Introduction
Colorectal cancer (CRC) represents the second
highest cause of cancer-associated mortality in developed countries
and is responsible for ~530,000 fatalities each year worldwide
(1,2).
Furthermore, CRC is the third most commonly diagnosed malignant
tumor, with more than 1,000,000 new cases every year globally
(3). The 5-year survival rate is
estimated at 65% in North America and 54% in Western Europe
(1,4).
The incidence of colorectal cancer is uncommon under the age of 50
years, predominantly in tumors induced by heredity and with a
family history, but the risk increases to the age of ~85 years. The
incidence of colorectal adenomas also rises with age. Indeed,
two-thirds of all colorectal cancers occurred in patients over the
age of 65 (5).
Regarding CRC management, comorbidities should be
taken into consideration, particularly in elderly patients, as
their incidence increases with age and strongly affects patient
prognosis (6,7). For example, a history of ischemic heart
disease may limit the employment of drugs, such as bevacizumab,
considering its association with the development of ischemic heart
disease (0.52–1.7% of the treated population) (8). Furthermore, this suggests a particular
caution is required for the use of 5-fluorouracil (5-FU) and
capecitabine, which can induce cardiotoxicity in 0–35% of the
treated population, which often presents as myocardial ischemia,
but to a lesser extent cardiac arrhythmias, hyper and hypotension,
left ventricular dysfunction, cardiac arrest and sudden death
(9). However, comorbidities are not
the only factor to consider when planning CRC management.
Functional status is also widely known to affect patient survival
and treatment tolerance in oncology. Geriatric studies have
reported that patients with functional limitations are at higher
risk of functional decline or mortality over 2 years after
functional limitations development compared with their more
functional counterparts (10). Frail,
elderly patients are often easily identifiable, and the treatment
approach is primarily palliative. This may occasionally involve the
cautious use of chemotherapy (11).
The present study describes the case of an elderly
man with metastatic CRC (mCRC) who was successfully treated with
single-agent capecitabine chemotherapy, underlining the positive
activity of the drug in terms of safety and obtaining a complete
response.
Case report
In September 2008, a 77-year-old man was referred to
our department following an anterior rectal-sigmoid resection
performed at Surgical Division of Second University of Naples, with
a histological confirmation of adenocarcinoma of the rectum and
multiple liver metastases. At diagnosis, the Eastern Cooperative
Oncology Group (ECOG) (12)
performance status score was 2. Excluding the presence of cancer,
the medical history of the patient included the following
conditions: Type II diabetes complicated by retinopathy with loss
of vision, skin ulcers and arterial disease, which was treated with
insulin; arterial hypertension, which was treated with
angiotensin-converting-enzyme inhibitors and calcium antagonists,
but was poorly controlled; transitory ischemic attack (TIA), which
occurred in 2006 during acetylsalicylic acid treatment; and
hyperlipidemia, which occurred during treatment with statins.
In May 2008, due to severe abdominal pain in the
left iliac region, weight loss (8 kg in 3 months) and anemia
(hemoglobin level, 9.2 g/dl; normal range, 13.5 −17 g/dl in the
male population), the patient underwent abdominal ultrasonography,
which revealed multiple, hyperechoic nodules with the maximum
diameter of 3.5 cm in the right and left liver lobes. Subsequently,
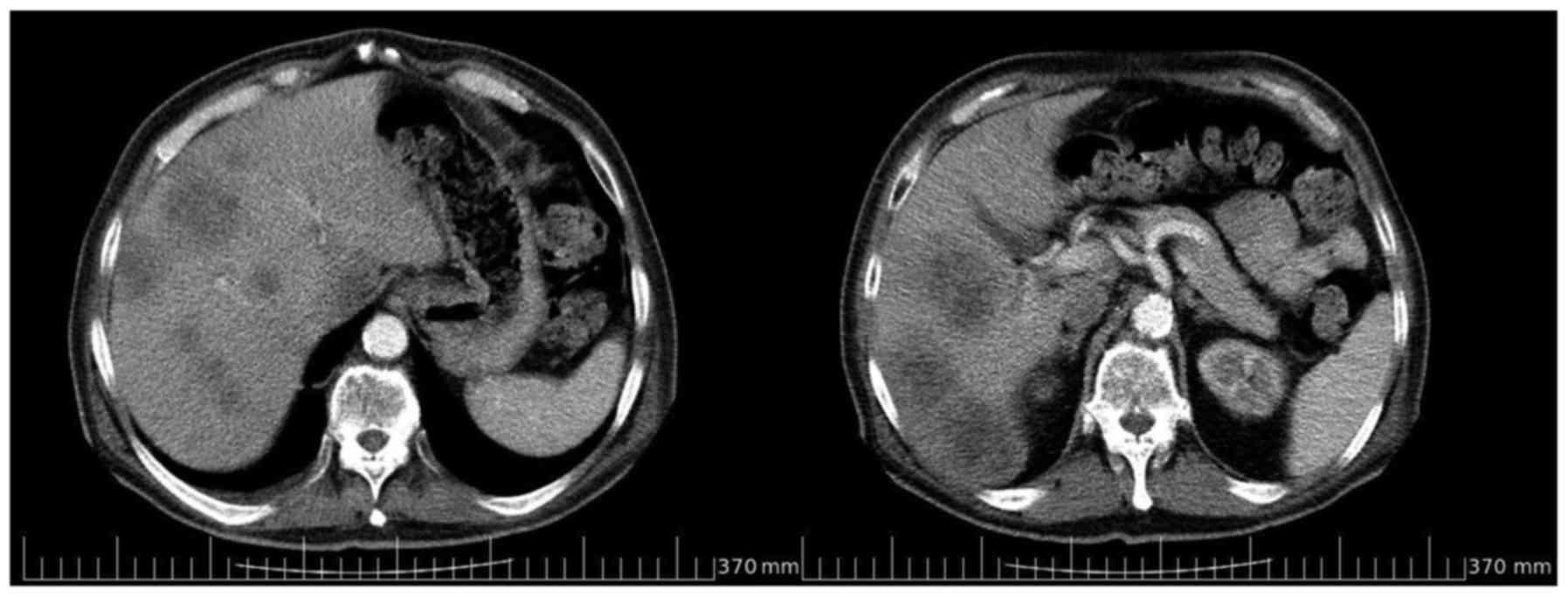
a computed tomography (CT) scan confirmed abnormal liver density,
the presence of multiple, solid nodules in the left and right
lobes, and wall thickening of the sigmoid colon (Fig. 1). These findings required further
evaluation via colonoscopy examination. The colonoscopy was
conducted up until the rectosigmoid junction due to an intestinal
constriction caused by a large lesion with the maxium diameter of 4
cm, partially occluding the intestinal lumen, ulcerated and
bleeding when touched; two biopsies were performed, which were each
positive for moderately differentiated (G2) ulcerated
adenocarcinoma. Laboratory tests were performed and the tumor
marker levels were as follows: Carcinoembryonic antigen (CEA),
337.2 ng/ml (normal range, 0–5 ng/ml); carbohydrate antigen
(CA)19-9, 109.7 U/ml (normal range 0–45 U/ml); and CA125, 98.2 U/ml
(normal range: 0–35 U/ml). In June 2008, the patient underwent
resection of the sigmoid tract due to bowel obstruction.
Histological examination was indicative of
moderately-differentiated (G2) ulcerated adenocarcinoma,
chromogranin negative, with stromal desmoplasia and invasion of the
intestinal wall to the adipose tissue of the root mesenteric
artery. A total of 16 lymph nodes were resected and metastasis from
adenocarcinoma was identified in 4 of them. According to the
tumor-node-metastasis classification, the adenocarcinoma was staged
as pT3N2M1 G2 (13). Due to a
post-surgical delay, the patient underwent observation in the
Department of Clinical and Experimental Medicine ‘F. Magrassi-A.
Lanzara’, Division of Medical Oncology, Second University of Naples
School of Medicine in September 2008. Considering the presence of
several comorbidities and according to the clinical condition of
the patient, single-agent treatment with oral capecitabine was
administered at the reduced dose of 825 mg/mq twice a day, for a
total dose of 3,000 mg/day, on days 1–14 every 3 weeks (the
standard dose routinely employed in our department was 1,250 mg/mq
twice a day on days 1–14 every 3 weeks).
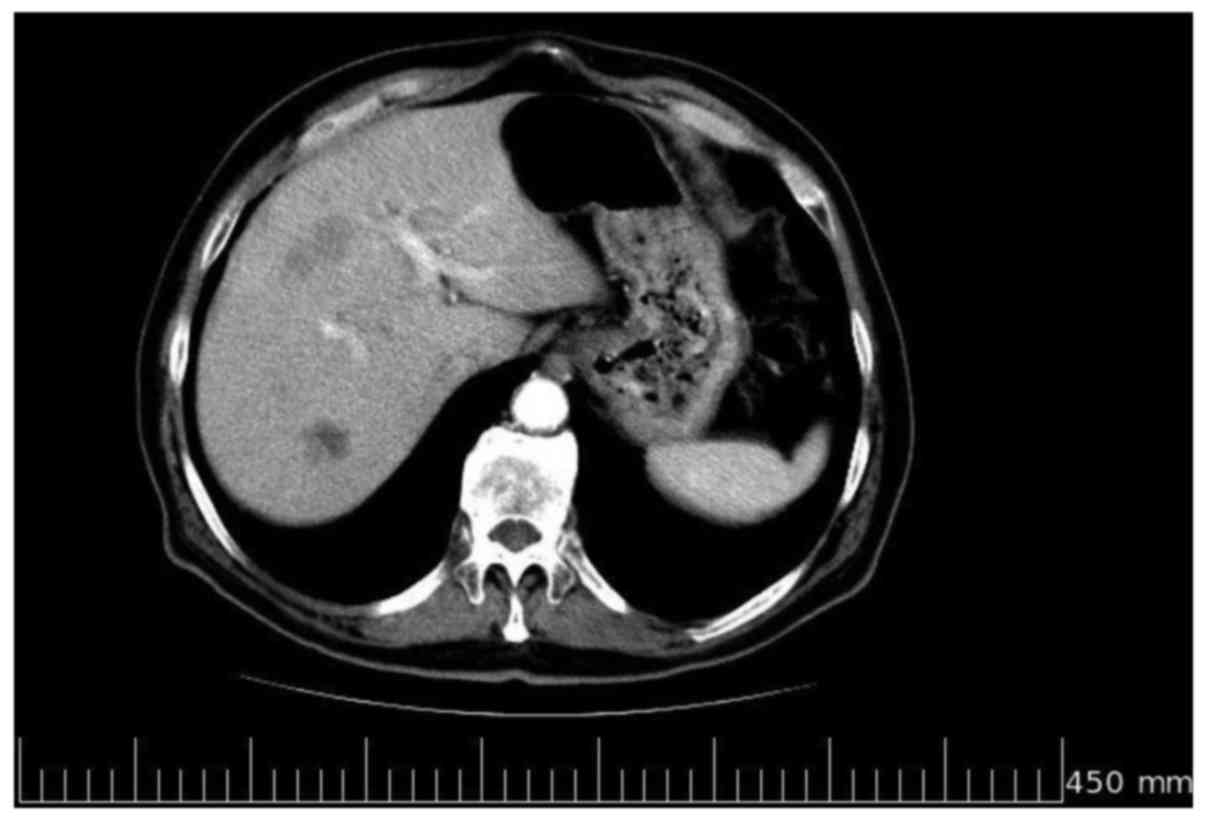
In January 2009, at the end of the sixth
chemotherapy cycle, a contrast-enhanced CT scan revealed a
reduction of >75% in the number and size of the multiple, solid
lesions evidenced in each liver lobe in the previous examinations
(Fig. 2). Further tumor marker
laboratory tests confirmed this response, indicating significantly
decreased levels compared with the pre-operative baselines (CEA,
7.2 ng/ml vs. 337.2 ng/ml; CA19-9, 7.3 U/ml vs. 109.7 U/ml; CA125,
31.3 U/ml vs. 98.2 U/ml). No hematological, gastrointestinal and/or
hand-foot syndrome toxicity was recorded. This response, in
addition to the positive tolerability and safety of the treatment
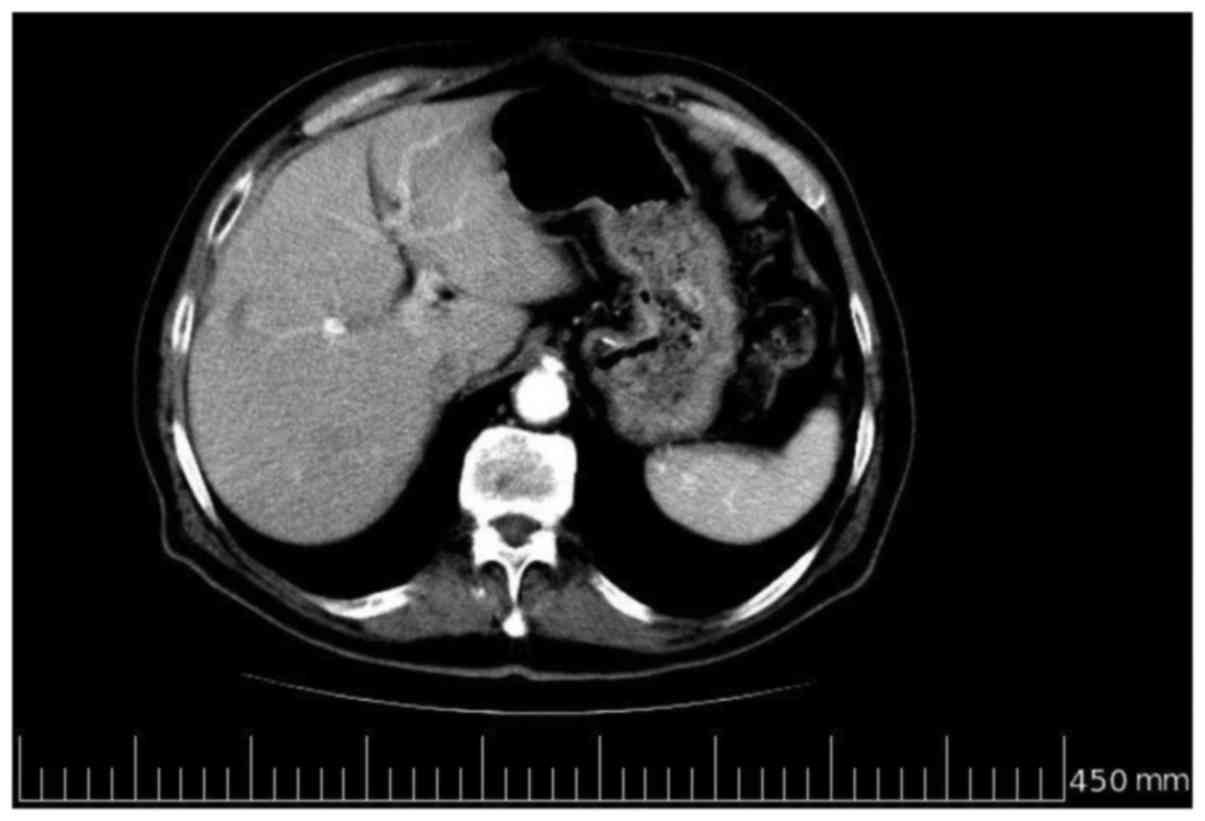
resulted in the continuation of the therapy. In June 2009, after 6
further cycles of the chemotherapy, a further total-body
contrast-enhanced CT scan was performed, which revealed evidence of
a complete response, with no evidence of liver metastases (Fig. 3). Due to the length of treatment and
the evidence of a complete response, chemotherapy was discontinued,
whilst clinical-instrument examination was continued with
contrast-enhanced CT every 6 months, abdominal ultrasound
examination and annual colonoscopy and laboratory haematological
examination and tumor marker level assessment (CEA, CA19.9 and
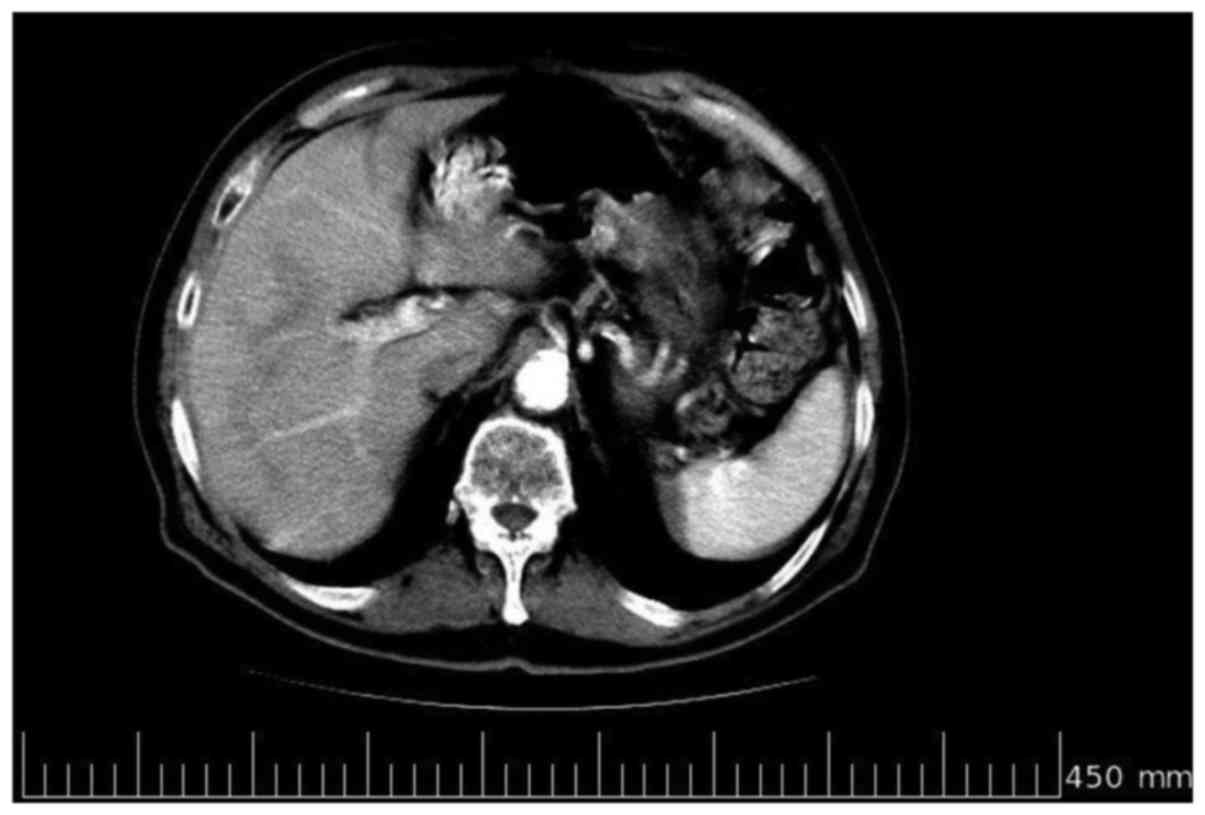
CA125) every 3 months. In July 2010, a contrast-enhanced CT scan
confirmed the complete response (Fig.
4). However, the patient succumbed to a diabetes-related
complication in October 2010 following 29 months of overall
survival. Ethical approval for the publication on the present case
report was obtained from the patient's family.
Discussion
Approximately 20% of patients with CRC present with
an advanced stage of the disease at diagnosis (1,2,4). For this reason, prolongation of
progression-free survival, palliation of symptoms, improvement of
quality of life, and only rarely, complete recovery, represent the
most feasible targets that may be pursued in advanced-stage
patients. Several lines of evidence have indicated that patients
with mCRC benefit, in terms of survival, from the three most active
chemotherapeutic agents [leucovorin (LV)/5-FU, irinotecan and
oxaliplatin] dispensed either sequentially or concomitantly
(14–16). However, the current literature does
not offer enough data regarding the use of chemotherapy in frail,
elderly patients with mCRC.
A primary concern raised by the present study is how
the term ‘elderly’ is defined. Clinical trial settings consider the
appropriate cut-off age of an elderly individual as ≥70 years
(17); however, daily practice has
demonstrated that individuals >70 years may often have less
clinical conditions and a better metabolic balance than younger
individuals. Taking this into account, it may be more appropriate
to discuss patients in terms of being ‘fit’ or ‘unfit’. Balducci
and Extermann (18) performed a
comprehensive geriatric assessment that examined facets of health
and functionality, which resulted in the identification of three
relevant categories: i) ‘Fit’, ii) ‘intermediate’ and iii) ‘frail’
patients. It was determined that fit, elderly patients could be
treated with the same schedules used in younger patients. Notably,
this subgroup could benefit from first-line strategies, such as the
folinic acid, 5-FU and oxaliplatin regimen, and the folinic acid,
5-FU and irinotecan regimen, while the feasibility of adding
targeted agents, including cetuximab and bevacizumab, requires
further investigation.
In the present case, the choice of a
chemotherapeutical regimen using capecitabine as a single agent was
supported by several parameters, including the age of the patient,
the presence of comorbidities and the current life expectancy.
Capecitabine is a third-generation, oral prodrug of 5-FU, produced
to closely simulate prolonged intravenous administration of 5-FU
(19). Previous studies have
demonstrated that it is an effective and well-tolerated first-line
monotherapy in elderly patients with CRC, and it has become a valid
alternative to LV/5-FU-based combinations, also in association with
irinotecan or oxaliplatin (20,21).
In the present study, the decision to take a
chemotherapeutical approach with a low dose of capecitabine (850
mg/mq twice a day) was due to the presence of type II diabetes
complicated by retinopathy with loss of vision, skin ulcers and
arterial disease. This decision was supported by the study by
Cripps et al (22), which
demonstrated that unfit, elderly patients may benefit from a lower
dose of capecitabine compared with the standard dose, due to a
potentially lower incidence of toxicities. In particular, Jung
et al showed that a metronomic dose of capecitabine, with a
dose ranging between 1,000 and 2,000 mg daily, without interruption
in elderly patients for whom combination chemotherapy or even
monotherapy is not feasible, offers a good toxicity profile and
good tumor control (23). In a large
observational study, 1,249 elderly patients received oral
capecitabine as single agent or in combination with other
chemotherapeutical agents in first line treatment:
Capecitabine-based combination was administered in 56% of patients
in the overall population. The median treatment duration was ~5
months. Severe toxicity occurred rarely, without any difference
regarding age groups. The most common hematological toxicity was
anemia. Gastrointestinal side effects and hand-food-syndrome were
the most frequent non-hematological toxicities. The overall
response rate (ORR) was significantly increased in the patient
group ≤75 years compared to patients >75 years of age (38 vs.
32%, p=0.019). Median progression free survival (PFS; 9.7 vs. 8.2
months, p=0.00021) and overall survival (OS; 31.0 vs. 22.6 months,
p<0.0001) was decreased in elderly patients (24). In a phase II trial of 51 patients aged
>70 years with advanced CRC, capecitabine was effective and well
tolerated, with an ORR of 24%, PFS of 7 months and OS of 11 months.
Grade 3/4 adverse events were observed in 12% of patients (25).
The current study considered K-Ras mutational status
evaluation and the use of cetuximab to be unnecessary, as this
would have required biweekly access to the day hospital, which was
not recommended in this frail patient. Bevacizumab was also not
recommended due to the possibility of arterial hypertension and the
medical history of TIA.
Treatment was well tolerated in the present case
with no evidence of grade 3–4 toxicity, and the patient exhibited
good compliance to oral administration. These conditions
contributed to a complete response of the liver metastases and an
overall survival time of 29 months. The literature suggests that
the median overall survival time for patients with mCRC, following
combinational or sequential use of active drugs and molecules, is
~24 months (26).
The present study evaluated the incidence and
severity of adverse events in elderly patients compared with
younger patients through an analysis of the literature. Previous
studies have reported that elderly patients have a similar
incidence rate of drug-related toxicities compared with younger
patients (20–22), probably due to lower functional
reserve of single organ. A previous study conducted by ECOG
analyzed drug toxicities in 19 trials of advanced cancer in
different sites, including CRC. Of the 1,210 cases analyzed,
including 174 patients who were ≥70 years, toxicities were uncommon
and no differences were observed across the cut-off age. However,
the rate of toxicity increased in frail patients (27). Similarly, Cascinu et al
(28) did not identify any
differences in toxicity in a study of 120 patients with advanced
cancer at 6 different sites, including CRC, using a cut-off age of
70 years.
In conclusion, the present case confirms the
efficacy of capecitabine chemotherapy in elderly patients with
mCRC, even when used as a single agent. The current case indicated
that advanced age alone is not a sufficient reason to withhold or
limit treatment, including chemotherapy. The possibility that
lifestyle and the presence of comorbidities may increase the
biological age of a patient should also be taken into account when
planning treatment. Chemotherapy is hazardous in patients with a
poor performance status, therefore therapeutic choices must be
modified case by case. However, the use of chemotherapy in the fit,
elderly population may be feasible without increasing either
mortality or morbidity, whilst also achieving similar side effects
and response rates as observed in younger patients.
References
|
1
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitry E, Bouvier AM, Esteve J and Faivre
J: Improvement in colorectal cancer survival: A population-based
study. Eur J Cancer. 41:2297–2303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rougier P and Mitry E: Epidemiology,
treatment and chemoprevention in colorectal cancer. Ann Oncol.
14:(Suppl 2). ii3–ii5. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan, and oxaliplatin in the course of treatment. J Clin
Oncol. 22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holt PR, Kozuch P and Mewar S: Colon
cancer and the elderly: From screening to treatment in management
of GI disease in the elderly. Best Pract Res Clin Gastroenterol.
23:889–907. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Extermann M, Overcash J, Lyman GH, Parr J
and Balducci L: Comorbidity and functional status are independent
in older cancer patients. J Clin Oncol. 16:1582–1587.
1998.PubMed/NCBI
|
|
7
|
Meyerhardt JA, Catalano PJ, Haller DG,
Mayer RJ, Macdonald JS, Benson AB III and Fuchs CS: Impact of
diabetes mellitus on outcomes in patients with colon cancer. J Clin
Oncol. 21:433–440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen XL, Lei YH, Liu CF, Yang QF, Zuo PY,
Liu CY, Chen CZ and Liu YW: Angiogenesis inhibitor bevacizumab
increases the risk of ischemic heart disease associated with
chemotherapy: A meta-analysis. PLoS One. 8:e667212013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polk A, Vistisen K, VaageNilsen M and
Nielsen DL: A systematic review of the pathophysiology of
5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol.
15:472014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saliba D, Elliott M, Rubenstein LZ,
Solomon DH, Young RT, Kamberg CJ, Roth C, MacLean CH, Shekelle PG,
Sloss EM and Wenger NS: The Vulnerable Elders Survey: A tool for
identifying vulnerable older people in the community. J Am Geriatr
Soc. 49:1691–1699. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fried LP, Tangen CM, Walston J, Newman AB,
Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al:
Cardiovascular Health Study Collaborative Research Group: Frailty
in older adults: Evidence for a phenotype. J Gerontol A Biol Sci
Med Sci. 56:M146–M156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lan YT, Yang SH, Chang SC, Liang WY, Li
AF, Wang HS, Jiang JK, Chen WS, Lin TC and Lin JK: Analysis of the
seventh edition of American Joint Committee on colon cancer
staging. Int J Colorectal Dis. 27:657–663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grothey A and Goldberg RM: A review of
oxaliplatin and its clinical use in colorectal cancer. Expert Opin
Pharmacother. 5:2159–2170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mohelnikova-Duchonova B, Melichar B and
Soucek P: FOLFOX/FOLFIRI pharmacogenetics: The call for a
personalized approach in colorectal cancer therapy. World J
Gastroenterol. 20:10316–10330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edwards MS, Chadda SD, Zhao Z, Barber BL
and Sykes DP: A systematic review of treatment guidelines for
metastatic colorectal cancer. Colorectal Dis. 14:e31–e47. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Extermann M, Albrand G, Chen H, Zanetta S,
Schonwetter R, Zulian GB, Cantor A and Droz JP: Are older French
patients as willing as older American patients to undertake
chemotherapy? J Clin Oncol. 21:3214–3219. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balducci L and Extermann M: Management of
cancer in the older person: A practical approach. Oncologist.
5:224–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang F, Wang FH, Bai L and Xu RH: Role of
capecitabine in treating metastatic colorectal cancer in Chinese
patients. Onco Targets Ther. 7:501–511. 2014.PubMed/NCBI
|
|
20
|
Chu E, Eng C, Abbruzzese J, et al:
Efficacy and safety of capecitabine for colorectal cancer. Am J
Oncol Rev. 2:(Suppl 3). 1–28. 2003.
|
|
21
|
Escudero P, Verge V, Feliu J, SanzLacalle
J, Lopez R, Gomez MJ, Aparicio A, Yubero A, Lorenzo A and
Gonzalez-Baron M: A study of capecitabine in elderly patients as
first line treatment in advanced or metastatic colorectal cancer.
Proc Am Soc Clin Oncol. 22:abstract 3114. 2003.
|
|
22
|
Cripps MC, Vincent M, Jonker D, Kerr I,
Dingle B, Martin LA, Mathews J, Biagi J, Knight G and Lam W: Dose
reduced first line capecitabine monotherapy in older and less fit
patients with advanced colorectal cancer (ACRC). J Clin Oncol.
23:35772005.PubMed/NCBI
|
|
23
|
Jung YH, Lee WJ, Byeon JH, Lee IK, Han CW
and Woo IS: Metronomic chemotherapy with capecitabine for
metastatic colorectal cancer in very elderly patients. Korean J
Intern Med. Mar 10–2016.(Epub ahead of print). View Article : Google Scholar
|
|
24
|
Stein A, Quidde J, Schröder JK, Göhler T,
Tschechne B, Valdix AR, Höffkes HG, Schirrmacher-Memmel S,
Wohlfarth T, Hinke A, Engelen A and Arnold D: Capecitabine in the
routine first-line treatment of elderly patients with advanced
colorectal cancer - results from a non-interventional observation
study. BMC Cancer. 16:822016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feliu J, Escudero P, Llosa F, Bolaños M,
Vicent JM, Yubero A, Sanz-Lacalle JJ, Lopez R, Lopez-Gómez L,
Casado E, Gómez-Reina MJ and González-Baron M: Capecitabine as
first-line treatment for patients older than 70 years with
metastatic colorectal cancer: An oncopaz cooperative group study. J
Clin Oncol. 23:3104–3111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohhara Y, Fukuda N, Takeuchi S, Honma R,
Shimizu Y, Kinoshita I and DosakaAkita H: Role of targeted therapy
in metastatic colorectal cancer. World J Gastrointest Oncol.
8:642–655. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Begg CB and Carbone PP: Clinical trials
and drug toxicity in the elderly: The experience of the Eastern
Cooperative Oncology Group. Cancer. 52:1986–1992. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cascinu S, Del Ferro E and Catalano G:
Toxicity and therapeutic response to chemotherapy in patients aged
70 years or older with advanced cancer. Am J Clin Oncol.
19:371–374. 1996. View Article : Google Scholar : PubMed/NCBI
|