise the definition of PBL to include systemic NHL, as it is
difficult to prove that the breast is the primary site of
carcinogenesis (14). Therefore, the
traditional criteria of PBL were used in the present study
(14).
Clinically, the results of the present study were
consistent with the published literature; the typical presentation
was with a solitary, unilateral breast lump by a female aged
between 50 and 60 years old (3–5,10–11). The
most frequent histology is DLBCL and the median tumor diameter is 4
cm, although masses of <20 cm have been reported (5). In contrast to previous studies, the left
breast was involved more frequently (44.8 vs. 41.4%) in the present
study (3,5,9,12,13). In
the present study, patients with PBL exhibited a 5-year OS rate of
74.4%. The 5-year OS rate has previously been reported to be
between 48 and 75% (5,12,13), and
is likely associated with the distribution of clinical
characteristics and management approaches taken.
CNS relapse occurs in between 5 and 16% of patients
with primary breast DLBCL (4,5,11,13). Increased rates of CNS relapse (3-year
cumulative incidence, 23.6 vs. 1.4%; P<0.001) have been observed
in a matched-pair analysis of primary breast and nodal DLBCL
following treatment with R-CHOP (22). The 3-year OS rates were similar
between the primary breast and nodal DLBCL groups (82.2 vs. 90.7%;
P=0.345). The authors concluded that following treatment with
rituximab, the clinical outcome of patients with primary breast
DLBCL may no longer be inferior to those with nodal DLBCL. In a
prospective study by Avilés et al (23), 0/32 patients with PBL developed CNS
relapses following treatment with rituximab and dose-dense
chemotherapy after a median follow-up of 64.5 months (range, 43–71
months). The majority of primary breast DLBCL CNS relapses occur
<2 years subsequent to treatment completion (13). In the present study, 4 patients
received CNS prophylaxis and 11 patients received treatment with
rituximab. No patients developed CNS relapses after a median
follow-up time of 66.8 (range, 25.4–110.0) months. This is likely
due to the limited number of patients, retrospective nature of the
study, and administration of intrathecal chemotherapy and
rituximab.
None of the treatments used, including surgery,
chemotherapy, radiotherapy and rituximab, were associated with OS
and PFS rates (Table III). However,
assessment of the association between treatment type and survival
was limited due to the retrospective nature of the present study
and the limited number of patients included. The only randomized
comparison to date demonstrated a significantly improved survival
rate in patients who received combined chemotherapy and
radiotherapy, compared with chemotherapy or radiotherapy alone
(24). In the present study, one
patient, who presented with bilateral breast involvement, developed
a relapse of the left breast following a lumpectomy and
chemotherapy. Additionally, a meta-analysis demonstrated that
radical surgery offers no benefit to patients with PBL (25). Although chemotherapy is now routinely
supplemented with rituximab in patients with DLBCL (26), there are no prospective randomized
clinical trials for the treatment of patients with PBL with
rituximab.
PBL appears to be a rare disease and it is therefore
difficult to characterize. However, the results of the present
study suggest that the overall prognosis of patients with PBL is
reasonable, and that the incidence of local relapse is low
following chemotherapy alone or in combination with other
treatments. Further studies into the development of effective
agents for use in treatment-resistant patients are required.
References
|
1
|
Dobrotina AF and Zlotnikova ZB:
Generalized sarcomatosis (lymphosarcomatosis) in pregnancy with
unusual bilateral involvement of the breasts. Vopr Onkol.
5:613–616. 1959.(In Russian). PubMed/NCBI
|
|
2
|
Aviv A, Tadmor T and Polliack A: Primary
diffuse large B-cell lymphoma of the breast: Looking at
pathogenesis, clinical issues and therapeutic options. Ann Oncol.
24:2236–2244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caon J, Wai ES, Hart J, Alexander C,
Truong PT, Sehn LH and Connors JM: Treatment and outcomes of
primary breast lymphoma. Clin Breast Cancer. 12:412–419. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeanneret-Sozzi W, Taghian A, Epelbaum R,
Poortmans P, Zwahlen D, Amsler B, Villette S, Belkacémi Y, Nguyen
T, Scalliet P, et al: Primary breast lymphoma: Patient profile,
outcome and prognostic factors. A multicentre Rare Cancer Network
study. BMC Cancer. 8:862008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ryan G, Martinelli G, Kuper-Hommel M,
Tsang R, Pruneri G, Yuen K, Roos D, Lennard A, Devizzi L, Crabb S,
et al: Primary diffuse large B-cell lymphoma of the breast:
Prognostic factors and outcomes of a study by the International
Extranodal Lymphoma Study Group. Ann Oncol. 19:233–241. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Talwalkar SS, Miranda RN, Valbuena JR,
Routbort MJ, Martin AW and Medeiros LJ: Lymphomas involving the
breast: A study of 106 cases comparing localized and disseminated
neoplasms. Am J Surg Pathol. 32:1299–1309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Surov A, Holzhausen HJ, Wienke A, Schmidt
J, Thomssen C, Arnold D, Ruschke K and Spielmann RP: Primary and
secondary breast lymphoma: Prevalence, clinical signs and
radiological features. Br J Radiol. 85:e195–e205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arber DA, Simpson JF, Weiss LM and
Rappaport H: Non-Hodgkin's lymphoma involving the breast. Am J Surg
Pathol. 18:288–295. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uesato M, Miyazawa Y, Gunji Y and Ochiai
T: Primary non-Hodgkin's lymphoma of the breast: Report of a case
with special reference to 380 cases in the Japanese literature.
Breast Cancer. 12:154–158. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Validire P, Capovilla M, Asselain B,
Kirova Y, Goudefroye R, Plancher C, Fourquet A, Zanni M, Gaulard P,
Vincent-Salomon A and Decaudin D: Primary breast non-Hodgkin's
lymphoma: A large single center study of initial characteristics,
natural history, and prognostic factors. Am J Hematol. 84:133–139.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yhim HY, Kang HJ, Choi YH, Kim SJ, Kim WS,
Chae YS, Kim JS, Choi CW, Oh SY, Eom HS, et al: Clinical outcomes
and prognostic factors in patients with breast diffuse large B cell
lymphoma: Consortium for Improving Survival of Lymphoma (CISL)
study. BMC Cancer. 10:3212010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao YB, Sun XF, He YN, Liu CJ and Liu H:
Clinicopathological features of thirty patients with primary breast
lymphoma and review of the literature. Med Oncol. 32:4482015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosein PJ, Maragulia JC, Salzberg MP,
Press OW, Habermann TM, Vose JM, Bast M, Advani RH, Tibshirani R,
Evens AM, et al: A multicentre study of primary breast diffuse
large B-cell lymphoma in the rituximab era. Br J Haematol.
165:358–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheah CY, Campbell BA and Seymour JF:
Primary breast lymphoma. Cancer Treat Rev. 40:900–908. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wiseman C and Liao KT: Primary lymphoma of
the breast. Cancer. 29:1705–1712. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hugh JC, Jackson FI, Hanson J and Poppema
S: Primary breast lymphoma. An immunohistologic study of 20 new
cases. Cancer. 66:2602–2611. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the committee on Hodgkin's
disease staging classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
18
|
Harris NL, Jaffe ES, Diebold J, Flandrin
G, Muller-Hermelink HK, Vardiman J, Lister TA and Bloomfield CD:
The World Health Organization classification of hematological
malignancies report of the clinical Advisory Committee Meeting,
Airlie House, Virginia, November 1997. Mod Pathol. 13:193–207.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miller TP, Dahlberg S, Cassady JR,
Adelstein DJ, Spier CM, Grogan TM, LeBlanc M, Carlin S, Chase E and
Fisher RI: Chemotherapy alone compared with chemotherapy plus
radiotherapy for localized intermediate- and high-grade
non-Hodgkin's lymphoma. N Engl J Med. 339:21–26. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheson BD, Horning JS, Coiffier B, Shipp
MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A,
Hagenbeek A, et al: Report of an international workshop to
standardize response criteria for non-Hodgkin's lymphomas. NCI
Sponsored International Working Group. J Clin Oncol. 17:1244–1253.
1999.PubMed/NCBI
|
|
21
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yhim HY, Kim JS, Kang HJ, Kim SJ, Kim WS,
Choi CW, Eom HS, Kim JA, Lee JH, Won JH, et al: Matched-pair
analysis comparing the outcomes of primary breast and nodal diffuse
large B cell lymphoma in patients treated with rituximab plus
chemotherapy. Int J Cancer. 131:235–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avilés A, Castañeda C, Neri N, Cleto S and
Nambo MJ: Rituximab and dose dense chemotherapy in primary breast
lymphoma. Haematologica. 92:1147–1148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Avilés A, Delgado S, Nambo MJ, Neri N,
Murillo E and Cleto S: Primary breast lymphoma: Results of a
controlled clinical trial. Oncology. 69:256–260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jennings WC, Baker RS, Murray SS, Howard
CA, Parker DE, Peabody LF, Vice HM, Sheehan WW and Broughan TA:
Primary breast lymphoma: The role of mastectomy and the importance
of lymph node status. Ann Surg. 245:784–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Habermann TM, Weller EA, Morrison VA,
Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI,
Peterson BA and Horning SJ: Rituximab-CHOP versus CHOP alone or
with maintenance rituximab in older patients with diffuse large
B-cell lymphoma. J Clin Oncol. 24:3121–3127. 2006. View Article : Google Scholar : PubMed/NCBI
|