Introduction
According to the WHO classification, glioblastoma
multiforme is the most common highly invasive primary glial tumor
of the human brain, which is more common in the second half of life
and occurs predominantly in men (1).
Glioblastoma multiforme accounts for >50% of all primary brain
tumors and ~20% of all intracranial tumors (2). Current treatment includes radical
removal of the tumor; surgical treatment may be complemented by
irradiation at a dose of up to 70 Gy and chemotherapy, which may
increase the duration of relapse-free survival (3). The preferred chemotherapeutic drug is
temozolomide (4). The prognosis for
patients is poor and the median survival time is 12–14 months.
Despite the best efforts of oncologists, only 10% of patients
survive more than 18 months from the initial point of diagnosis,
and the overall 5-year survival rate is close to zero (5).
A significant cause of recurrent glioblastoma is due
to infiltration of brain parenchyma by the tumor cells (6). During the process of invasive growth,
the neoplastic cells migrate far beyond the neoplastic node,
meaning that traditional surgical methods are ineffective (7). Attempts to solve this issue by
increasing the dose of radiation leads to the development of brain
damage (8). Classical cytostatics are
not effective at targeting cells outside the phases of mitosis
(9). The application of modern
targeted therapies has had no apparent success. Furthermore, the
choice of drugs is limited by the selective permeability of the
blood-brain barrier and the use of systemic chemotherapy is limited
by the general condition of the patient (10).
The effectivity of chemotherapy may be significantly
extended through the introduction of cellular and biopolymer
technologies (11). Aboody et
al (12) described the phenomenon
of ‘molecular adhesion’. During this process, stem cells migrate
following tumor cells that begin to metastasize to the brain
parenchyma. Once the target is reached, the stem cells attach to
the neoplastic cells (12). Such stem
cells may be used for targeted delivery of therapeutic agents to
the brain parenchyma. Stem cells may be conjugated with
immunoliposomes or nanocapsules that contain drug substance
(13). The implantation of such cells
in a biopolymer gel that fills the defect of brain tissue following
removal of the tumor provides a direct treatment for any remaining
neoplastic cells, leading to prevention of relapse. The drug
substance may be in biodegradable containers, and incorporated into
a biopolymer gel. The specific wall thickness of the containers
will provide a systematic release of the drug substance, thereby
avoiding the toxic effects of chemotherapy (14).
Application of biomedical technologies extends the
capabilities of chemotherapy and has led to the search for novel
drugs that possess the ability to inhibit proliferation and induce
cell death in glial tumors. The present study focused on a group of
alkaloids, which are based on a pentacyclic system of
pyrido[1,2-a:3,4-b']diindola known as fascaplysin alkaloids
(15). The most well-known member of
this group is the red pigment fascaplysin, which was first isolated
from a marine sponge Fascaplysinopsis sp. in 1988 (15). This compound has a broad spectrum of
biological activity, combining antimicrobial, antifungal, antiviral
and antitumor activity (16). The
action of fascaplysin is mediated by the activation of BH3
interacting-domain death agonist protein, which alters the
conformation of proapoptotic protein bcl-2-like protein 4 leading
to the release of cytochrome c, therefore activating
caspases 3, 8, 9 and triggering apoptosis (17). Activation of the receptor family
ligands of tumor necrosis factor and suppression of the
anti-apoptotic B-cell lymphoma 2 (Bcl-2) protein family causes the
death of vascular endothelial cells, which inhibits the formation
of neoplastic vascular networks (17). These properties mean that fascaplysin
may be a promising novel substance for the treatment of
glioblastoma. Antitumor effects of fascaplysin alkaloids have been
observed in a number of cell lines, including HeLa, BEL-7402,
THP-1, SNU-C4, SK-MEL-28, DLD-1 and MDA-MB-231, as well as a number
of solid tumors; however, it has not been considered as a potential
agent for the treatment of glioblastoma (18,19).
Modern cellular and polymer technologies allow the creation of
local concentrations of substances that are capable of rendering a
strong directional impact on neoplastic cells (20).
The aims of the present study were to establish
effective concentrations of fascaplysin by evaluating its
anti-proliferative and cytotoxic activity against C6 glioma cells
in vitro, to study the characteristics and mechanisms of the
antitumor action of fascaplysin and to evaluate fascaplysin's
effectiveness in comparison with other chemotherapy drugs.
Materials and methods
Materials
The present study used the red pigment fascaplysin
(12,13-dihydro-13-oxopyrido [1,2-a:3,4-b']diindol-5-ium chloride),
the C6 malignant glioma cell line and human fibroblasts.
Fascaplysin
All experiments described in the present study were
performed with synthesized fascaplysin. Fascaplysin was synthesized
according to the two-step synthesis protocol as described
previously (21). The main spectral
characteristics (infra-red, mass spectrometry, 1H and
13C nuclear magnetic resonance) of the synthesized
fascaplysin were identical to those published for the natural
product (22).
C6 glioma cells
Glioma cells are the most commonly used model of
human glioblastoma. For the present experiment, C6 glioma cells
(American Type Culture Collection, Manassas, VA, USA; CCL-107),
previously frozen at −80°C, were thawed at 37°C for 10 min and
washed to remove dimethyl sulfoxide (Ameresco, Inc., Framingham,
MA, USA; Am-O231-0.5), and were maintained in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.;
41965-039) containing 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.; 16000-036) and 100X antibiotic-antimycotic
solution (Gibco; Thermo Fisher Scientific, Inc.; 15240-062). Prior
to experimental use, cells were pelleted by centrifugation (120 ×
g at 20°C for 3 min), fresh medium was added, and cells were
resuspended and seeded into culture flasks. The culture was
continued until a monolayer formed. Cells were removed using
enzymatic dissociation by TrypLE™ Express (Gibco; Thermo Fisher
Scientific, Inc.; 12604-021) at 37°C for 10 min, followed by
centrifugation (120 × g for 3 min at 20°C). Subsequently,
fresh medium was added and cells were resuspended. Immediately
prior to the commencement of experiments, the cells underwent
immunocytochemical characterization.
Immunocytochemical study of C6 glioma
cells
Prior to performing the immunocytochemical analysis,
cells were evaluated for viability by staining with 0.4% trypan
blue (Gibco; Thermo Fisher Scientific, Inc.; 15250-061) and the
cells were counted using a hemocytometer. Cells were seeded in a
24-well plate (Greiner Bio-One Ltd., Stonehouse, UK; 662892) at
25×103 cells per well and cultured in DMEM for 2 days at
37°C in an atmosphere of 5% CO2. Cells were fixed
according to the following methodology: 4% paraformaldehyde for 20
min, at 4°C, followed by washing with a stock solution composed of
phosphate-buffered saline (PBS; pH 7.4; Gibco; Thermo Fisher
Scientific, Inc., 10010-023), 0.2% Tween 20 (Sigma-Aldrich, St.
Louis, MO, USA; P9416), 0.2% Triton-X-100 (Sigma-Aldrich; T8787)
and 0.3% bovine serum albumin (Sigma-Aldrich; A2058) 3 times, each
for 10 min.
Immunocytochemical staining was performed according
to the following protocol: The primary antibodies against
oligodendrocyte 4 (mouse monoclonal; dilution, 1:50; MAB1326;
R&D Systems, Inc., Minneapolis, MN, USA), glial fibrillary
acidic protein (GFAP; mouse polyclonal; dilution, 1:50; ab7260;
Abcam, Cambridge, MA, USA), p53 (mouse monoclonal; dilution, 1:100;
AHO0152; Thermo Fisher Scientific, Inc.), anti-tubulin-β-III (mouse
monoclonal; dilution, 1:100; ab7751 Abcam), nestin (polyclonal
rabbit; dilution, 1:100; N5413; Sigma-Aldrich), cluster of
differentiation (CD)133 (rabbit polyclonal; dilution, 1:100;
PA5-38014; Invitrogen; Thermo Fisher Scientific, Inc.) S100 (rabbit
polyclonal; dilution, 1:100; ab868; Abcam) were incubated with
cells for 18–20 h at 4°C. Following incubation, the cells were
washed by stock solution 3 times, each for 10 min. Secondary
antibodies, Alexa Fluor® 488-conjugated rabbit
anti-mouse polyclonal immunoglobulin (Ig) G (dilution, 1:500;
A11059; Thermo Fisher Scientific, Inc.) or Alexa Fluor®
633-conjugated goat anti-rabbit polyclonal IgG (dilution, 1:500;
A21071; Thermo Fisher Scientific, Inc.), were incubated with the
cells for 2 h at 37°C. Following incubation, the samples were
washed with the stock solution 3 times, each for 10 min, and cell
nuclei were stained with 4′,6-diamidino-2-phenylindole (Molecular
Probes; Thermo Fisher Scientific, Inc.; D1306) for 7 min at 22°C.
Subsequently, an additional wash with stock solution was performed
2 times, each for 10 min, and cells were enclosed in
Mowiol® 4–88 (Sigma-Aldrich; 324590). Controls were
performed where cells where incubated with secondary antibodies
without prior use of the primary antibodies. Primary and secondary
antibodies were used according to the manufacturer's protocol.
Culture of human fibroblasts
Primary cultures of human fibroblasts were used
(Thermo Fisher Scientific, Inc.; S-004-5S). Cells were previously
frozen at −80°C. The cells were thawed according and cultured in
complete DMEM/Ham's F12 (Gibco; Thermo Fisher Scientific, Inc.,
11330-032) containing 10% FBS, 2 mM L-glutamine (Gibco; Thermo
Fisher Scientific, Inc.; 25030-081), 0.8% glucose (Gibco; Thermo
Fisher Scientific, Inc.; A2494001), 0.2 units/ml insulin (Gibco;
Thermo Fisher Scientific, Inc.; 12585-014) and 100X
antibiotic-antimycotic solution.
Investigation into cytotoxicity and
cell death caused by fascaplysin
Plates (24-well) were seeded with C6 glioma cells
(7×104 per well) and incubated for 48 h at 37°C in an
atmosphere of 5% CO2 to establish concentrations of
fascaplysin that exerted a cytotoxic effect. The first row of the
plate wells was used as a control, and a solution of fascaplysin
was added to the remaining wells at concentrations of 2, 1.5 and 1
µM. Counting of cells was performed in an automated manner using a
Cell-IQ® MLF system (CM Technologies GmbH, Elmshorn,
Germany).
C6 glioma cells and human fibroblasts
(5×103) were cultured in three 96-well plates (Greiner
Bio-One; 662120), filled with DMEM containing 10% FBS, 10 ng/ml
fibroblast growth factor (Gibco; Thermo Fisher Scientific, Inc.;
PHG0023), 10 ng/ml epidermal growth factor (Gibco; Thermo Fisher
Scientific, Inc.; PHG0311 L) and 100X antibiotic-antimycotic
solution at 37°C in an atmosphere of 5% CO2 to establish
effective concentrations of fascaplysin that exerted an
anti-proliferative effect. The first row of the plate wells was
used as a control, and a solution of fascaplysin was added to the
remaining wells at concentrations of 0.5, 0.05 and 0.005 µM.
The first 96-well plate was incubated for 96 h at
37°C in an atmosphere of 5% CO2. Counting of live and
dead cells was performed in an automated manner using a Cell-IQ MLF
system. The second 96-well plate was used for staining in order to
observe the apoptosis of C6 glioma cells following 6 h of exposure
to fascaplysin with the terminal deoxynucleotidyl transferase dUTP
nick end labeling (TUNEL) method, using the Click-iT TUNEL Alexa
Fluor® 488 Imaging Assay (Molecular Probes; Thermo
Fisher Scientific, Inc.; C10245). The third 96-well plate with C6
glioma cells was used to study the mechanisms of cell death by flow
cytometry following 6 h of exposure to fascaplysin.
Flow cytometry
The present study used two methods to assess the
level of apoptosis in C6 glioma cells. The first method was based
on the simultaneous use of two fluorescent dyes, which were
YO-PRO-1 iodide (Molecular Probes; Thermo Fisher Scientific, Inc.;
Y3603) and propidium iodide (PI; Sigma-Aldrich; P4864). The dye
ligand is nucleic acid, which is localized in the cytoplasm of
cells (23). Penetration of YO-PRO-1
into the cell occurs via P2X purinoceptor 7 ligand-dependent ion
channels that are not active in intact cells (24). Activation occurs when apoptosis
commences and coincides with the violation of the asymmetry of the
lipid composition of the membrane surface, which means that
accumulation of YO-PRO-1 is an event that is indicative for the
early stages of apoptosis (24).
To detect the later stages of apoptosis, besides
using the dye YO-PRO-1, the cells were additionally stained with
PI. PI does not have any specific carriers integrated into the
membrane and is able to penetrate into the cytoplasm and nucleus
only through damaged membranes; this typically is able to take
place in the final stages of apoptosis during the formation of
apoptotic bodies or cell necrosis (25). Thus, living cells in the sample were
not stained by YO-PRO-1 or PI. Stained cells that have entered into
apoptosis, will be positive only to YO-PRO-1, whereas cells that
are in the later stages of apoptosis will be effectively stained by
both dyes.
Cell staining was performed in 12×75 mm cytometric
tubes (Globe Scientific, Inc., Paramus, NJ, USA; 110410) for
cytometric study. A total of 5 µl YO-PRO-1 solution was added to
100 µl of the cell suspension (1×106 cells/ml) to give a
final dye concentration of 250 nM. Subsequently, 10 µl of propidium
iodide solution was added to the samples to give a final
concentration of PI that was equal to 1 µg/ml. Staining was
performed at room temperature for 10 min in the dark. Upon
completion of staining, 100 µl of PBS was added to the samples and
samples were analyzed by a flow cytometer (BD Accuri™ C6; BD
Biosciences, Franklin Lakes, NJ, USA).
At least 10,000 single cells were analyzed for each
sample. Prior to the experiment, the cells were treated using
enzymatic dissociation by TrypLE™ (Gibco; Thermo Fisher Scientific,
Inc.; 12604-021) at 37°C for 5 min, followed by centrifugation (120
× g for 3 min, at 20°C). Cell aggregates were discriminated
from the analysis. Analysis of the results was performed using
Kaluza® software (version 1.3; Beckman Coulter, Inc.,
Brea, CA, USA).
The second staining approach was based on the
simultaneous use of fluorescein diacetate (FDA; Sigma-Aldrich;
F7378) and PI (26). It is thought
that an effect of triggering apoptosis in cells is reduced activity
of intracellular enzymes, primarily intracellular esterases
(hydrolase class of enzymes) that are responsible for the
hydrolytic cleavage of esters to alcohols and acids, with the
participation of water molecules (25). FDA dye is activated by esterase and
also exhibits lipophilic properties, allowing spontaneous
penetration into cells from the culture medium. Following reaction
with the enzymes a loss of hydroxyl groups occurs, which is
accompanied by the accumulation of the dye in the cell and
induction of fluorescence in response to laser light (27).
Thus, living cells possess bright fluorescence,
whereas fluorescence of the FDA in cells undergoing death is
considerably reduced. Additional PI staining of these cells allows
discrimination between the early and late stages of apoptosis as PI
is able to penetrate into the cytoplasm only in cases of violation
of the integrity of the surface membrane.
Cell staining was performed in 12×75 mm cytometric
tubes. A total of 1 µl of FDA was added to 100 µl of the cell
suspension (1×106 cells/ml) to give a final dye
concentration of 100 ng/ml. Staining was performed at room
temperature for 10 min in the dark, and following staining cells
were washed once by PBS containing 2% FBS (Gibco; Thermo Fisher
Scientific, Inc.; 16000-036). Cells were centrifuged at 350 ×
g for 7 min at 20°C. The cell pellet was resuspended in 200
µl PBS, after which 10 µl of propidium iodide was added to the
samples, to give a final concentration of 1 µg/ml. Following
completion of incubation (10 min at 20°C), samples were analyzed on
a flow cytometer (BD Accuri™ C6). At least 10,000 single cells were
analyzed for each sample.
Comparison of fascaplysin with other
chemotherapeutic drugs
An assessment of the antitumor effects of
fascaplysin compared with other chemotherapeutic drugs was
performed. A total of 3×104 C6 glioma cells were seeded
into 24-well plates (Greiner Bio-One Ltd.; 662160). Subsequently,
0.5 µg/ml dexamethasone, 0.5 µg/ml temozolomide and 0.5 µg/ml
fascaplysin were added to the wells. A row of untreated wells with
glioma C6 cells was used as a control. A cell count was performed
automatically using the Cell-IQ® MLF system.
Dexamethasone
The present study used 4 mg/ml dexamethasone
(Galenika a.d., Zemun, Serbia). Dexamethasone is a mandatory
component of the regimen used for the treatment of glial tumors
(28).
Temozolomide
Temozolomide is the standard chemotherapeutic
treatment for invasive gliomas (29).
The effectiveness of this drug is due to alkylation of guanine at
position O6 and N7, which is disturbs the structure and synthesis
of DNA (30). The drug
Temodal® (Schering-Plough Labo nv; Merck Sharpe &
Dohme, Hoddesdon, UK) was used in the present study.
Sample visualization
The FluoView FV 1200 MPE confocal laser scanning
microscope (Olympus Corporation, Tokyo, Japan) and LSM 710 confocal
system (Zeiss GmbH, Jena, Germany) were used to visualize samples.
Image processing was executed using ImageJ software version 4.1
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed using analysis of
variance, followed by Tukey's post-hoc test. Data are expressed as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference. All statistical tests were
performed using GraphPad Prism version 4 (GraphPad Software, Inc.,
La Jolla, CA, USA).
Results
Immunocytochemistry of glioma C6
C6 glioma cells were stained with antibodies to
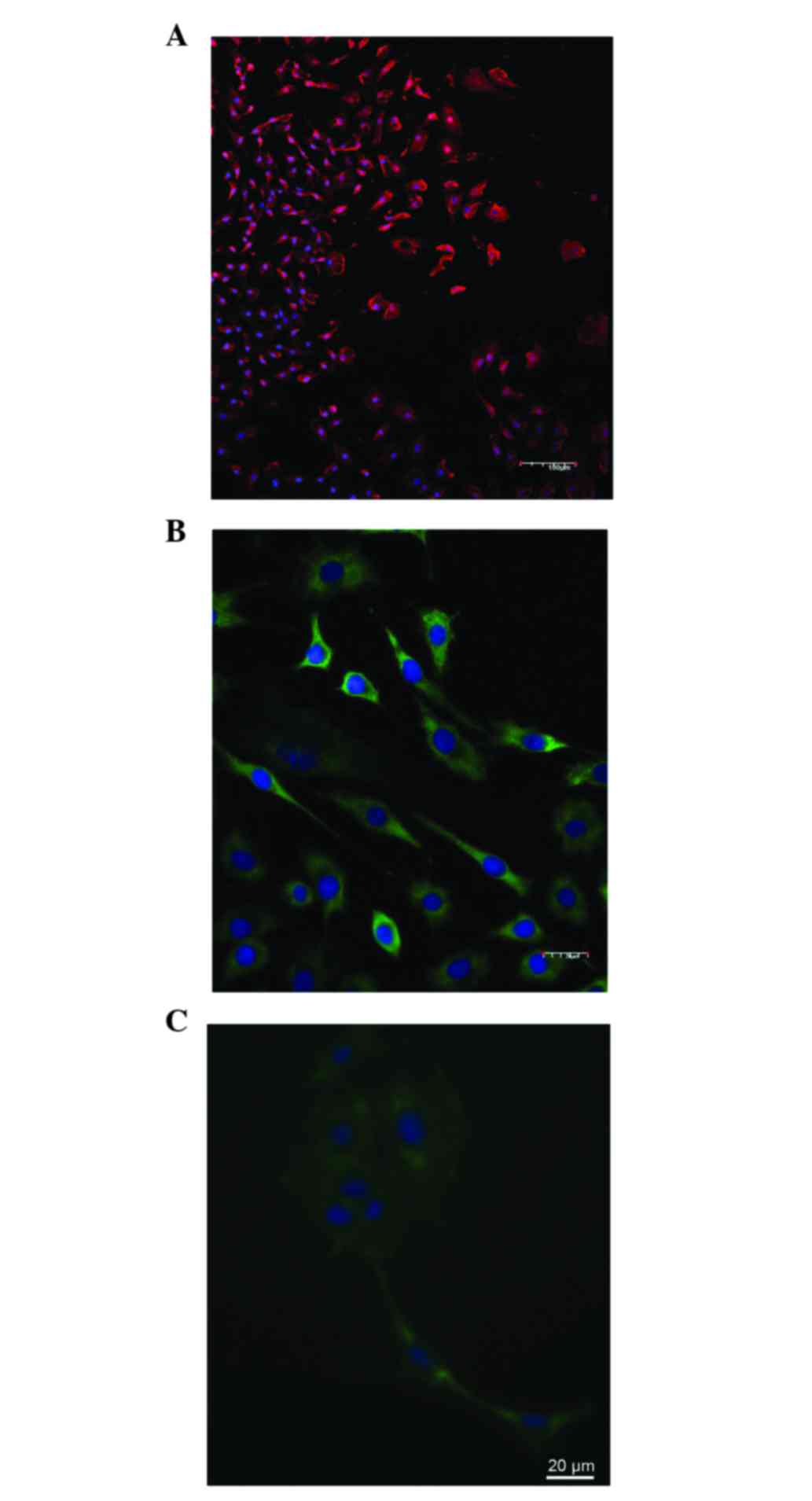
nestin (96.7±2.8%) and p53 (88.4±3.8%; Fig. 1A-C), and the number of GFAP-positive
cells was very low (4.5±1.4%). Tumor cells rapidly adhered to the
bottom of the plate, and flattened and proliferated (Table I). Following 1 day of culture the
number of nestin-positive cells significantly decreased (52.5±6.5%;
P=0.01), and the cells became round and polygonal in shape. Cells
stained positively for the calcium binding protein S100
(68.8±4.8%), oligodendrocyte 4 (46.2±3.7%) and tubulin-β-III
(55.2±4.8%), as well as GFAP (38.2±3.5%); the number of cells
stained positively for mutant p53 protein remained relatively
unchanged.
 | Table I.Immunocytochemical staining of C6
glioma cells prior to and following the adhesion of cells. |
Table I.
Immunocytochemical staining of C6
glioma cells prior to and following the adhesion of cells.
| Marker | Stained cells prior
to adhesion, % | Stained adherent
cells, % |
|---|
| Nestin | 96.7±6.8 | 52.5± 6.5 |
| Oligodendrocyte
4 | – | 46.2±3.7 |
| S100 | – | 68.8±4.8 |
| Tubulin-β-III | – | 55.2±4.8 |
| GFAP | 4.5±1.4 | 38.2±3.5 |
| p53 | 88.4±3.8 | 85.9±2.6 |
Investigation of cytotoxic and
cytostatic effects of fascaplysin in vitro
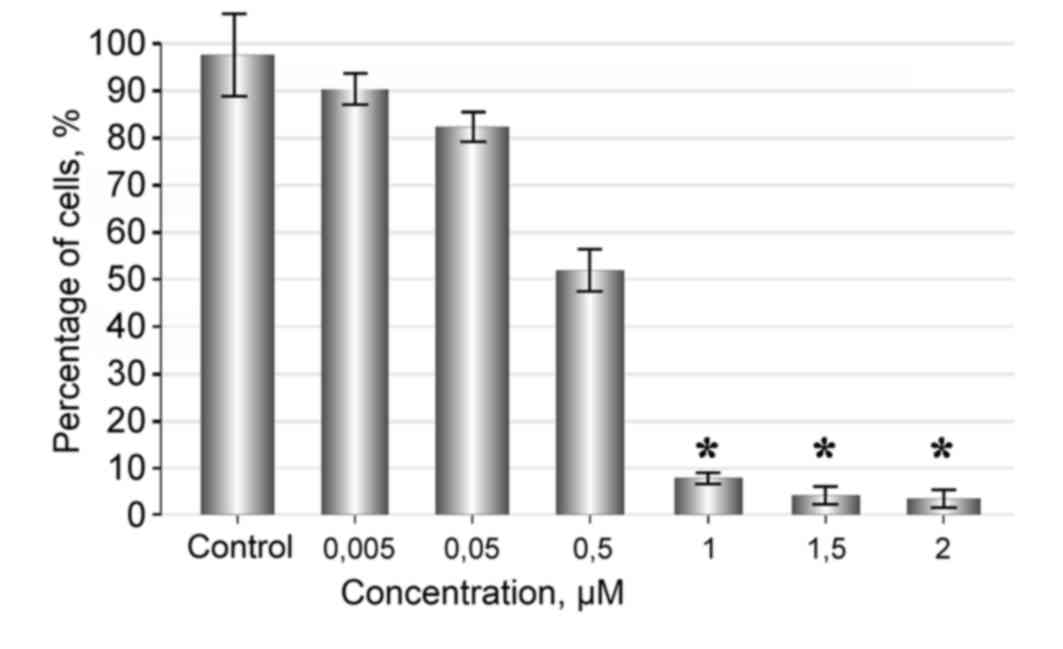
Fascaplysin at a concentration of 2-1 µM strongly
inhibited proliferation and induced death of glioma cells. The
cytotoxic effect became more marked with an increasing
concentration of fascaplysin in the culture medium. Following 48 h
of observation, the number of live cells in the sample treated with
1, 1.5 and 2 µM of fascaplysin was minimal (Fig. 2).
Fascaplysin at a concentration of 0.005 µM had no
significant effect on the rate of tumor cell proliferation and
showed no significant difference compared with the control
(P=0.22). Tumor cells were attached and flattened on the bottom of
the plate, where they proliferated and formed various morphological
forms, including spindle-shaped and polygonal cells, which formed a
monolayer. When the concentration of fascaplysin was increased to
0.5 µM, a marked anti-proliferative effect was observed, and with
increasing concentrations of the substance a cytotoxic effect was
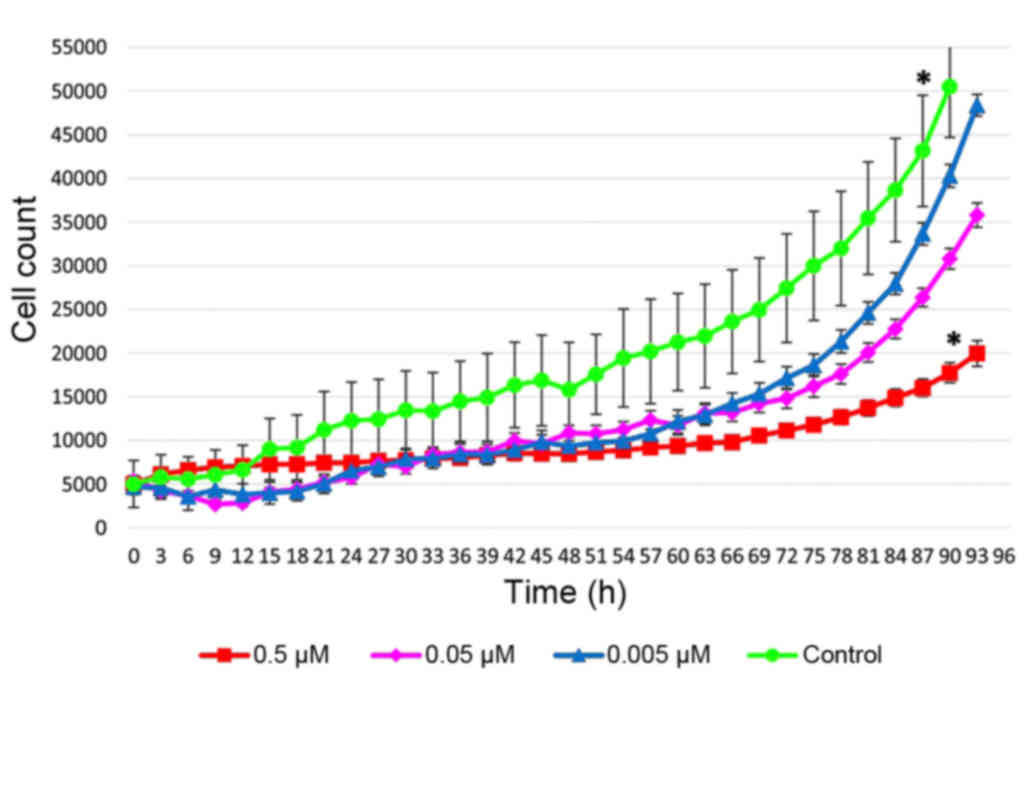
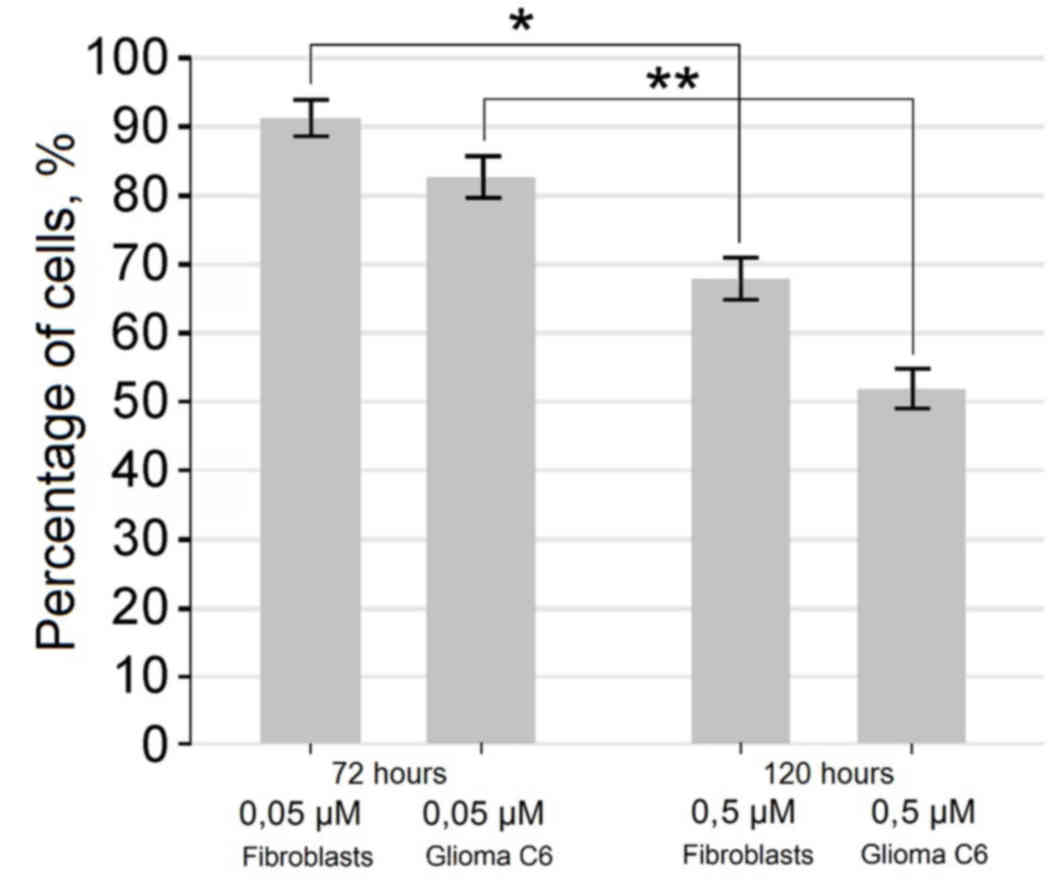
also observed (Figs. 2 and 3). Exposure of cells for 72 h in a medium
containing 0.5 µM of fascaplysin slowed the rate of fibroblast
proliferation, but did not lead to a significant increase in the
number of dead cells compared with glioma C6 cells treated with
fascaplysin (P=0.094), indicating the preferential cytostatic and
cytotoxic effects. Following 120 h in culture containing 0.5 µM of
fascaplysin, morphological features of apoptosis were detected in
~50% of C6 glioma cells (Fig. 4). At
the same time, following 120 h in culture containing 0.5 µM of
fascaplysin, the dynamic of fibroblast growth was higher (~70%)
compared with glioma C6 cells treated with fascaplysin at 120 h,
indicating preferential cyctostatic and cytotoxic effects on tumor
cells.
A total of 3 h subsequent to the beginning of the
experiment, in the culture of glioma containing 0.5 µM fascaplysin,
there was a marked decrease in the rate of proliferation, as well
as changes in cell shape to predominantly circular or oval and a
sharp deterioration in adhesiveness. After 6 h, the initial signs
of nuclear fragmentation were observed in neoplastic cells.
Staining by the TUNEL method revealed a large number of fluorescent
apoptotic cells, which indicated oligonucleosome DNA degradation
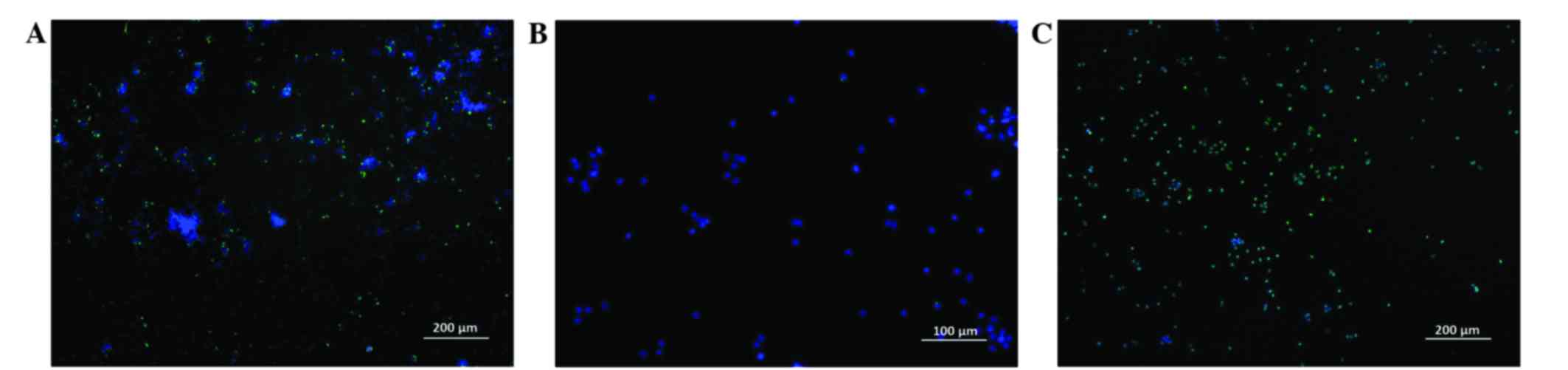
(Fig. 5).
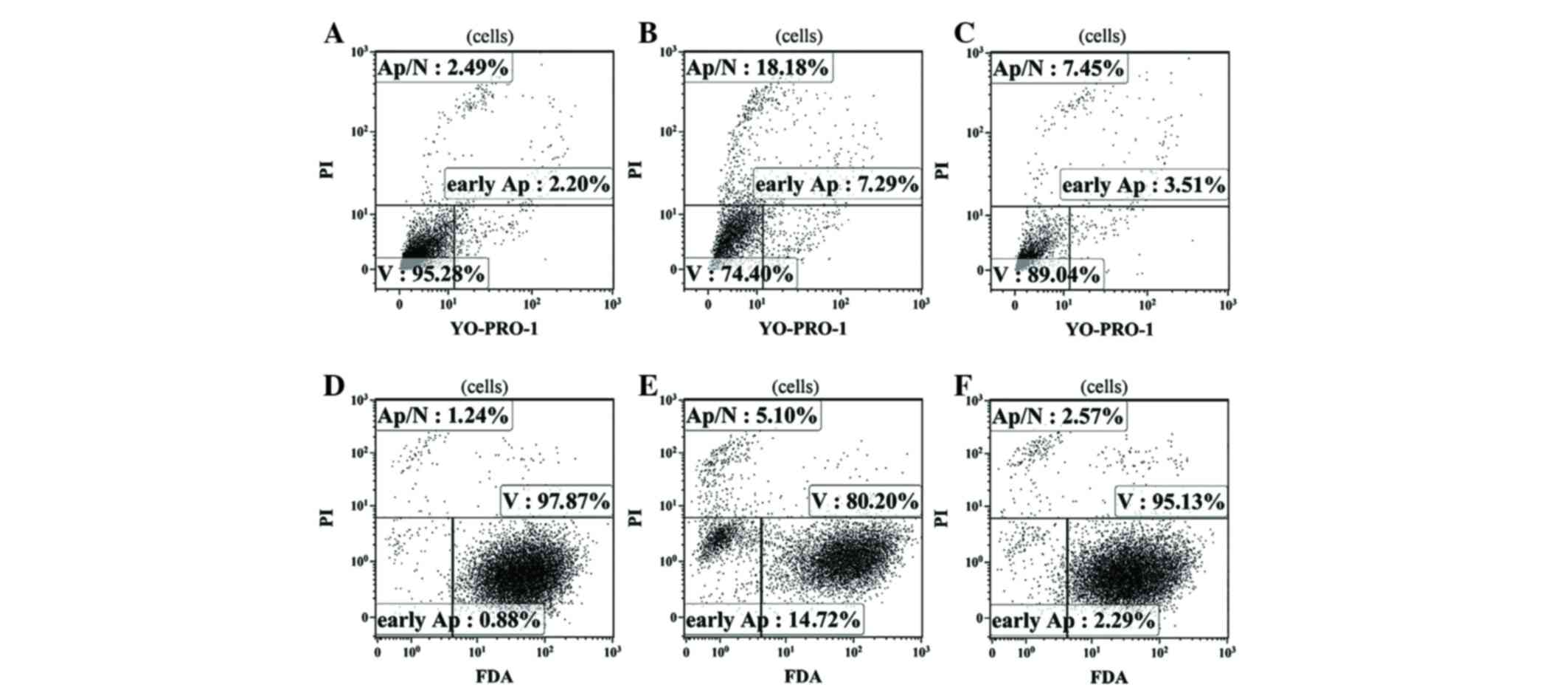
According to the results of flow cytometry performed
using two independent methods to assess the level of apoptosis,
fascaplysin at a final concentration of 0.5 µM significantly
increased the relative content of glioma cells at various stages of
apoptosis (P<0.001). The number of apoptotic glioma cells
stained with YO-PRO-1 (early apoptosis) and the number of dead
cells stained with PI (late apoptosis) at a given concentration of
fascaplysin was maximal. Furthermore, the number of live cells that
were intensely stained by FDA at a concentration of 0.5 µM
fascaplysin was minimal (Fig. 6;
Table II).
 | Table II.Ratio of live and dead cells in C6
glioma cell culture with 0.5 µM fascaplysin according to flow
cytometry data. |
Table II.
Ratio of live and dead cells in C6
glioma cell culture with 0.5 µM fascaplysin according to flow
cytometry data.
| Time, h | Live C6 glioma
cells, % FDA stained | Apoptotic C6 glioma
cells, % YO-PRO-1 stained |
|---|
| 24 | 65.12±9.4 | 24.61±5.4 |
| 48 | 48.13±11.2 | 31.7±7.3 |
| 72 | 24.6±14.2 | 53.7±8.3 |
It should also be noted that the decrease in FDA
fluorescence in C6 glioma cells during the early stages of
apoptosis, when cells have not yet lost their plasma membrane
integrity, indicates a shortage of adenosine triphosphate (ATP) in
the cytoplasmic compartment of the cell, as the early stages of
apoptosis are considered to be ATP-dependent (31). Therefore, it may be assumed that a
direct and/or indirect influence of fascaplysin treatment on cells
is associated with impaired mitochondrial membrane potential and a
decrease in the efficiency of ATP-synthetase, which is accompanied
by a decrease in the overall level of ATP in the cytoplasm and
induction of apoptosis.
The results of the present study confirm the data
obtained by the Cell-IQ MLF system. Reducing the final
concentration of fascaplysin to 0.005 µM was accompanied by a
decrease in the number of cells undergoing apoptosis.
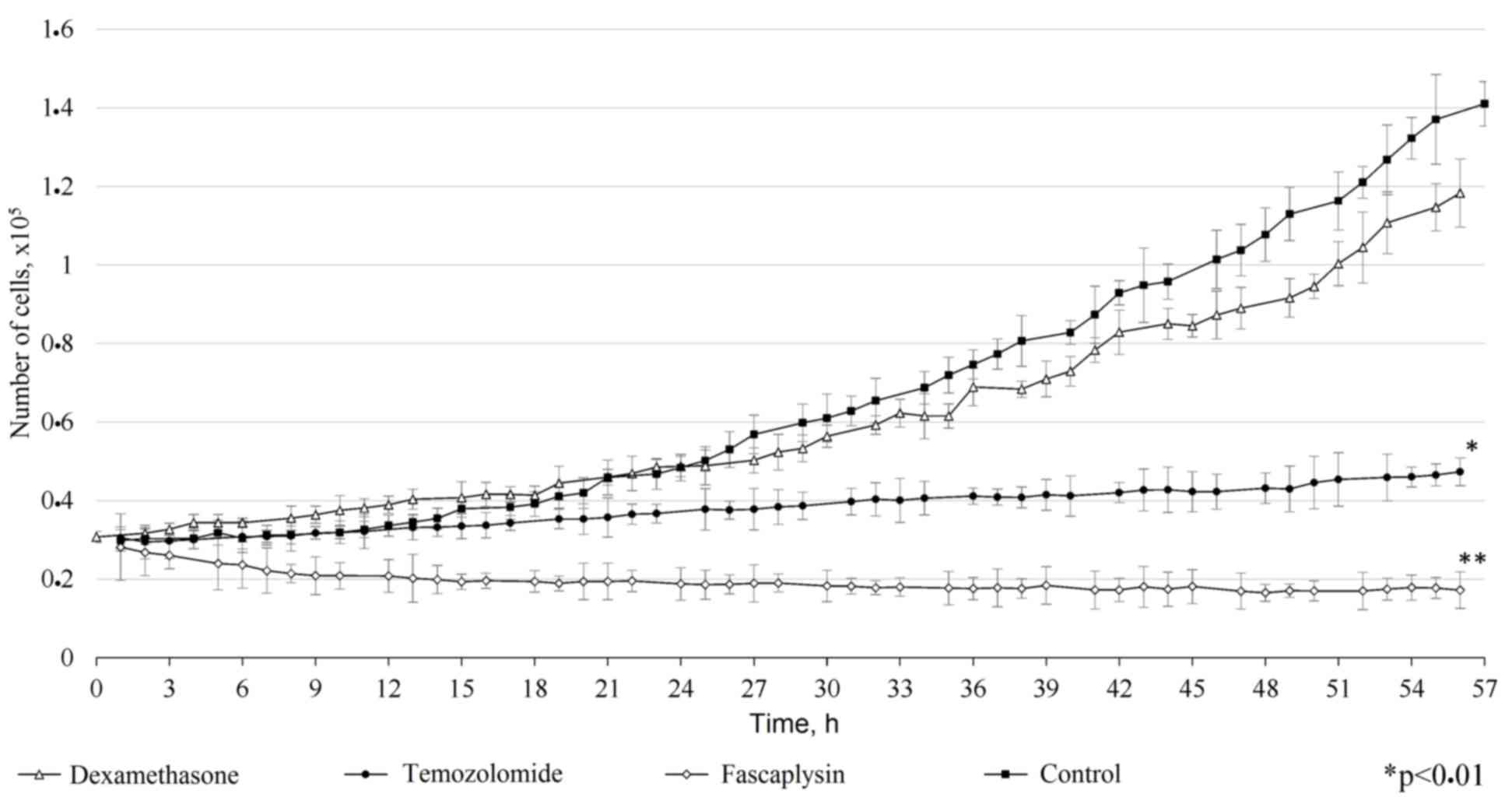
Comparison of the antitumor effect of
fascaplysin with other drugs
Comparison of the antitumor effect of fascaplysin
with other drugs used for the treatment of tumors revealed a number
of features. In vitro, dexamethosone and temozolomide
demonstrated inferior cytotoxic efficiency compared with
fascaplysin. Dexamethasone weakly inhibited the growth of glioma
cells and did not show a significant difference compared with the
control (P=0.15). Addition of dexamethasone to the culture medium
did not alter the morphology of the tumor cells, but increased the
time of adhesion to the surface of the cultural plate. Temozolomide
prevented the proliferation of tumor cells, but its cytotoxic
effect was dependent on the exposure time. Overall, the inhibition
of tumor cell growth by fascaplysin was more marked compared with
the other drugs investigated (Fig. 7;
P<0.001).
Discussion
Glioblastoma multiforme is one of the most
aggressive human brain tumors (32).
An important reason for the development of resistance to treatment
in glioblastoma is associated with tumor stem cells (33). A number of arguments have provided
evidence in favor of the origin of tumor stem cells of glioblastoma
from the neural stem cells of the human brain. This is indicated by
the similarity in basic cell surface markers and other
immunocytochemical clusters of differentiation in tumor cells
compared with neural stem cells, which has been reported to be
63.5% (34).
Adhesion to the substrate is a strategically
important regulatory mechanism of tumor stem cells (35). In the present study, the majority of
free-floating ‘gliomaspheres’ were stained with antibodies against
nestin, which is found in intermediate filaments and is one of the
key markers of neural stem cells (36). An important distinction of C6 glioma
cells from neural stem cells is the presence of the mutant protein
p53 (37). The ability to transform
into other cell immunophenotypes indicates multipotency, which is
also a key feature of stem cells (38). Thus, the culture of C6 glioma cells
used in the present study contained a high number of stem cells,
allowing the assumption that the described method will be effective
for treatment of cells of this type.
Invasion is one of the main features of glioblastoma
progression (39). One of the most
important conditions for the initiation of the invasive process is
the predominance of proliferation over apoptosis, allowing the
tumor to accumulate the necessary quantitative potential for
penetration into the surrounding tissues and organs (39). The tumor acquires the capacity to
invade surrounding tissues as a result of genetic and biochemical
changes, which allow cells to undergo proliferation (40). Fascaplysin is characterized by a
strong anti-proliferative activity against C6 glioma cells. This
effect is in direct proportion to the exposure time and the
concentration of the substance. A gradual increase of fascaplysin
concentration in the culture of tumor cells from 0.005 to 0.5 µM
causes a distinct reliable cytostatic effect, and death of the
majority of the cell population with a further increase in time of
exposure in the culture medium. Increasing the concentration of
fascaplysin up to 2 µM is accompanied by increased apoptosis of
neoplastic cells. It may be assumed that the local release of
fascaplysin will have a profound anti-proliferative action against
tumor cells infiltrating the brain, exceeding the effects of
irradiation and chemotherapy. Increasing the exposure time to
fascaplysin and increasing the local concentration leads to the
death of neoplastic cells and the inhibition of tumor growth in
contrast to the majority of modern drugs.
Creating local concentrations of fascaplysin by
targeted transport of substances in tumor foci may have a
pronounced cytoreduction effect, which is particularly important
for inoperable tumors (41). In the
present study, a feature of the cytostatic and cytoreduction
effects of fascaplysin was a predominant targeting of actively
proliferating tumor cells, with a lesser impact on fibroblasts. Our
present study further suggests that fascaplysin, as used in the
present study but released from capsules, will not have a negative
effect on fibroblasts and other cellular elements that penetrate
the modern biodegradable matrix and form loose connective tissue.
This may be identified in future studies by delivering fascaplysin
by capsules and the use of biodegradable matrices.
The antitumor effect of fascaplysin against C6
glioma cells surpasses tested substances. In theory, the activation
of glucocorticoid receptors by corticosteriods, such as
dexamethasone, shifts the balance from Bcl-2 family proteins in
favor of pro-apoptotic proteins, inhibiting the migration of tumor
cells by influencing the Akt/mammalian target of rapamycin/Ras
homolog gene family, member A signaling pathway (42). Dexamethosone typically inhibits the
growth of tumor cells. However, the addition of dexamethasone to
the culture medium in the present study had virtually no effect on
the rate of proliferation of glioma cells. This substance is
potentially applicable for local anti-relapse therapy of
glioblastoma multiforme, but it is significantly inferior in
efficiency to temozolomide (43).
Temozolomide is the most commonly used drug in the treatment of
gliomas and metastatic tumors (44).
It has a strong cytotoxic effect due to alkylation of guanine at
position O6 and optional alkylation in position N7 (45). In a previous study by the present
authors, temozolomide increased the life expectancy of rats with
experimentally-induced glioblastoma (46). The combined use of methods of targeted
delivery of fascaplysin and traditional chemotherapeutic agents
requires further research, as this may potentially result in strong
antitumor responses.
In conclusion, the present study suggests the
following: Fascaplysin in a concentration from 0.05 to 1 µM has a
marked cytostatic effect against C6 glioma cells, which is replaced
by tumor cell death via apoptosis with increasing exposure time.
Fascaplysin causes the death of C6 glioma cells at doses exceeding
0.5 µM. The anti-proliferative effect of fascaplysin is
significantly superior to temozolomide. However, the most important
finding of the present study is the ability of fascaplysin to
inhibit the growth of and kill poorly differentiated multipotent
tumor cells. Compared with other substances investigated in
vitro, fascaplysin showed marked inhibition of cell growth and
proliferation, inducing apoptosis in tumor cells.
Acknowledgements
The present study was funded by the Russian Science
Foundation (grant no., 14-15-00084). The research concerning
fascaplysin synthesis, standardization and preparation for in
vitro experiments was supported by the Russian Scientific Fund
(grant no., 14-50-00034).
References
|
1
|
Carlsson SK, Brothers SP and Wahlestedt C:
Emerging treatment strategies for glioblastoma multiforme. EMBO Mol
Med. 6:1359–1370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Omuro A and DeAngeles LM: Glioblastoma and
others malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anton K, Baehring JM and Mayer T:
Glioblastoma multiforme: Overview of current treatment and future
perspectives. Hematol Oncol Clin North Am. 26:825–853. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Konovalov AN, Potapov AA, Loshakov VA,
Olyushin VE, Ulitin AY, Kornienko VN, Pronin IN, Shishkina LV,
Golanov AV, Tanqskiu SV, Urakov SV and Kobqkov GL: Association of
Neurosurgeons of Russia: The standards, guidelines and options in
the treatment of brain tumors in adults (2009). Association of
Neurosurgeons of Russia; Moscow, Russia: 2009, (In Russian).
|
|
5
|
Louis DN, Perry A, Burger P, Ellison DW,
Reifenberger G, von Deimling A, Aldape K, Brat D, Collins VP,
Eberhart C, et al: International Society of Neuropathology -
Haarlem consensus guidelines for nervous system tumor
classification and grading. Brain Pathol. 24:429–435. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paw I, Carpenter RC, Watabe K, Debinski W
and Lo HW: Mechanisms regulating glioma invasion. Cancer Lett.
362:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molina JR, Hayashi Y, Stephens C and
Georgescu MM: Invasive glioblastoma cells acquire stemness and
increased Akt activation. Neoplasia. 12:453–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barani IJ and Larson DA: Radiation therapy
of glioblastoma. Cancer Treat Res. 163:49–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitchison TJ: The proliferation rate
paradox in antimitotic chemotherapy. Mol Biol Cell. 23:1–6. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu YY, Gao P, Sun Y and Duan YR:
Development of targeted therapies in treatment of glioblastoma.
Cancer Biol Med. 12:223–237. 2015.PubMed/NCBI
|
|
11
|
Tiwari G, Tiwari R, Sriwastawa B, Bhati L,
Pandey S, Pandey P and Bannerjee SK: Drug delivery systems: An
updated review. Int J Pharm Investig. 2:2–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aboody KS, Brown A, Rainov NG, Bower KA,
Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, et al:
Neural stem cells display extensive tropism for pathology in adult
brain: Evidence from intracranial gliomas. Proc Natl Acad Sci USA.
97:12846–12851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou J, Atsina KB, Himes BT, Strohbehn GW
and Saltzman WM: Novel delivery strategies for glioblastoma. Cancer
J. 18:89–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barber FA: Complications of Biodegradable
Materials: Anchors and Interference Screws. Sports Med Arthrosc.
23:149–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roll DM, Ireland CM, Lu HSM and Clardy J:
Fascaplysin, an unusual antimicrobial pigment from the marine
sponge Fascaplysinopsis sp. J Org Chem. 53:3276–3278. 1988.
View Article : Google Scholar
|
|
16
|
Bharate SB, Manda S, Mupparapu N, Battini
N and Vishwakarma RA: Chemistry and biology of fascaplysin, a
potent marine-derived CDK-4 inhibitor. Mini Rev Med Chem.
12:650–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuzmich AS, Fedorov SN, Shastina VV,
Shubina LK, Radchenko OS, Balaneva NN, Zhidkov ME, Park JI, Kwak JY
and Stonik VA: The anticancer activity of 3- and
10-bromofascaplysins is mediated by caspase-8, −9, −3-dependent
apoptosis. Bioorg Med Chem. 18:3834–3840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu XL, Zheng YL, Chen HM, Yan XJ, Wang F
and Xu WF: Anti-proliferation of human cervical cancer HeLa cell
line by fascaplysin through apoptosis induction. Yao Xue Xue Bao.
44:980–986. 2009.(In Chinese). PubMed/NCBI
|
|
19
|
Hamilton G: Cytotoxic effects of
fascaplysin against small cell lung cancer cell lines. Mar Drugs.
12:1377–1389. 2013. View Article : Google Scholar
|
|
20
|
Phillips MA, Gran ML and Peppas NA:
Targeted nanodelivery of drugs and diagnostics. Nano Today.
5:143–159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhidkov ME and Kaminskii VA: A new method
for the synthesis of marine alkaloid fascaplysin based on the
microwave-assisted Minisci reaction. Tetrahedron Lett.
54:3530–3532. 2014. View Article : Google Scholar
|
|
22
|
Kirsch G, Köng GM, Wright AD and Kaminsky
R: A new bioactive sesterterpene and antiplasmodial alkaloids from
the marine sponge hyrtios cf. erecta. J Nat Prod. 63:825–829. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Idziorek T, Estaquier J, De Bels F and
Ameisen JC: YOPRO-1 permits cytofluorometric analysis of programmed
cell death (apoptosis) without interfering with cell viability. J
Immunol Methods. 185:249–258. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujisawa S, Romin Y, Barlas A, Petrovic
LM, Turkekul M, Fan N, Xu K, Garcia AR, Monette S, Klimstra DS, et
al: Evaluation of YO-PRO-1 as an early marker of apoptosis
following radiofrequency ablation of colon cancer liver metastases.
Cytotechnology. 66:259–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rieger AM, Nelson KL, Konowalchuk JD and
Barreda DR: Modified annexin V/propidium iodide apoptosis assay for
accurate assessment of cell death. J Vis Exp. 50:2512011.
|
|
26
|
Kudryavtsev IV, Khaidukov SV, Zurochka AV
and Chereshev VA: Flow cytometry in experimental biology. RIO Ural
Branch of the Russian Academy of Sciences; Yekaterinburg, Russia:
2012, (In Russian).
|
|
27
|
Hughes D and Mehmet H: Cell Proliferation
and Apoptosis. 1st. Taylor and Francis; Abingdon, UK: 2002
|
|
28
|
Kostaras X, Cusano F, Kline GA, Roa W and
Easaw J: Use of dexamethasone in patients with high-grade glioma: A
clinical practice guideline. Curr Oncol. 21:e493–e503. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chamberlain MC: Temozolomide: Therapeutic
limitations in the treatment of adult high-grade gliomas. Expert
Rev Neurother. 10:1537–1544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen TC, Cho HY, Wang W, Wetzel SJ, Singh
A, Nguyen J, Hofman FM and Schönthal AH: Chemotherapeutic effect of
a novel temozolomide analog on nasopharyngeal carcinoma in vitro
and in vivo. J Biomed Sci. 22:712015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeiss CJ: The apoptosis-necrosis
continuum: Insights from genetically altered mice. Vet Pathol.
40:481–495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Zhang W, Cao WD, Cheng G and
Zhang YQ: Glioblastoma multiforme: Molecular characterization and
current treatment strategy (Review). Exp Ther Med. 3:9–14.
2012.PubMed/NCBI
|
|
33
|
Kalkan R: Glioblastoma stem cells as a new
therapeutic target for glioblastoma. Clin Med Insights Oncol.
9:95–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bryukhovetskiy A, Shevchenko V, Kovalev S,
Chekhonin V, Baklaushev V, Bryukhovetskiy I and Zhukova M: To the
novel paradigm of proteome-based cell therapy of tumors: Through
comparative proteome mapping of tumor stem cells and
tissue-specific stem cells of humans. Cell Transplant. 23:(Suppl
1). S151–S170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khalili AA and Ahmad MR: A Review of Cell
Adhesion Studies for Biomedical and Biological Applications. Int J
Mol Sci. 16:18149–18184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park D, Xiang AP, Mao FF, Zhang L, Di CG,
Liu XM, Shao Y, Ma BF, Lee JH, Ha KS, et al: Nestin is required for
the proper self-renewal of neural stem cells. Stem Cells.
28:2162–2171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barth RF and Kaur B: Rat brain tumor
models in experimental neuro-oncology: The C6, 9L, T9, RG2, F98,
BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 94:299–312. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang PY, Yang YJ, Xue Y, Fu J, Zhang CX,
Wang Y, Yang Y and Shi H: Cancer stem cells: Targeting tumors at
the source. Eur Rev Med Pharmacol Sci. 19:1821–1828.
2015.PubMed/NCBI
|
|
39
|
Xie Q, Mittal S and Berens ME: Targeting
adaptive glioblastoma: An overview of proliferation and invasion.
Neuro Oncol. 16:1575–1584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paltsev MA, Ivanov AA and Severin SE:
Cellular interactions (2nd). Medicine. Moscow: 2003.(In
Russian).
|
|
41
|
Wolinsky JB, Colson YL and Grinstaff MW:
Local drug delivery strategies for cancer treatment: gels,
nanoparticles, polymeric films, rods, and wafers. J Control
Release. 159:14–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng XG and Yue SW: Dexamethasone disrupts
cytoskeleton organization and migration of T47D human breast cancer
cells by modulating the AKT/mTOR/RhoA pathway. Asian Pac J Cancer
Prev. 15:10245–10250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Laquintana V, Trapani A, Denora N, Wang F,
Gallo JM and Trapani G: New strategies to deliver anticancer drugs
to brain tumors. Expert Opin Drug Deliv. 6:1017–1032. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Friedman HS, Kerby T and Calvert H:
Temozolomide and treatment of malignant glioma. Clin Cancer Res.
6:2585–2597. 2000.PubMed/NCBI
|
|
45
|
Zhang J, Stevens MF and Bradshaw TD:
Temozolomide: Mechanisms of action, repair and resistance. Curr Mol
Pharmacol. 5:102–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bryukhovetsky IS: The effectiveness in
vivo of a stem cell drug on glioblastoma rat's model after
chemotherapy. Russian Journal of Biotherapy. 13:51–58. 2014.(In
Russian).
|