Introduction
A number of epidemiological and clinical studies
have presented evidence that chronic inflammation caused by
microbial infection, as well as chemical irritants, significantly
increases cancer risk (1,2). Obesity and post-partum involution are
chronic inflammatory states in mammary glands, and are associated
with increased breast cancer risk (3). Furthermore, non-steroidal
anti-inflammatory drugs (NSAID), which are nonselective
Prostaglandin G/H synthase 2, or cyclooxygenase-2 (COX-2)
inhibitors, significantly reduce the risk of mammary
carcinogenesis, recurrence, and motility of breast cancer (4). In the tumor microenvironment, various
inflammatory cells, such as tumor-associated macrophages, are
recruited to form a pro-tumor inflammatory environment.
Inflammatory cells produce a variety of mediators, including growth
factors, chemokines and cytokines, which induce tumor growth,
invasion, and angiogenesis (5,6).
Proinflammatory cytokines are a major determinant
for the invasiveness of tumor cells. Among these cytokines,
interleukin-1β (IL-1β) is abundant in tumor tissues and stimulates
tumor growth, invasion, carcinogenesis and host-tumor association
(7). IL-1β-knockout mice are
resistant to the development of chemically induced tumors (8), and exhibit suppressed tumor invasion and
angiogenesis (9,10). On the other hand, stomach-specific
IL-1β overexpression induces gastric inflammation and cancer
(11). These results identify IL-1β
as one of the essential components mediating
inflammation-associated tumor progression. IL-1β stimulation of
tumor cells activates multiple signaling pathways involving protein
kinase B, mitogen activated protein (MAP) kinase, and nuclear
factor-κB (12). Activation of these
signaling molecules is required for IL-1β-mediated production of
matrix metalloproteinase (MMP)-9, a matrix degrading enzyme that is
regarded as a critical regulator in IL-1β-induced tumor invasion
(13–15). A number of studies have investigated
the association between MMP expression and the prognosis of breast
cancer patients. Most recently, a meta-analysis by Ren et al
(16) reported that MMP-9
overexpression in serum was associated with poor patient prognosis
in breast cancer.
Proto-oncogene tyrosine-protein kinase Src (Src) is
a non-receptor tyrosine kinase that is comprised of SH3, SH2, and
kinase domains. Extracellular stimuli including cytokines, growth
factors and integrin engagement, activate Src, which in turn,
phosphorylates various target proteins to regulate cell
proliferation, differentiation, and migration (17,18). Among
these target proteins, focal adhesion kinase 1 (FAK) is essential
for the regulation of signal transduction, cell adhesion and
migration carried out by Src (19).
FAK is composed of an N-terminal FERM domain, a central kinase
domain, and a C-terminal focal adhesion targeting domain.
Additionally, FAK localizes to the site of cell-extracellular
matrix contact (20). There are six
major tyrosine phosphorylation sites in FAK, and two of them, the
Tyr397 and Tyr925 sites, are important for FAK-dependent signaling
(21). Src interacts with the
phosphorylated Tyr397 of FAK and phosphorylates Tyr925, which in
turn associates with signaling molecules such as growth factor
receptor-bound protein 2 (Grb2) to induce activation of the
Ras-dependent/MAP kinase pathway (19,22).
It has previously been reported by this group that
the enhancement of cell invasion caused by nitric oxide stimulation
is mediated by Src and FAK kinase activation in MCF-7 breast cancer
cells (23). To expand on these
findings, the current study aimed to examine the role Src and FAK
serve in the IL-1β-mediated cell invasion of MCF-7 cells, and
identify whether Src and FAK kinase are involved in MMP-9
production and cell invasion.
Materials and methods
Antibodies, cytokines and
chemicals
Recombinant murine IL-1b and recombinant human IL-1b
were purchased from PeproTech EC (London, UK). The PP2 kinase
inhibitor (PP2) was purchased from EMD Millipore (Billerica, MA,
USA). Anti-FAK (cat. no. sc-154; 1:1,000), anti-phospho-Erk (cat.
no. sc-7383; 1:1,000) and anti-Erk2 (cat. no. sc-558; 1:1,000)
antibodies were all obtained from Santa Cruz Biotechnology (Dallas,
TX, USA); anti-phosphotyrosine antibody (pTyr20; cat. no. 610012;
1:1,000) was purchased from BD Transduction Laboratories™ (BD
Biosciences, Franklin Lakes, NJ USA); anti-phospho-FAK antibody
(pTyr397; cat. no. 44624G; 1:500) was purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA); and
anti-phospho-Src (pTyr416; cat. no. 6943; 1:1,000) and
anti-phospho-FAK (pTyr925; cat. no. 3284; 1:1,000) antibodies were
both obtained from Cell Signaling Technology (Danvers, MA,
USA).
Cell culture, plasmid construction,
and transfection
The human breast cancer cell line, MCF-7, was
obtained from the Japanese Collection of Research Bioresources Cell
Bank (Osaka, Japan), and cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (Biowest
Europe, Nuaillé, France) and 5 mg/ml human insulin (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany). Subsequently, knockout of FAK
was performed to establish homozygous null FAK knockout fibroblast
cells, following a previously documented procedure by Ilić et
al (24). To establish
FAK-wild-type (FAK-Wt), FAK-kinase dead (KD), FAK-Y397F, and
FAK-Y925F cells, wild-type and mutant FAKs were cloned into a
pBabepuro vector and transfected into FAK-Ko cells.
Assay of gelatin-degrading MMPs by
zymography
The activity of MMPs in the conditioned media was
assayed by zymography as described previously (21). Briefly, cells were incubated in
serum-free medium for 6 h followed by stimulation with or without
IL-1b for 16 h. Conditioned media were collected, clarified by
centrifugation, and subjected to electrophoresis with sodium
dodecyl sulphate-polyacrylamide gels copolymerized with gelatin.
Gels were washed and incubated with reaction buffer (50 mM
Tris-HCl, pH 7.4, 0.02% NaN3, 10 mM CaCl2)
for 16 h at 37°C, stained with Coomassie brilliant blue, and
subsequently destained.
FAK siRNA and transfection
The sequence of FAK siRNA is
5′-CCACCUGGGCCAGUAUUAUTT-3, and the sequence for luciferase siRNA
is 5′-CUUACGCUGAGUACUUCGATT-3′. Cells were transfected with 20 nM
of each siRNA using Lipofectamine™ RNAi/MAX (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturers'
protocol.
Invasion assay
MCF-7 cells were assayed for their invasiveness by a
modified Boyden chamber method as described previously (21). Cells were serum-starved for 6 h and
pretreated with varying concentrations of IL-1b (0, 0.5, 1, 3, 5,
10 nM) for 12 h. Cells were subsequently resuspended in serum-free
DMEM and seeded onto Matrigel-coated filters with or without IL-1b.
Following incubation for 7 h, cells that had invaded the lower
surface of the filter were fixed, stained, and quantified by
counting three randomly selected fields under the microscope. The
mean ± standard deviation (SD) of three independent experiments was
calculated. To examine cell invasion in the absence of FAK
expression, cells were incubated with the indicated siRNAs for 30
h, serum-starved for 6 h and treated with IL-1b for 12 h before
undergoing an invasion assay. To investigate cell invasion with
PP2, cells were serum-starved for 6 h, treated with 10 µM PP2 for 1
h, stimulated with IL-1b for 12 h and subjected to an invasion
assay.
Statistical analysis
Values are expressed as the mean ± SD of three
independent experiments. Comparisons between groups were performed
using an unpaired Student's t-test. Statistical analysis was
performed using GraphPad Prism software (version 7.0; GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 were considered to
indicate a statistically significant difference.
Results
IL-1b induces invasion and MMP-9
production in MCF-7 cells
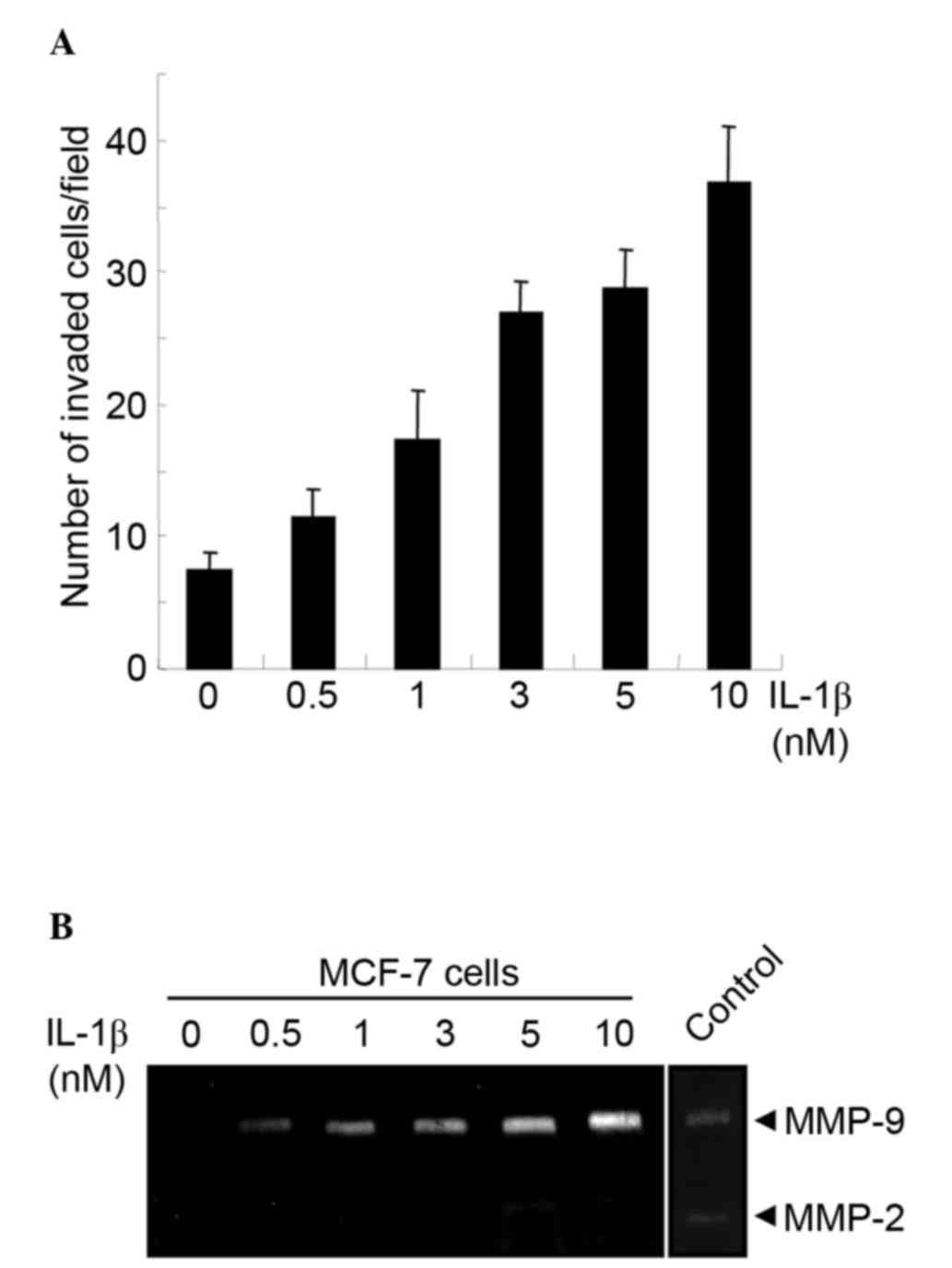
The effect of IL-1b stimulation on the invasiveness
of non-metastatic MCF-7 human breast cancer cells in vitro
was examined. The level of invasion by MCF-7 cells was low in the
absence of IL-1b stimulation. However, increasing the concentration
of IL-1b increased the invasiveness of MCF-7 cells in a
dose-dependent manner (Fig. 1A). MMPs
are matrix-degrading enzymes essential for tumor cell invasion.
Previous studies have demonstrated that IL-1b activates MMP-9
secretion (14). Therefore, the
production of MMP-9 in MCF-7 cells following IL-1b stimulation was
examined. MCF-7 cells were serum-starved, treated with the
indicated concentrations of human IL-1b for 16 h prior to gelatin
zymography. Although MMP-9 production was undetectable in MCF-7
cells in the absence of IL-1b, IL-1b stimulation increased MMP-9
expression in a dose-dependent manner (Fig. 1B). By contrast, the production of
MMP-2 was limited even at higher doses of IL-1b (Fig. 1B).
FAK and Src are activated by IL-1b
stimulation in MCF-7 cells
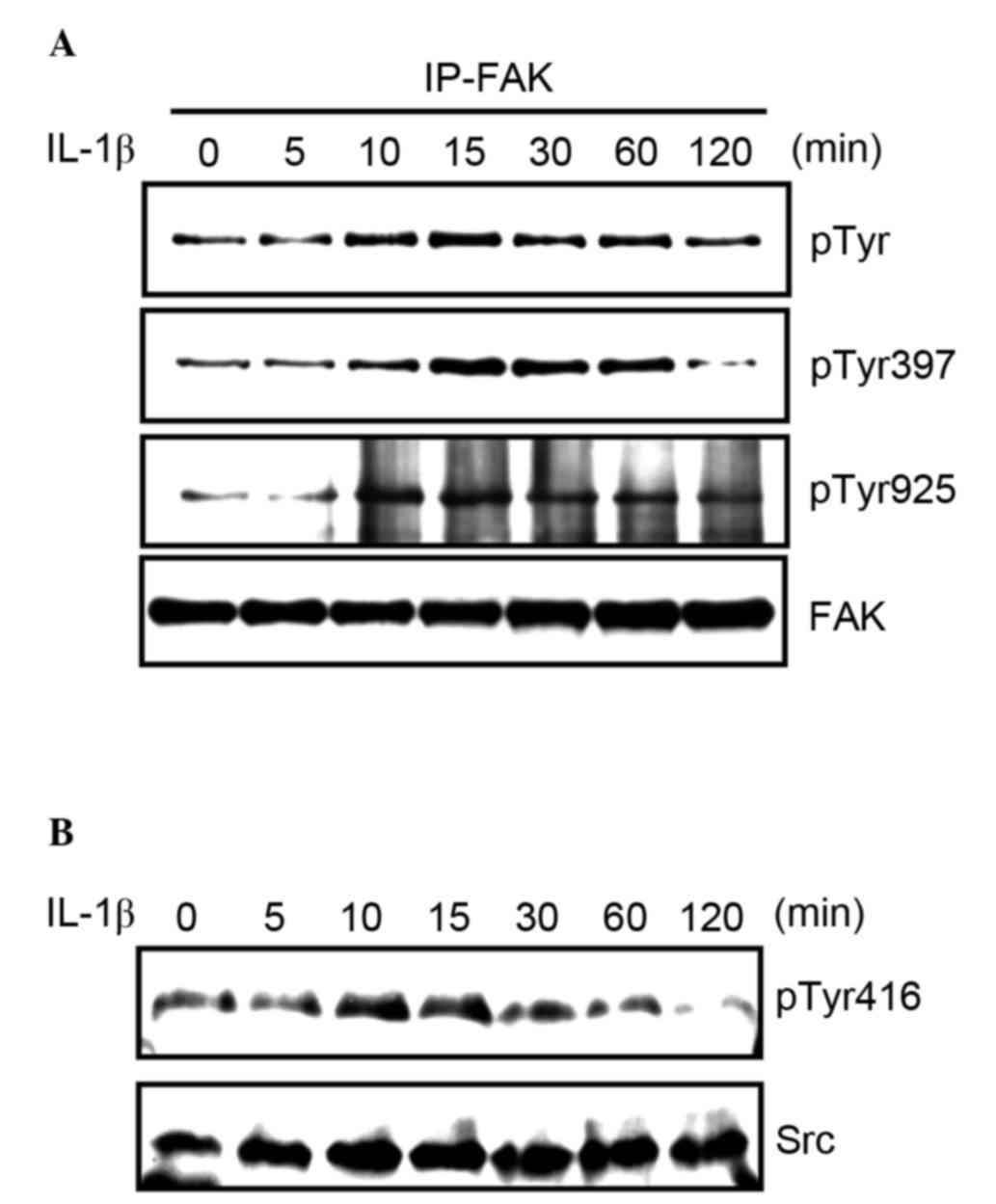
It was previously demonstrated by this group that
FAK activation is responsible for the increased production of MMP-9
by fibronectin and tumor necrosis factor α (25,26). Thus,
the current study investigated whether FAK was activated by IL-1b
stimulation. To measure tyrosine phosphorylation of FAK, MCF-7
cells were serum-starved, treated with 3 nM IL-1b, and lysed to
immunoprecipitate FAK, and western blotting was subsequently
carried out. Treatment of MCF-7 cells with IL-1b increased FAK
tyrosine phosphorylation in a time-dependent manner (Fig. 2A). Furthermore, the phosphorylation of
Tyr397 and Tyr925 in FAK was evaluated. Tyr397 is
auto-phosphorylated when the kinase is activated, and Tyr925, which
functions as a binding site for Grb2 to activate the Ras/ERK
pathway, is phosphorylated by Src. The phosphorylation of these
tyrosine residues was induced by IL-1b stimulation (Fig. 2A). Src regulates the phosphorylation
of Tyr925, therefore its activation following IL-1b stimulation was
assessed. The phosphorylation of Tyr416, which regulates the
catalytic activity of Src, was increased by IL-1b stimulation
(Fig. 2B).
FAK and Src are required for
IL-1b-induced MMP-9 production and invasion in MCF-7 cells
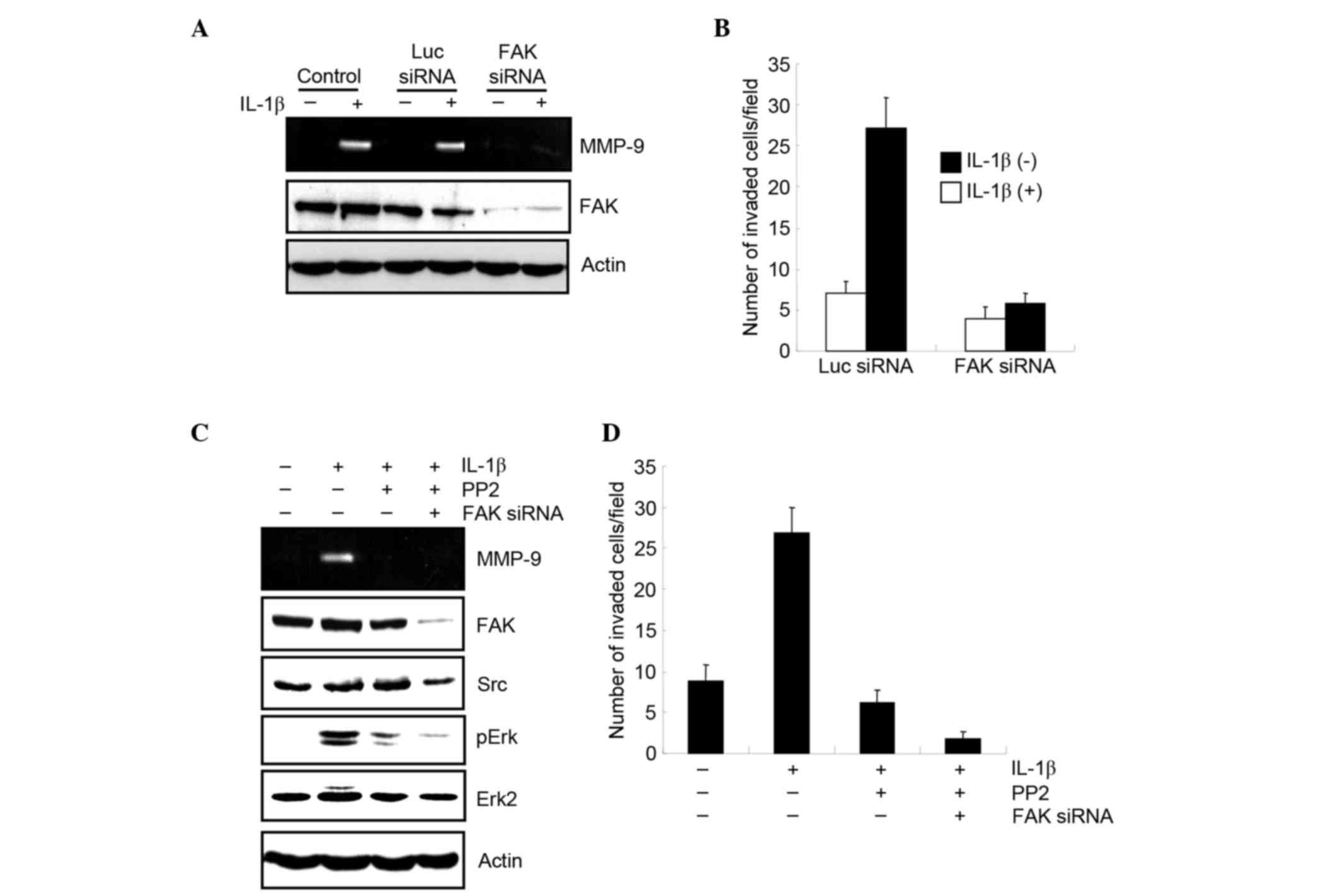
The effect of silencing FAK on the production of
MMP-9 in MCF-7 cells was investigated. MCF-7 cells were transfected
with FAK siRNA, and the production of MMP-9 after IL-1b treatment
was examined by zymography. Knockdown of FAK expression by siRNA
reduced the production of MMP-9 by IL-1b (Fig. 3A). The role of FAK in the
IL-1b-induced invasion of MCF-7 cells was then examined using a
modified Boyden chamber. MCF-7 cells were transfected with either a
luciferase or FAK siRNA, and after 30 h, they were starved and
stimulated with IL-1b. Cells were then loaded onto the upper
chamber and incubated with or without IL-1b. Following 7 h, cells
that had invaded the lower surface of the chamber were fixed,
stained and quantified by counting. The results demonstrated that
FAK siRNA significantly suppressed cell invasion (Fig. 3B).
To determine whether Src is required for
IL-1b-mediated production of MMP-9, the Src inhibitor PP2 was used.
MCF-7 cells were stimulated with IL-1b with or without PP2, MMP-9
production was examined, and it was observed that PP2 treatment
markedly suppressed MMP-9 production (Fig. 3C).
A previous study had demonstrated that the
activation of extracellular signal-related kinases (Erk) was
required for MMP-9 production (12).
The current study demonstrated that stimulation of MCF-7 cells with
IL-1b led activated Erk, which in turn was inhibited by PP2
treatment. In addition, the combined treatment of PP2 and FAK siRNA
reduced the phosphorylation of Erk, which is mediated by IL-1b
(Fig. 3C). This indicates that the
Src/FAK pathway is crucial for the activation of Erk by IL-1b, and
production of MMP-9.
The requirement of Src for IL-1b-induced invasion of
MCF-7 cells was examined using a modified Boyden chamber.
IL-1b-induced cell invasion was suppressed by PP2 treatment, and
the combined treatment of PP2 and FAK siRNA significantly reduced
cell invasion (P=0.0014; Fig. 3D).
Therefore, FAK and Src are both required for IL-1b-mediated MMP-9
production and cell invasion.
IL-1b-mediated MMP-9 production is
dependent on the activation of FAK
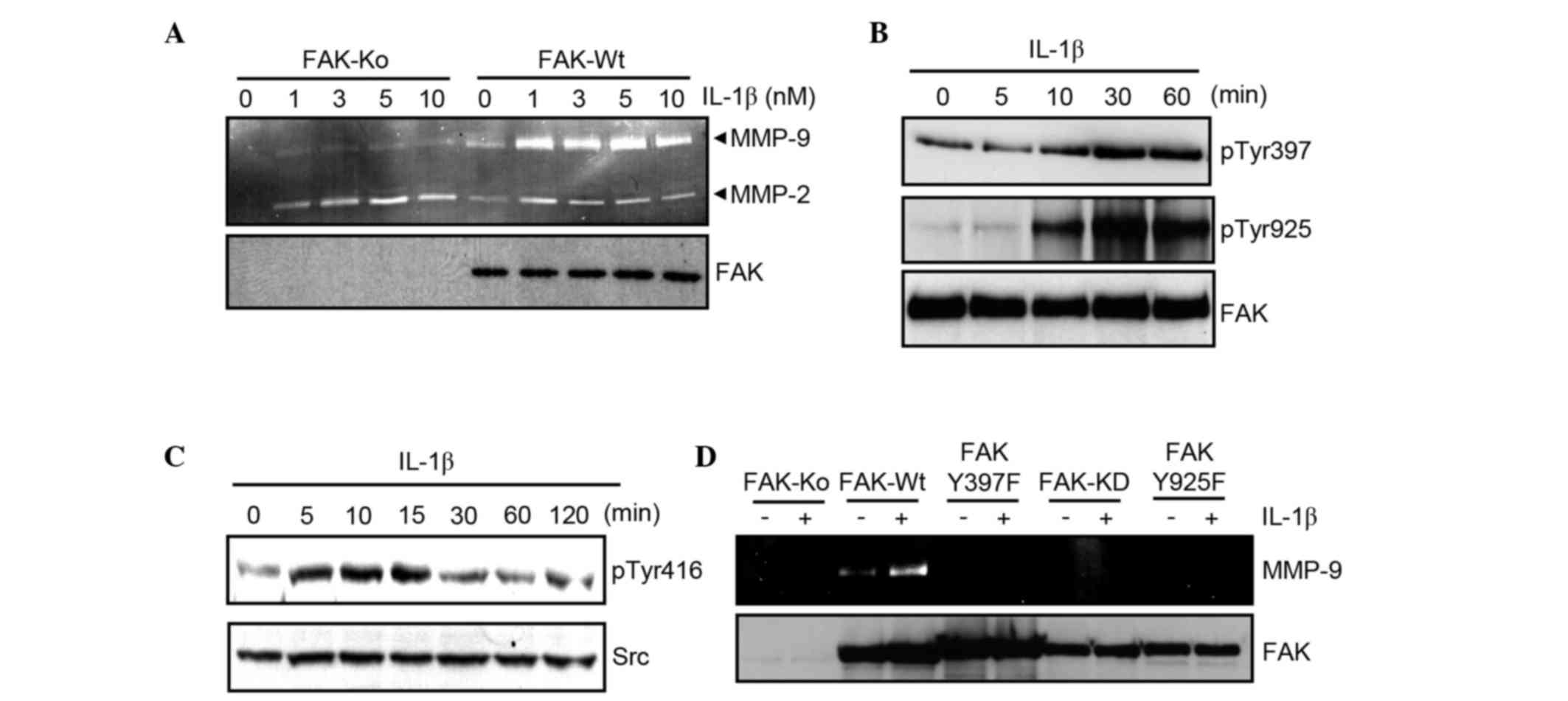
To confirm the role of FAK in IL-1b-induced MMP-9
production, FAK-Ko cells and FAK-Wt cells (generated by
transfecting FAK-Ko cells with wild-type FAK) were used. The cells
were serum-starved and stimulated with varying concentrations of
IL-1b (0, 1, 3, 5, and 10 nM) before undergoing gelatin zymography.
The results demonstrated that IL-1b stimulation increased the
production of MMP-9 by FAK-Wt cells (Fig.
4A). By contrast, an increase of MMP-2 production stimulated by
IL-1b was limited in FAK-Wt cells. FAK-Ko cells responded weakly to
IL-1b treatment, and the production of MMP-9 was markedly lower
than untreated FAK-Wt cells (Fig.
4A). These results indicate that FAK is crucial for
IL-1b-mediated MMP-9 production.
The effect of IL-1b stimulation in FAK-Wt cells was
subsequently examined, to identify whether FAK and Src were
activated. FAK-Wt cells were serum-starved, stimulated with 3 nM
IL-1b, and the expression of phosphorylated FAK and Src was
examined by western blot analysis. This demonstrated that IL-1b
enhanced the tyrosine phosphorylation of FAK and Src in FAK-Wt
cells (Fig. 4B and C).
Finally, to investigate the functional role of FAK
for MMP-9 production, cell lines expressing mutant FAK were
established. FAK-Y397F and FAK-Y925F are mutant FAKs in which
Tyr397 and Tyr925 are replaced with phenylalanine, respectively.
FAK-KD lacks kinase activity due to replacement of lysine 454 with
arginine. Cell lines expressing these mutants did not increase
MMP-9 expression in the presence of IL-1b (Fig. 4D). These data suggest a critical role
of FAK activity in IL-1b-dependent MMP-9 production.
Discussion
IL-1b is abundant at tumor sites, and it induces the
expression of various genes to facilitate malignant cell invasion
(7). Src and FAK are critical
regulators that control cell attachment, migration and signal
transduction which are associated with invasion and metastasis
(27–29). The present report investigated whether
Src and FAK are required for IL-1b-mediated MMP-9 production and
cell invasion. Using FAK-Ko mouse fibroblasts and FAK
siRNA-transfected MCF-7 cells, it was demonstrated that FAK is
essential for IL-1b-induced MMP-9 production and cell invasion.
Furthermore, suppression of Src activity by a chemical inhibitor
decreased IL-1b-induced MMP-9 production and cell invasion,
indicating that Src activation is required to induce MMP-9 via
stimulation of IL-1b. These results demonstrate that activation of
the Src/FAK pathway is crucial for cell invasion following IL-1b
stimulation.
MMPs are major proteolytic enzymes required for
tumor invasion and angiogenesis. Among MMPs, MMP-9 secretion is
observed in different types of cancer, and its production is
regulated by extracellular stimuli, such as growth factors and
cytokines (30). Although the
signaling pathways required for MMP-9 production differ depending
on the extracellular stimuli, Erk activation seems to be essential
(14,31). In the current study, it was observed
that inhibiting the Src/FAK pathway significantly reduced the
activation of Erk by IL-1b. It had previously been demonstrated
that phosphorylating Tyr397 and Tyr925 induces the activation of
the Ras/Erk pathway (19). In the
present study, cells expressing mutant FAK, which consisted of
Tyr397 and Try925 substituted to alanine, exhibited only a limited
increase of MMP-9 production following IL-1b stimulation. These
results suggest that activation of the Src/FAK pathway is required
for the Erk activation by IL-1b, which in turn promotes MMP-9
production.
Previous studies have indicated that Src and FAK are
related to cancer progression and invasion (32–34).
Elevated Src expression has been observed in different types of
cancer, including colon, breast, pancreatic and gastric cancer
(27). In addition, genetic analysis
has revealed an activating mutation in the C-terminus of Src in a
subset of metastatic colon cancers (35). Overexpression of FAK has been observed
in various types of invasive cancer, and FAK activity in malignant
cells is correlated with invasiveness (36). Both Src and FAK are critical for the
assembly of focal adhesions and cytoskeleton to induce tumor cell
invasion (37). In addition to these
critical functions, the current study indicated that Src and FAK
are required for IL-1b-induced cell invasion and MMP-9 production.
Taken together, these results indicate that the Src/FAK pathway
plays a pivotal role in inflammation-mediated tumor cell
invasion.
IL-1b promotes MMP-9 production and cell invasion in
non-metastatic MCF-7 breast cancer cells. Src and FAK activation
are important for MMP-9 production and cell invasion by IL-1b
stimulation and IL-1b-induced Erk activation is dependent on the
activation of the Src/FAK pathway. Systemic treatment of mice with
the IL-1 receptor antagonist (IL-1Ra), a physiological inhibitor of
IL-1 signaling, inhibits tumor growth and metastasis, indicating
that targeting IL-1b signaling is a promising therapy for cancer
(9). Both Src and FAK have been
extensively studied over the last decade. However, therapeutically
targeting Src and FAK has only generated substantial interest
recently (38).
In conclusion, the results of the present study
suggest that the inhibition of the Src/FAK pathway is an effective
treatment for inflammation-associated tumor growth and
invasion.
Acknowledgements
We thank the members and staff of the Division of
Cancer Biology for their technical assistance and helpful
discussion and S.K. Hanks for providing FAK mutants. This work was
supported by a grant from the Japan Society for the Promotion of
Science (grant no. 15K08302).
References
|
1
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hussain SP and Harris CC: Inflammation and
cancer: An ancient link with novel potentials. Int J Cancer.
121:2373–2380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hugo HJ, Saunders C, Ramsay RG and
Thompson EW: New insights on COX-2 in chronic inflammation driving
breast cancer growth and metastasis. J Mammary Gland Biol
Neoplasia. 20:109–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harris RE, Casto BC and Harris ZM:
Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J
Clin Oncol. 5:677–692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu H, Ouyang W and Huang C: Inflammation,
a key event in cancer development. Mol Cancer Res. 4:221–233. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Apte RN, Dotan S, Elkabets M, White MR,
Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y and Voronov E: The
involvement of IL-1 in tumorigenesis, tumor invasiveness,
metastasis and tumor-host interactions. Cancer Metastasis Rev.
25:387–408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Apte RN, Krelin Y, Song X, Dotan S, Recih
E, Elkabets M, Carmi Y, Dvorkin T, White RM, Gayvoronsky L, et al:
Effects of micro-environment- and malignant cell-derived
interleukin-1 in carcinogenesis, tumour invasiveness and
tumour-host interactions. Eur J Cancer. 42:751–759. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Voronov E, Shouval DS, Krelin Y, Cagnano
E, Benharroch D, Iwakura Y, Dinarello CA and Apte RN: IL-1 is
required for tumor invasiveness and angiogenesis. Proc Natl Acad
Sci USA. 100:2645–2650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Voronov E, Carmi Y and Apte RN: Role of
IL-1-mediated inflammation in tumor angiogenesis. Adv Exp Med Biol.
601:265–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tu S, Bhagat G, Cui G, Takaishi S,
KurtJones EA, Rickman B, Betz KS, PenzOesterreicher M, Bjorkdahl O,
Fox JG and Wang TC: Overexpression of interleukin-1beta induces
gastric inflammation and cancer and mobilizes myeloid-derived
suppressor cells in mice. Cancer Cell. 14:408–419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weber A, Wasiliew P and Kracht M:
Interleukin-1 (IL-1) pathway. Sci Signal. 3:cm12010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yokoo T and Kitamura M: Dual regulation of
IL-1 beta-mediated matrix metalloproteinase-9 expression in
mesangial cells by NF-kappa B and AP-1. Am J Physiol.
270:F123–F130. 1996.PubMed/NCBI
|
|
14
|
Amin AR Ruhul, Senga T, Oo ML, Thant AA
and Hamaguchi M: Secretion of matrix metalloproteinase-9 by the
proinflammatory cytokine, IL-1beta: A role for the dual signalling
pathways, Akt and Erk. Genes Cells. 8:515–523. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bauvois B: New factors of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.PubMed/NCBI
|
|
16
|
Ren F, Tang R, Zhang X, Madushi WM, Luo D,
Dang Y, Li Z, Wei K and Chen G: Overexpression of MMP family
members function as prognostic biomarker for breast cancer
patients: A systemic review and meta-analysis. PLoS One.
10:e01355442015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Playford MP and Schaller MD: The interplay
between Src and integrins in normal and tumor biology. Oncogene.
23:7928–7946. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roskoski R Jr: Src kinase regulation by
phosphorylation and dephosphorylation. Biochem Biophys Res Commun.
331:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mitra SK and Schlaepfer DD:
Integrin-regulated FAK-Src signaling in normal and cancer cells.
Curr Opin Cell Biol. 18:516–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hall JE, Fu W and Schaller MD: Focal
adhesion kinase: Exploring FAK structure to gain insight into
function. Int Rev Cell Mol Biol. 228:185–225. 2011. View Article : Google Scholar
|
|
21
|
Calalb MB, Polte TR and Hanks SK: Tyrosine
phosphorylation of focal adhesion kinase at sites in the catalytic
domain regulates kinase activity: A role for Src family kinases.
Mol Cell Biol. 15:954–963. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schlaepfer DD and Hunter T: Evidence for
in vivo phosphorylation of the Grb2 SH2-domain binding site on
focal adhesion kinase by Src-family protein-tyrosine kinases. Mol
Cell Biol. 16:5623–5633. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rahman MA, Senga T, Ito S, Hyodo T,
Hasegawa H and Hamaguchi M: S-nitrosylation at Cystein 498 of c-Src
tyrosine kinase regulates nitoric oxide-mediated cell invasion. J
Biol Chem. 285:3806–3814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ilić D, Furuta Y, Kanazawa S, Takeda N,
Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M and Yamamoto T:
Reduced cell motility and enhanced focal adhesion contact formation
in cells from FAK-deficient mice. Nature. 377:539–544. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mon NN, Hasegawa H, Thant AA, Huang P,
Tanimura Y, Senga T and Hamaguchi M: A role for focal adhesion
kinase signaling in tumor necrosis factor-alpha-dependent matrix
metalloproteinase-9 production in a cholangiocarcinoma cell line,
CCKS1. Cancer Res. 66:6778–6784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shibata K, Kikkawa F, Nawa A, Thant AA,
Naruse K, Mizutani S and Hamaguchi M: Both focal adhesion kinase
and c-Ras are required for the enhanced matrix metalloproteinase 9
secretion by fibronectin in ovarian cancer cells. Cancer Res.
58:900–903. 1998.PubMed/NCBI
|
|
27
|
Rajshankar D, Downey GP and McCulloch CA:
IL-1β enhances cell adhesion to degraded fibronectin. FASEB J.
26:4429–4444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frame MC: Src in cancer: Deregulation and
consequences for cell behavior. Biochim Biophy Acta. 1602:114–130.
2002.
|
|
29
|
Avizienyte E and Frame MC: Src and FAK
signalling controls adhesion fate and the epithelial-to-mesenchymal
transition. Curr Opin Cell Biol. 17:542–547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sen T, Dutta A, Maity G and Chatterjee A:
Fibronectin induces matrix metalloproteinase-9 (MMP-9) in human
laryngeal carcinoma cells by involving multiple signaling pathways.
Biochimie. 92:1422–1434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Siesser PM and Hanks SK: The signaling and
biological implications of FAK overexpression in cancer. Clin
Cancer Res. 12:3233–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Summy JM and Gallick GE: Src family
kinases in tumor progression and metastasis. Cancer Metastasis Rev.
22:337–358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mon NN, Ito S, Senga T and Hamaguchi M:
FAK signaling in neoplastic disorders: A linkage between
inflammation and cancer. Ann NY Acad Sci. 1086:199–212. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Irby RB, Mao W, Coppola D, Kang J, Loubeau
JM, Trudeau W, Karl R, Fujita DJ, Jove R and Yeatman TJ: Activating
SRC mutation in a subset of advanced human colon cancers. Nature
Genet. 21:187–190. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer - a new therapeutic opportunity. Nat Rev Cancer.
5:505–515. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brunton VG and Frame MC: Src and focal
adhesion kinase as therapeutic targets in cancer. Curr Opin
Pharmacol. 8:427–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoon H, Dehart JP, Murphy JM and Lim ST:
Understanding the role of FAK in cancer: Inhibitors, genetic
models, and new insights. J Histochem Cytochem. 62:114–128. 2015.
View Article : Google Scholar
|