Introduction
Although marked progress in recent decades has been
made in the treatment of cancer, pancreatic cancer remains a
lethal disease, with a 5-year survival rate of <6%
(1,2).
Personalized medicine and surgery is tailored to the individual
patient, and has the potential to improve the management of
pancreatic cancer (3,4). As pancreatic cancer is a malignant tumor
that exhibits heterogeneous biological characteristics, it may be
susceptible to treatment with personalized medicine (5). Global genomic analyses have revealed
various core signaling pathways in pancreatic cancer that may
represent ideal targets for personalized treatment, including
K-Ras, transforming growth factor β, c-Jun N-terminal kinases,
integrin, Wnt/Notch, Hedgehog, control of G1/S phase, apoptosis,
DNA damage control, small GTPases, invasion and homophilic cell
adhesion (4). It is necessary to
identify distinct pancreatic cancer subgroups with unique
characteristics in order to allow the selection of personalized
treatments.
Carbohydrate antigen 19-9 (CA19-9) is a
tumor-associated biomarker and its expression requires the presence
of sialylated Lewis antigen (6–10). It has
been extensively used as a pancreatic cancer biomarker at various
phases of pancreatic cancer management (6,8,9,11–13). The recommended upper limit for normal
serum CA19-9 expression is 37 U/ml, as determined by the standard
deviation of CA19-9 expression in the normal population (11,12,14).
Several studies have demonstrated that early- and advanced-stage
pancreatic cancer patients with normal serum CA19-9 expression (≤37
U/ml) had a significant survival advantage compared with patients
with elevated serum CA19-9 expression (>37 U/ml) (11,14,15).
However, the clinical features of pancreatic cancer occurring with
normal CA19-9 levels remain unknown.
In the present multicenter study, an extensive
analysis of the clinical, pathological and biological features of
patients with various stages of pancreatic cancer, who were
stratified by normal and elevated baseline serum CA19-9 levels, was
performed.
Materials and methods
Patients
All patients (1,912 cases) were selected from a
multicenter database constructed by the Shanghai Cancer Center of
Fudan University and the Shanghai Cancer Institute (Shanghai,
China); patients treated between December 2006 and March 2016 were
included. The protocol used in the present study conformed to the
ethical guidelines of The Declaration of Helsinki and was approved
by the Ethics Boards of the Shanghai Cancer Institute and Shanghai
Cancer Center. Written informed consent was obtained from all
patients participating in the study. Patients were stratified
according to their baseline serum CA19-9 level and type of
treatment received (surgery, chemotherapy, radiotherapy or best
supportive care). Survival time was calculated as the time between
the date of diagnosis and the date of the latest follow-up or
mortality (14). Follow-up
information was updated in April 2016.
The included patients were those who had
histological or cytological evidence of pancreatic adenocarcinoma.
Exclusion criteria included endocrine or acinar pancreatic
carcinoma, or intraductal papillary mucinous neoplasm associated
pancreatic adenocarcinoma. Patients lacking detailed information
for serum CA19-9 levels were also excluded. Tumors were staged
according to the 7th edition of the American Joint Committee on
Cancer (Chicago, IL, USA) classification (16). All patients with stage I or II
pancreatic cancer received curative-intent resection. Although
serum CA19-9 levels have been documented to be affected by altered
biliary excretion, such as with biliary tract obstruction (9), this effect was ignored as the subjects
in this study were subdivided into subgroups with CA19-9 levels ≤37
U/ml (CA19-9-normal) and ≥37 U/ml (CA19-9-elevated).
Statistical analysis
Continuous variables are presented as the mean ±
standard deviation. Time-to-event variables and the 2- and 5-year
survival rates were examined using the Kaplan-Meier estimator. The
treatment arms were compared using log-rank tests, and stratified
by serum CA19-9 levels and type of treatment received. Multivariate
analysis of the association between mortality and the clinical
characteristics of patients was performed by Cox proportional
hazards model. Continuous variables were compared using the
Student's t-test or the Wilcoxon rank-sum test, where
appropriate. Dichotomous variables were compared using the
χ2 test. STATA statistical software package (version
12.0; StataCorp LP, College Station, TX, USA) was used for all
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
Baseline characteristics
The present study analyzed a total of 1,912 cases
(588 of stage I–II and 1,324 of stage III–IV), of which the median
survival time was 8.7 months and the 5-year survival rate was 8.0%.
A total of 80.4% of patients exhibited baseline serum CA19-9 levels
of >37 U/ml. No statistically significant differences in patient
age (P=0.495), gender (P=0.670), tumor location (P=0.215), tumor
size (P=0.352), tumor grade (P=0.947), lymph node metastasis
(P=0.166), nerve invasion status (P=0.656) or vessel invasion
status (P=0.863) were observed between the CA19-9-normal and
CA19-9-elevated subgroups in patients of stage I–II. In addition,
no statistically significant differences in patient age (P=0.077),
gender (P=0.489), Eastern Cooperative Oncology Group (ECOG)
performance status (P=0.762), tumor location (P=0.487), tumor size
(P=0.119) or distant metastasis status (P=0.939) were observed
between the CA19-9-normal and CA19-9-elevated subgroups in patients
of stage III–IV.
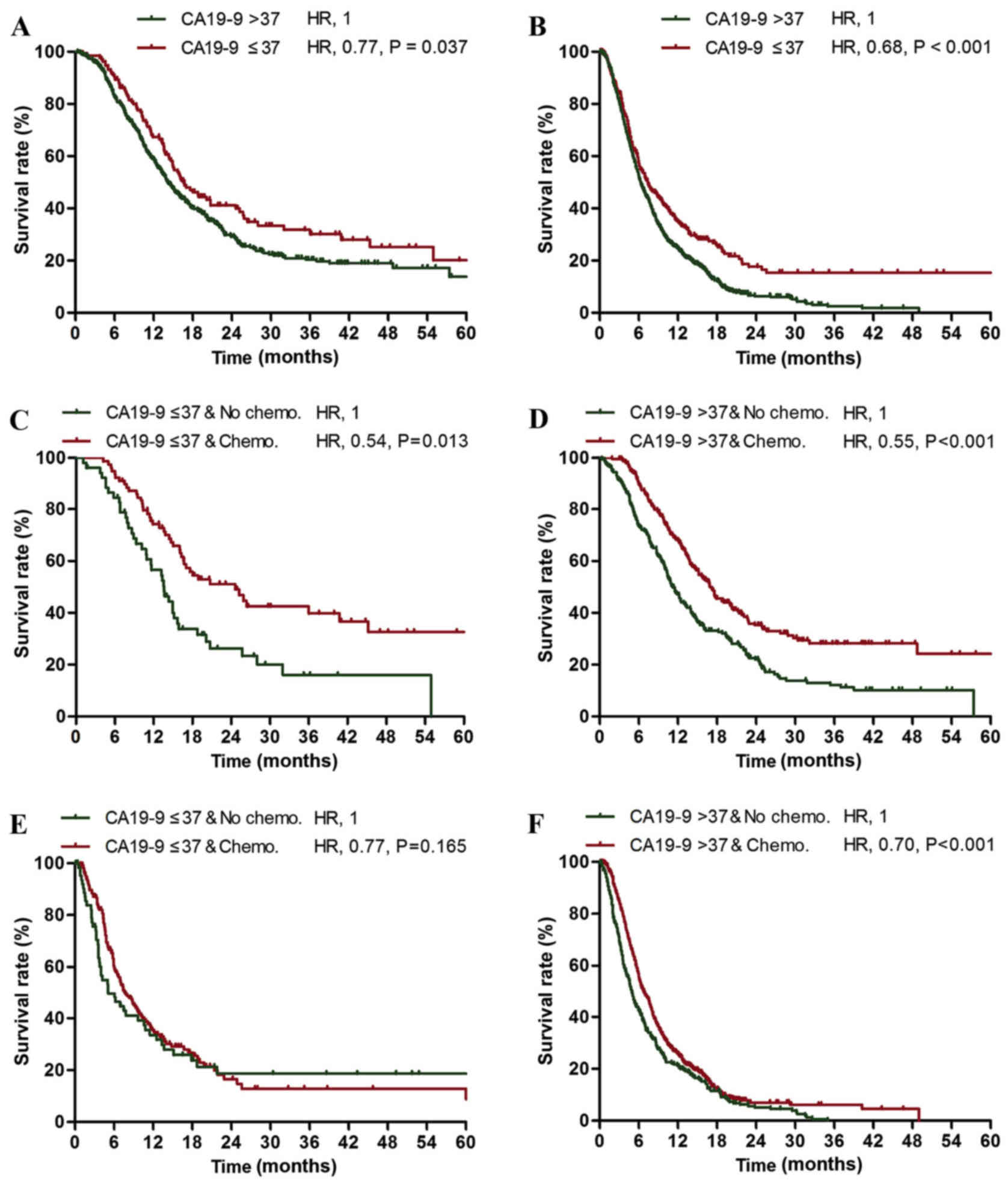
Improved prognosis in the
CA19-9-normal subgroup
Among the patients with stage I–II cancer, the
CA19-9-normal subgroup exhibited an increased survival rate
compared with the CA19-9-elevated subgroup (median survival times,
16.6 vs. 14.2 months; 2-year survival rates, 39.9 vs. 29.1%;
Fig. 1A). In addition, among the
stage III–IV patients, the CA19-9-normal subgroup exhibited an
increased survival rate compared with the CA19-9-elevated subgroup
(median survival times, 7.4 vs. 6.3 months; 2-year survival rates,
17.6 vs. 6.3%; Fig. 1B). The 5-year
survival rate of the stage III–IV CA19-9-normal subgroup was
increased compared with the stage I–II CA19-9-elevated subgroup
(15.4 vs. 13.8%; Fig. 1A and B).
Normal serum CA19-9 level was identified to be an independent
prognostic factor for mortality in patients with stage I–II and
stage III–IV pancreatic cancer [stage I–II, hazard ratio (HR)=0.77,
P=0.037; stage III–IV, HR=0.68, P<0.001; Table I].
 | Table I.Multivariate analysis (Cox
proportional hazards model) of the association between mortality
and the clinical characteristics of patients with stage I–II and
stage III–IV pancreatic cancer. |
Table I.
Multivariate analysis (Cox
proportional hazards model) of the association between mortality
and the clinical characteristics of patients with stage I–II and
stage III–IV pancreatic cancer.
|
| Stage
I–IIa | Stage
III–IVb |
|---|
|
|
|
|
|---|
| Clinical
characteristics | HR | P-value | HR | P-value |
|---|
| Age (>60 vs. ≤60
years) | 1.14 | 0.217 | 1.15 | 0.037 |
| Gender (female vs.
male) | 0.94 | 0.548 | 0.93 | 0.270 |
| Tumor location
(pancreatic head vs. all others) | 1.13 | 0.255 | 1.09 | 0.194 |
| ECOG performance
status (2–3 vs. 0–1) | / | / | 1.65 | <0.001 |
| Tumor size (>4 vs.
≤4 cm) | 1.79 | <0.001 | 1.15 | 0.042 |
| Nerve invasion status
(positive vs. negative) | 1.89 | 0.001 | / | / |
| Vessel invasion
status (positive vs. negative) | 1.59 | 0.002 | / | / |
| Lymph node metastasis
status (positive vs. negative) | 1.67 | <0.001 | / | / |
| Tumor grade (high vs.
low) | 1.32 | 0.012 | / | / |
| Distant metastasis
status (positive vs. negative) | / | / | 1.93 | <0.001 |
| Chemotherapy
(administered vs. not administered) | 0.58 | 0.001 | 0.71 | <0.001 |
| CA19-9 level (≤37
vs. >37 U/ml) | 0.77 | 0.037 | 0.68 | <0.001 |
Efficacy of gemcitabine-based
chemotherapy
Patients with stage I–II pancreatic cancer who
underwent neoadjuvant therapy, adjuvant radiotherapy or
non-gemcitabine-based adjuvant chemotherapy were excluded from the
evaluation of the response to gemcitabine-based chemotherapy. In
addition, patients with stage III–IV pancreatic cancer who
underwent radiotherapy or non-gemcitabine based chemotherapy were
excluded from the evaluation of response to gemcitabine-based
chemotherapy. No statistically significant differences were
observed in the proportion of gemcitabine-based chemotherapy
administered in the stage I–II CA19-9-normal subgroup compared with
the stage I–II CA19-9-elevated subgroup (59.8 vs. 55.7%; P=0.397),
or in the stage III–IV CA19-9-normal subgroup compared with the
stage III–IV CA19-9-elevated subgroup (71.9 vs. 72.0%; P=0.976).
The stage I–II CA19-9-normal (HR=0.54; P=0.013) and CA19-9-elevated
(HR=0.55; P<0.001) subgroups exhibited significantly increased
survival rates following treatment with gemcitabine compared with
the untreated subgroups (Table II;
Fig. 1C and D). However, the stage
III–IV CA19-9-normal subgroup exhibited no significant change in
survival rate following treatment with gemcitabine compared with
the untreated subgroup (HR=0.77; P=0.165; Table III; Fig.
1E), while the stage III–IV CA19-9-elevated subgroup exhibited
a significant increase in the 2-year and 5-year survival rates
following treatment with gemcitabine compared with the untreated
subgroup (HR=0.70; P<0.001; Table
III; Fig. 1F) sp16.
 | Table II.Association between mortality and the
clinical characteristics of patients with stage I–II pancreatic
cancer stratified by baseline CA19-9 levels according to Cox
proportional hazards model. |
Table II.
Association between mortality and the
clinical characteristics of patients with stage I–II pancreatic
cancer stratified by baseline CA19-9 levels according to Cox
proportional hazards model.
|
| CA19-9 ≤37
U/ml | CA19-9 >37
U/ml |
|---|
|
|
|
|
|---|
| Clinical
characteristics | HR | P-value | HR | P-value |
|---|
| Age (>60 vs. ≤60
years) | 1.37 | 0.198 | 1.08 | 0.528 |
| Gender (female vs.
male) | 0.66 | 0.103 | 1.00 | 0.983 |
| Tumor location
(pancreatic head vs. all others) | 1.25 | 0.396 | 1.10 | 0.439 |
| Tumor size (>4
vs. ≤4 cm) | 2.13 | 0.046 | 1.75 | 0.001 |
| Nerve invasion
status (positive vs. negative) | 2.50 | 0.052 | 1.83 | 0.004 |
| Vessel invasion
status (positive vs. negative) | 2.55 | 0.019 | 1.56 | 0.007 |
| Lymph metastasis
status (positive vs. negative) | 0.66 | 0.234 | 1.97 | <0.001 |
| Tumor grade (high
vs. low) | 1.39 | 0.202 | 1.33 | 0.024 |
| Chemotherapy
(administered vs. not administered) | 0.54 | 0.013 | 0.55 | <0.001 |
 | Table III.Association between the mortality
rate and clinical characteristics of patients with stage III–IV
pancreatic cancer according to Cox proportional hazards model. |
Table III.
Association between the mortality
rate and clinical characteristics of patients with stage III–IV
pancreatic cancer according to Cox proportional hazards model.
|
| CA19-9 ≤37
U/ml | CA19-9 >37
U/ml |
|---|
|
|
|
|
|---|
| Clinical
characteristics | HR | P-value | HR | P-value |
|---|
| Age (>60 vs. ≤60
years) | 1.44 | 0.028 | 1.10 | 0.173 |
| Gender (female vs.
male) | 1.01 | 0.954 | 0.92 | 0.245 |
| Tumor location
(pancreatic head vs. all others) | 0.93 | 0.687 | 1.13 | 0.093 |
| ECOG performance
status (0–1 vs. 2–3) | 0.54 | 0.005 | 0.68 | <0.001 |
| Tumor size (>4
vs. ≤4 cm) | 1.17 | 0.377 | 1.18 | 0.035 |
| Distant metastasis
status (positive vs. negative) | 1.88 | 0.001 | 1.96 | <0.001 |
| Chemotherapy
(administered vs. not administered) | 0.77 | 0.165 | 0.70 | <0.001 |
Discussion
CA19-9 is the most important tumor marker in
pancreatic cancer and is aberrantly secreted by the majority of
pancreatic tumors (6,9,11–15,17,18).
However, a distinct subset of patients with pancreatic cancer
present with normal serum CA19-9 levels and are occasionally Lewis
antigen-positive. These patients exhibit decreased or no CA19-9
secretion, independent of Lewis antigen genotype (18). In the present study, patients with
CA19-9-normal pancreatic cancer were demonstrated to be a distinct
subgroup that has a more favorable prognosis and unique therapeutic
response.
The improved prognosis and distinct therapeutic
response of the CA19-9-normal subgroup cannot be attributed to
tumor burden or stage, but to its biological behavior. CA19-9, also
known as sialylated Lewis a antigen, has been reported to promote
metastasis by binding E-selectin, which is expressed on the surface
of endothelial cells (7,19). Several studies have demonstrated that
CA19-9 promotes pancreatic cancer cell metastasis (20–22),
suggesting that CA19-9 is a therapeutic target in the treatment of
pancreatic cancer. However, further studies are required to confirm
this hypothesis.
Chemotherapy is frequently utilized in the treatment
of pancreatic cancer. In the present study, it was observed that
patients with stage I–II pancreatic cancer exhibited a
significantly increased survival rate following gemcitabine-based
adjuvant chemotherapy, compared with the untreated patients. In
addition, gemcitabine-based chemotherapy was effective against
stage III–IV pancreatic tumors with elevated CA19-9 expression.
However, patients with stage III–IV CA19-9-normal pancreatic cancer
exhibited a poor response to gemcitabine-based chemotherapy. Novel
chemotherapeutic agents and regimens are required for the treatment
of advanced stage pancreatic cancer with normal CA19-9
expression.
The present study did not determine the clinical and
pathological characteristics of Lewis antigen-negative patients
with pancreatic cancer. However, several studies have observed
similar survival rates in patients with resectable pancreatic
adenocarcinoma with undetectable and normal CA19-9 levels (14,15,23).
Furthermore, abnormal Lewis antigens (including types a and b) and
the Lewis enzyme have been detected in normal and neoplastic tissue
samples from patients typed as Lewis antigen
a−b− on red blood cells (18,24–27). For
example, Orntoft et al (27)
detected Lewis antigens in 3/6 cancer-bearing patients using
immunohistology and immunochemistry; however all 6 patients were
typed as Lewis antigen a−b− according to
hemagglutination assays. These observations further support that
normal serum CA19-9 expression should be viewed as a distinct
subgroup of pancreatic cancer, independently of Lewis antigen
status.
In conclusion, CA19-9-normal pancreatic cancer is a
less aggressive subgroup of pancreatic cancer that has distinct
clinical, pathological and biological characteristics. The
characterization of this subgroup may have a great impact on the
overall management of pancreatic cancer. Clinical trials should be
separately conducted on patients with pancreatic cancer who are
subdivided by baseline serum CA19-9 levels. Despite its large
sample size, the present study is limited by its retrospective
nature; therefore prospective randomized clinical studies are
required to confirm the results.
Acknowledgements
The authors would like to thank Professor Jianfeng
Luo from the School of Public Health of Fudan University (Shanghai,
China), Dr Zengying Liu and Dr Jing Huang from Life Technologies
Comapny (Carlsbad, CA, USA), Dr Jing Wang from the Shanghai Cancer
Institute, Professor Dong Xiang from the Shanghai Blood Center
(Shanghai, China), and Professor Menghong Sun from the Tissue Bank
of the Shanghai Cancer Center, for their technical assistance. The
present study was supported by the Shanghai Rising Star Program
(grant no. 14QA1400900) and the National Science Foundation of
China (grant no. 81372649).
References
|
1
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamburg MA and Collins FS: The path to
personalized medicine. N Engl J Med. 363:301–304. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ko AH and Tempero MA: Personalized
medicine for pancreatic cancer: A step in the right direction.
Gastroenterology. 136:43–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones S, Zhang X, Parsons DW, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et
al: Core signaling pathways in human pancreatic cancers revealed by
global genomic analyses. Science. 321:1801–1806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goonetilleke KS and Siriwardena AK:
Systematic review of carbohydrate antigen (CA 19–9) as a
biochemical marker in the diagnosis of pancreatic cancer. Eur J
Surg Oncol. 33:266–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kannagi R: Carbohydrate antigen sialyl
Lewis a-its pathophysiological significance and induction mechanism
in cancer progression. Chang Gung Med J. 30:189–209.
2007.PubMed/NCBI
|
|
8
|
Pleskow DK, Berger HJ, Gyves J, Allen E,
McLean A and Podolsky DK: Evaluation of a serologic marker, CA19-9,
in the diagnosis of pancreatic cancer. Ann Intern Med. 110:704–709.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schlieman MG, Ho HS and Bold RJ: Utility
of tumor markers in determining resectability of pancreatic cancer.
Arch Surg. 138:951–955. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tempero MA, Uchida E, Takasaki H, Burnett
DA, Steplewski Z and Pour PM: Relationship of carbohydrate antigen
19–9 and Lewis antigens in pancreatic cancer. Cancer Res.
47:5501–5503. 1987.PubMed/NCBI
|
|
11
|
Ferrone CR, Finkelstein DM, Thayer SP,
Muzikansky A, FernandezdelCastillo C and Warshaw AL: Perioperative
CA19-9 levels can predict stage and survival in patients with
resectable pancreatic adenocarcinoma. J Clin Oncol. 24:2897–2902.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Humphris JL, Chang DK, Johns AL, Scarlett
CJ, Pajic M, Jones MD, Colvin EK, Nagrial A, Chin VT, Chantrill LA,
et al: The prognostic and predictive value of serum CA19.9 in
pancreatic cancer. Ann Oncol. 23:1713–1722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kondo N, Murakami Y, Uemura K, Hayashidani
Y, Sudo T, Hashimoto Y, Nakashima A, Sakabe R, Shigemoto N, Kato Y,
et al: Prognostic impact of perioperative serum CA 19–9 levels in
patients with resectable pancreatic cancer. Ann Surg Oncol.
17:2321–2329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berger AC, Garcia M Jr, Hoffman JP, Regine
WF, Abrams RA, Safran H, Konski A, Benson AB III, MacDonald J and
Willett CG: Postresection CA 19–9 predicts overall survival in
patients with pancreatic cancer treated with adjuvant
chemoradiation: A prospective validation by RTOG 9704. J Clin
Oncol. 26:5918–5922. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berger AC, Meszoely IM, Ross EA, Watson JC
and Hoffman JP: Undetectable preoperative levels of serum CA 19–9
correlate with improved survival for patients with resectable
pancreatic adenocarcinoma. Ann Surg Oncol. 11:644–649. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Magnani JL, Steplewski Z, Koprowski H and
Ginsburg V: Identification of the gastrointestinal and pancreatic
cancer-associated antigen detected by monoclonal antibody 19–9 in
the sera of patients as a mucin. Cancer Res. 43:5489–5492.
1983.PubMed/NCBI
|
|
18
|
Luo G, Guo M, Jin K, Liu Z, Liu C, Cheng
H, Lu Y, Long J, Liu L, Xu J, et al: Optimize CA19-9 in detecting
pancreatic cancer by Lewis and Secretor genotyping. Pancreatology.
pii:S1424–3903. 2016.(Epub ahead of print).
|
|
19
|
Takada A, Ohmori K, Yoneda T, Tsuyuoka K,
Hasegawa A, Kiso M and Kannagi R: Contribution of carbohydrate
antigens sialyl Lewis A and sialyl Lewis X to adhesion of human
cancer cells to vascular endothelium. Cancer Res. 53:354–361.
1993.PubMed/NCBI
|
|
20
|
Aubert M, Panicot L, Crotte C, Gibier P,
Lombardo D, Sadoulet MO and Mas E: Restoration of alpha(1,2)
fucosyltransferase activity decreases adhesive and metastatic
properties of human pancreatic cancer cells. Cancer Res.
60:1449–1456. 2000.PubMed/NCBI
|
|
21
|
Aubert M, PanicotDubois L, Crotte C,
Sbarra V, Lombardo D, Sadoulet MO and Mas E: Peritoneal
colonization by human pancreatic cancer cells is inhibited by
antisense FUT3 sequence. Int J Cancer. 88:558–565. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iwai K, Ishikura H, Kaji M, Sugiura H,
Ishizu A, Takahashi C, Kato H, Tanabe T and Yoshiki T: Importance
of E-selectin (ELAM-1) and sialyl Lewis(a) in the adhesion of
pancreatic carcinoma cells to activated endothelium. Int J Cancer.
54:972–977. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hartwig W, Strobel O, Hinz U, Fritz S,
Hackert T, Roth C, Büchler MW and Werner J: CA19-9 in potentially
resectable pancreatic cancer: Perspective to Adjust Surgical and
Perioperative Therapy. Ann Surg Oncol. 20:2188–2196. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Björk S, Breimer ME, Hansson GC, Karlsson
KA and Leffler H: Structures of blood group glycosphingolipids of
human small intestine. A relation between the expression of
fucolipids of epithelial cells and the ABO, Le and Se phenotype of
the donor. J Biol Chem. 262:6758–6765. 1987.PubMed/NCBI
|
|
25
|
Hamada E, Taniguchi T, Baba S and Maekawa
M: Investigation of unexpected serum CA19-9 elevation in
Lewis-negative cancer patients. Ann Clin Biochem. 49:266–272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirano K, Kawa S, Oguchi H, Kobayashi T,
Yonekura H, Ogata H and Homma T: Loss of Lewis antigen expression
on erythrocytes in some cancer patients with high serum CA19-9
levels. J Natl Cancer Inst. 79:1261–1268. 1987.PubMed/NCBI
|
|
27
|
Orntoft TF, Holmes EH, Johnson P, Hakomori
S and Clausen H: Differential tissue expression of the Lewis blood
group antigens: Enzymatic, immunohistologic, and immunochemical
evidence for Lewis a and b antigen expression in Le(a-b-)
individuals. Blood. 77:1389–1396. 1991.PubMed/NCBI
|