Introduction
Lung cancer is one of the most frequently diagnosed
types of cancer and the leading cause of cancer-associated
mortality in males worldwide (1). The
majority of lung cancer cases are classed as non-small cell lung
cancer (NSCLC), which accounts for ~85% of all lung cancer cases.
Surgical resection, chemotherapy and radiotherapy are the primary
therapeutic methods for the treatment of NSCLC; however, the 5-year
survival rate in patients with NSCLC is <15% (2), thus it is essential that novel
diagnostic and therapeutic methods are developed. Recent clinical
studies indicate the potential of immunotherapy for the treatment
of lung cancer (3,4).
The selection of a good therapeutic target is
necessary to achieve a beneficial outcome through cancer
immunotherapy. Antigens encoded by cancer-germline genes are
considered potential targets for cancer immunotherapy, as they are
expressed exclusively within immune-privileged germ cells and
various malignancies (5).
Immunotherapy that targets cancer-germline genes, such as MAGE-A3,
represents a potential therapeutic approach for the treatment of
NSCLC (6). In addition, MAGE-A3
antigen-specific T-cell receptor (TCR) modified T-cell therapy has
been demonstrated to effectively kill lung cancer cells (7).
As of 2013, 265 cancer-germline genes have been
identified, 105 of which are located on the X chromosome (8); however, their biological function in
tumors remains poorly understood. Cancer-germline genes are
considered important factors in oncogenesis, influencing the
immortality, invasiveness, immune evasion capability and metastatic
capacity of tumor cells (9).
Downregulation of cancer-germline gene expression could modify
tumor cell morphology, adhesion and migration (10,11).
Malignant tissues frequently coexpress several cancer-germline
genes, whose expression levels are associated with advanced tumor
stage and poor patient prognosis (9).
Antigens encoded by cancer-germline genes are considered potential
markers of poor prognosis and attractive targets for immunotherapy
in various cancers (12,13). Lung cancer frequently exhibits
expression of these genes. Several studies have investigated the
mRNA and protein expression of cancer-germline genes in lung cancer
(14–19). Cancer-germline gene expression is
induced through promoter demethylation by DNA demethylation agents
(15,20,21).
MAGE-A3 and MAGE-C2 (MAGE-A3/C2) have been recognized as attractive
potential targets for cancer immunotherapy (22–27).
Previous studies have indicated that overexpression of MAGE-A3/C2
is associated with tumor metastasis and poor clinical outcome
(28,29). However, the expression and prognostic
significance of MAGE-A3/C2 in NSCLC remains unclear.
In the present study, the frequency of MAGE-A3/C2
expression at the mRNA and protein level in NSCLC samples was
detected. The association between MAGE-A3/C2 expression and the
clinicopathological characteristics, in addition to the overall
survival, of patients with NSCLC was subsequently evaluated. In
addition, the underlying mechanisms through which MAGE-A3
expression influences NSCLC cell biology were investigated in
vitro.
Materials and methods
Cell line and tumor samples
The human NSCLC cell line A549 was purchased from
Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai, China),
and was cultured in RMPI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
calf serum (FBS; Hyclone; GE Healthcare, Logan, UT, USA),
penicillin (100 U/ml), and streptomycin (100 µg/ml). The cell
culture was maintained at 37°C with 5% CO2 in a
humidified atmosphere. A total of 206 NSCLC specimens and paired
adjacent healthy lung tissue samples were collected at the First
Affiliated Hospital of Zhengzhou University (Zhengzhou, China)
between November 2012 and December 2013 (first cohort). The sample
collection process was approved by the First Affiliated Hospital of
Zhengzhou University Ethics Committee and informed consent was
obtained from each patient. None of the patients received
preoperative chemotherapy or radiotherapy. A total of 76
formalin-fixed, paraffin-embedded (FFPE) NSCLC tissue samples and 7
corresponding adjacent healthy tissue samples were obtained from
the Department of Pathology at the First Affiliated Hospital of
Zhengzhou University from patients who were diagnosed with NSCLC
between September 2008 and August 2009 (second cohort). Lung cancer
is staged according to the system advocated by the American Joint
Committee on Cancer and the Union for International Cancer Control,
which as of 2010 is in its 7th edition (30,31).
Details of the clinicopathological characteristics of patients from
the two cohorts are detailed in Table
I.
 | Table I.Clinicopathological characteristics
of patients with non-small cell lung cancer. |
Table I.
Clinicopathological characteristics
of patients with non-small cell lung cancer.
|
| Number of patients
(%) |
|---|
|
|
|
|---|
| Clinicopathological
characteristic | Cohort 1
(n=206) | Cohort 2
(n=76) |
|---|
| Age at diagnosis
(years old) |
|
|
|
<65 | 143 (69) | 50 (66) |
|
≥65 | 63
(31) | 26 (34) |
| Gender |
|
|
Male | 141 (68) | 50 (66) |
|
Female | 65
(32) | 26 (34) |
| Histological
grade |
|
| G1 | 26
(13) | 18 (24) |
| G2 | 115 (55) | 44 (58) |
| G3 | 65
(32) | 14 (18) |
| Histological
type |
|
|
Squamous cell carcinoma | 75
(36) | 36 (47) |
|
Adenocarcinoma | 131 (64) | 40 (53) |
| Lymph node
metastasis |
|
|
Absent | 70
(34) | 36 (47) |
|
Present | 136 (66) | 40 (53) |
| Tumor stage |
|
|
I–II | 158 (72) | 42 (55) |
|
III–IV | 48
(28) | 34 (45) |
Reverse transcription-polymerase chain
reaction (RT-PCR) and quantitative (q)PCR
Total RNA was isolated using TRIzol reagent
according to the manufacturer's instructions (Invitrogen; Thermo
Fisher Scientific, Inc.). The concentration and purity of all
samples were verified using NanoDrop2000 (Thermo Fisher Scientific,
Inc.). cDNA was obtained using a PrimeScript™ RT reagent kit
(Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
instructions. Briefly, samples containing l µg total RNA were
incubated with 1 µl gDNA Eraser, 2 µl 5X gDNA eraser buffer and
RNase-free dH2O at 42°C for 2 min. After adding the
enzyme mix, the reaction was incubated at 37°C for 15 min. The cDNA
was amplified using Premix Tap (Takara Bio, Inc.) and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a
loading control. The initial step was performed at 94°C for 5 min,
the amplification was performed for 35 cycles of denaturation at
95°C for 30 sec, annealing at 58°C for 30 sec and elongation at
72°C for 30 sec. Following the last cycle, a terminal elongation
step (5 min at 72°C) was added and then the samples were kept at
4°C. The PCR products were separated on 1.5% agarose gel stained
with ethidium bromide and recorded. The expected sizes of GAPDH,
MAGE-A3 and MAGE-C2 RT-PCR products were 233, 239 and 189 bp,
respectively. PCR products from samples positive for MAGE-A3/C2
were sequenced by Sangon Biotech Co., Ltd. (Shanghai, China). qPCR
was performed using SYBR Premix Ex Taq II (Takara Bio, Inc.) and an
Mx3005P qPCR system (Agilent Technologies, Inc., Santa Clara, CA,
USA), in order to quantify the mRNA expression of MAGE-A3 and
epithelial-mesenchymal transition (EMT) markers in A549 cells
transfected with scrambled small interfering RNA (siRNA) and siRNA
targeting MAGE-A3 after 48 h. The thermal cycling conditions
included an initial denaturation for 30 sec at 95°C and 40 cycles
consisting of an annealing step at 95°C for 5 sec and an extension
step at 60°C for 20 sec. Each sample was analyzed in triplicate.
The abundance of mRNA for each gene of interest was normalized to
GAPDH. Each sample obtained from three independent experiments was
used for analysis of relative gene expression using the 2-ΔΔCq
method (32). mRNAs being quantified
and their primers sequences are illustrated in Table II.
 | Table II.Primers used for polymerase chain
reaction analysis. |
Table II.
Primers used for polymerase chain
reaction analysis.
| Gene | Primer
sequences | Product size
(bp) |
|---|
| GAPDH |
|
|
|
Forward |
5′-GGAGCCAAAAGGGTCATCATCTC-3′ | 233 |
|
Reverse |
5′-GAGGGGCCATCCACAGTCTTCT-3′ |
|
| MAGE-A3 |
|
|
|
Forward |
5′-AGTCCGAGTTCCAAGCAG-3′ | 239 |
|
Reverse |
5′-GCAGGTGGCAAAGATGTA-3′ |
|
| MAGE-C2 |
|
|
|
Forward |
5′-TGAGTTAGAAGACTGGGTAGATGC-3′ | 189 |
|
Reverse |
5′-ATGCTCTCGGTAAGATTTGGTATC-3′ |
|
|
HLA-A2-internala |
|
|
|
Forward |
5′-GCGCCGTGGAAGAGGGTCG-3′ | 236 |
|
Reverse |
5′-CCCGTCCCAATACTCCCGA-3′ |
|
| HLA-A2-outer |
|
|
|
Forward |
5′-GGTCCGGAGTATTGGGACG-3′ | 511 |
|
Reverse |
5′-GTGCTTGGTGGTCTGAGCT-3′ |
|
| E-cadherin |
|
|
|
Forward |
5′-TGCCCAGAAAATGAAAAAGG-3′ | 200 |
|
Reverse |
5′-GTGTATGTGGCAATGCGTTC-3′ |
|
| N-cadherin |
|
|
|
Forward |
5′-ACAGTGGCCACCTACAAAGG-3′ | 200 |
|
Reverse |
5′-CCGAGATGGGGTTGATAATG-3′ |
|
| Vimentin |
|
|
|
Forward |
5′-GAGAACTTTGCCGTTGAAGC-3′ | 163 |
|
Reverse |
5′-GCTTCCTGTAGGTGGCAATC-3′ |
|
| SLUG |
|
|
|
Forward |
5′-GGGGAGAAGCCTTTTTCTTG-3′ | 158 |
|
Reverse |
5′-TCCTCATGTTTGTGCAGGAG-3′ |
|
Immunohistochemistry (IHC)
MAGE-A3/C2 protein staining was performed using
4-µm-thick sections of FFPE tissues. Sections were treated with 3%
H2O2 and 5% bovine serum albumin for 30 min
at room temperature, and then incubated with anti-human MAGE-A3
(1:150 dilution; catalog no. ab140678; Abcam, Cambridge, UK) and
MAGE-C2 (1:400 dilution; catalog no. ab209667, Abcam) primary
antibodies overnight at 4°C. Following incubation with horseradish
peroxidase-conjugated secondary antibody for 1 h at room
temperature, the sections were washed and counterstained with
hematoxylin and visualized under a microscope (Olympus, Tokyo,
Japan). Samples were considered positive for MAGE-A3/C2 following
the detection of any nuclear and/or cytoplasmic antibody signal in
the tumor cells. Samples with complete absence of antibody signal
were considered negative for each MAGE tested. Detection of
MAGE-A3/C2 expression in testis tissue was used as a positive
control and samples that underwent the same process with the
absence of primary antibody treatment were used as a negative
control.
Flow cytometry and sequence-specific
primer (SSP)-PCR
Expression of human leukocyte antigen (HLA)-A2 on
peripheral blood mononuclear cells (PBMCs) from cohort 1 patients
was detected using flow cytometry and verified through SSP-PCR.
PBMCs were incubated with phycoerythrin-conjugated mouse anti-human
HLA-A2 monoclonal antibody (catalog no. 343305; BD Biosciences,
Franklin Lakes, NJ, USA), at an appropriate concentration
(~5×105 cells were resuspended in 100 µl PBS and added
to 2 µl anti-human HLA-A2 antibody) according to the manufacturer's
instructions, for 15 min at 4°C in the dark. Samples were analyzed
by flow cytometry (BD FACSCanto II; BD Biosciences). The results
were also verified by SSP-PCR using two pairs of HLA-A2 primers
(Table II).
siRNA-mediated gene knockdown
Scrambled negative control siRNA (Scrambled siRNA
sense, 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and antisense, 5′-ACG UGA
CAC GUU CGG AGA ATT-3′) and siRNA targeting MAGE-A3 (si-MAGE-A3
sense, 5′-CAG UGA UCC UGC AUG UUA UTT-3′ and antisense, 5′-AUA ACA
UGC AGG AUC ACU GTT-3′) were provided by Shanghai GenePharma
(Shanghai, China). Transfection siRNA into NSCLC cells was
performed using Lipofectamine® 3000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) at a final concentration of 36 nM.
The efficiency of siRNA knockdown was subsequently confirmed using
qPCR (as aforementioned).
Cell apoptosis and colony formation
assays
Following NSCLC cell siRNA transfection, as
described above, for 48 h, 1×105 transfected cells were
collected and centrifuged at 300 × g at room temperature for
10 min. Thereafter, cells incubated with AlexaFluor647 Annexin V
(BioLegend, Inc. San Diego, CA, USA) for 15 min at 4°C in the dark,
and propidium iodide (PI; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) was added. Samples were immediately analyzed by
flow cytometry (BD FACSCanto II; BD Biosciences). For the colony
formation assay, cells transfected with specific or scrambled
si-RNA were plated on 6-well plates (Costar; Corning, Inc., NY,
USA) at a density of 1,000/well maintained in RPMI-1640 medium
containing 10% FBS and 4 µg/ml heparin (Sigma-Aldrich; Merck
Millipore), B27 (1:50 dilution; Gibco; Thermo Fisher Scientific,
Inc.), 20 ng/ml EGF, 20 ng/ml basic fibroblast growth factor (both
Sigma-Aldrich; Merck Millipore), 100 IU/ml penicillin and 100 µg/ml
streptomycin for 7 days. The colonies were counted under a low
magnification microscope (Leica Microsystems, GmbH, Wetzlar,
Germany) and a group of >30 cells or a diameter of >1 mm in
each well was defined as a colony.
Cell migration assay
Cell migration was assessed in 24-well Boyden
Chambers (Corning, Inc.) according to the manufacturer's protocol.
Cells that migrated to the underside of the membranes of each
insert were counted at ×100 magnification in five random areas
under a low magnification microscope (Leica Microsystems GmbH).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). Pearson's
chi-squared test was used to evaluate the association between
MAGE-A3/C2 expression and the clinicopathological characteristics
of patients with NSCLC. The Kaplan-Meier estimator was performed to
evaluate the overall survival of patients. Univariate and
multivariate Cox's proportional hazard regression model analysis
were used to evaluate the prognostic significance of MAGE-A3/C2
expression in NSCLC. All experiments were repeated three times and
results are expressed as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
Association between MAGE-A3/C2 mRNA
expression and the clinicopathological characteristic of patients
with NSCLC
MAGE-A3/C2 mRNA expression was analyzed in 206 lung
cancer tissue and paired adjacent lung tissue samples from patients
with NSCLC (cohort 1) using RT-PCR. MAGE-A3/C2 was not expressed in
paired adjacent healthy lung tissue, but was frequently expressed
in corresponding NSCLC tissues (Fig.
1A). The RT-PCR products from three MAGE-A3/C2 positive samples
were subsequently sequenced. The obtained sequences had high
intra-isolate and inter-isolate nucleotide consistency. Expression
of MAGE-A3 and MAGE-C2 mRNA was identified in 73 and 53% of NSCLC
cases, respectively. The association between MAGE-A3/C2 expression
and the clinicopathological characteristics of patients with NSCLC
are illustrated in Table III.
Positive MAGE-A3 mRNA expression was significantly associated with
lymph node metastasis and stage III–IV disease. A higher frequency
of MAGE-A3 was found in patients with lymph node metastasis at
diagnosis (84%) compared with patients without lymph node
metastasis at diagnosis (68%) (P=0.012). In total, 60% of patients
with stage I, 68% of patients with stage II and 90% of patients
with stage III–IV disease expressed MAGE-A3 (P=0.006). However,
MAGE-C2 mRNA expression was not significantly associated with any
clinicopathological characteristics. These results indicate that
MAGE-A3 is associated with the development and progression of
NSCLC, suggesting it is a biomarker of poor patient prognosis.
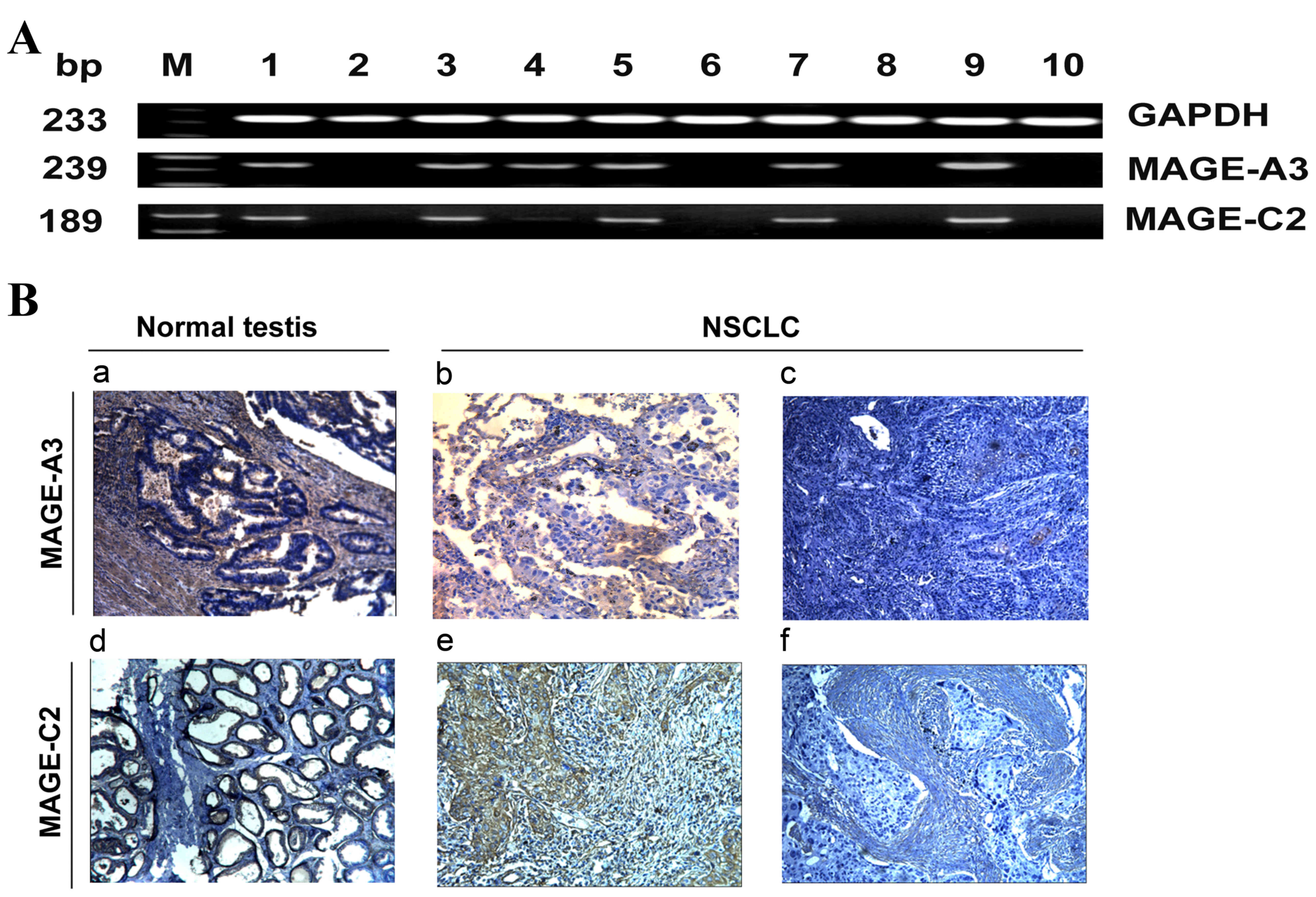 | Figure 1.Representative mRNA and protein
expression of MAGE-A3/C2 in NSCLC tissues. (A) Agarose gel
electrophoresis of reverse transcription polymerase chain reaction
results for MAGE-A3/C2 mRNA expression in NSCLC samples. GAPDH was
used as an internal control. Lanes: M, marker; 1, normal testis
tissue used as a positive control; 2, healthy lung tissue; 3–10,
NSCLC tissues. (B) Immunohistochemical analysis of MAGE-A3/C2
protein expression in NSCLC specimens. (B) Tumor staining with
monoclonal antibodies against MAGE-A3/C2. a, MAGE-A3 positive
normal testis sample (positive control); b, MAGE-A3-positive NSCLC
sample; c, MAGE-A3-negative NSCLC sample; d, MAGE-C2-positive
normal testis sample (positive control); e, MAGE-C2-positive NSCLC
samples; f, MAGE-C2-negative NSCLC sample. Magnification, ×200.
MAGE, melanoma-associated antigen; NSCLC, non-small cell lung
cancer; bp, base pairs. |
 | Table III.Association between MAGE-A3/C2 mRNA
expression and the clinicopathological characteristics of patients
with non-small cell lung cancer in cohort 1. |
Table III.
Association between MAGE-A3/C2 mRNA
expression and the clinicopathological characteristics of patients
with non-small cell lung cancer in cohort 1.
|
| Cancer germline
gene-expressing samples (%) |
|---|
|
|
|
|---|
| Clinicopathological
characteristic | MAGE-A3 | P-value | MAGE-C2 | P-value | MAGE-A3/C2
coexpression | P-value |
|---|
| Age at diagnosis
(years) |
|
|
|
|
|
|
|
<65 | 74 | 0.733 | 57 | 0.072 | 50 | 0.002a |
|
≥65 | 71 |
| 44 |
| 33 |
|
| Gender |
|
|
|
|
|
|
|
Male | 76 | 0.238 | 50 | 0.109 | 43 | 0.228 |
|
Female | 68 |
| 60 |
| 50 |
|
| Histological
grade |
|
|
|
|
|
|
| G1 | 69 | 0.694 | 50 | 0.872 | 39 | 0.310 |
| G2 | 72 |
| 52 |
| 42 |
|
| G3 | 77 |
| 55 |
| 52 |
|
| Histological
type |
|
|
|
|
|
|
|
Squamous cell carcinoma | 76 | 0.624 | 47 | 0.112 | 40 | 0.192 |
|
Adenocarcinoma | 72 |
| 57 |
| 47 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
Present | 84 | 0.012a | 53 | 0.554 | 53 | 0.061 |
|
Absent | 68 |
| 53 |
| 440 |
|
| Tumor stage |
|
|
|
|
|
|
| I | 66 | 0.006a | 52 | 0.853 | 39 | 0.014a |
| II | 79 |
| 54 |
| 54 |
|
|
III–IV | 90 |
| 56 |
| 56 |
|
| HLA-A2
expression |
|
|
|
|
|
|
|
Positive | 77 | 0.270 | 56 | 0.272 | 47 | 0.503 |
|
Negative | 70 |
| 51 |
| 42 |
|
Coexpression analysis of MAGE-A3 and MAGE-C2 in
NSCLC tissue revealed that at least one of the MAGEs analyzed were
expressed in 82% of samples. Coexpression of MAGE-A3 and MAGE-C2
was identified in 45% of samples (data not shown). The frequency of
coexpression was significantly higher in patients that were <65
years old (P=0.002) and those with advanced disease stage (P=0.014)
(Table III). A statistically
significant pattern of coexpression between MAGE-A3 and MAGE-C2 was
also identified (P=0.0001; data not shown). These results suggest
that 82% of patients with NSCLC would be eligible for
antigen-specific immunotherapeutic approaches targeting MAGE-A3 or
MAGE-C2.
Percentage of samples coexpressing
HLA-A2 and MAGE-A3/C2
Among cohort 1 patients with NSCLC, 47% of samples
expressed HLA-A2, 37% coexpressed HLA-A2 and MAGE-A3, and 26%
coexpressed HLA-A2 and MAGE-C2 (data not shown). A previous study
revealed that 29% of patients with NSCLC coexpressed HLA-A2 and
MAGE-A3 in a Japanese cohort (12).
Patients with coexpression of HLA-A2 and MAGE-A3/C2 may benefit
from HLA-A2 restricted peptide vaccination or antigen-specific TCR
modified T cell therapy. The redirection of T cell targeting
through TCR gene modification has been demonstrated to be a
valuable strategy for cancer immunotherapy (33).
Expression of MAGE-A3 mRNA is
increased in male smokers with NSCLC
MAGE-A3 mRNA expression was identified in 76%
of male patients (cohort 1; n=141; Table III). Furthermore, 72% (101/141) of
male patients had a prolonged smoking history (>20 years).
MAGE-A3 mRNA was demonstrated to be expressed in 81% of male
smokers with NSCLC and 63% of male non-smokers. MAGE-A3 mRNA
expression was significantly correlated with the pack-year smoking
history of the male patients (P=0.019; data not shown), indicating
that male patients with a history of smoking may have a poorer
prognosis compared with male non-smokers and could benefit from
MAGE-A3 targeted immunotherapy.
Correlation between MAGE-A3/C2
expression and the overall survival of patients with NSCLC
To assess the association between MAGE-A3/C2 protein
expression and the overall survival of patients with NSCLC, the
protein expression of MAGE-A3 and MAGE-C2 in NSCLC cancer tissues
(cohort 2) was detected using IHC. Representative results are
illustrated in Fig. 1B. MAGE-A3/C2
protein was absent in healthy adjacent lung tissue samples. In
cancerous tissue samples, 58% (44/76) and 53% (40/76) were positive
for MAGE-A3 and MAGE-C2 protein expression, respectively (data not
shown). The majority of immunoreactivity to MAGE-A3/C2 was observed
in the cytoplasm, with fewer nuclei stained. The association
between MAGE-A3/C2 protein expression and the clinicopathological
characteristics of patients with NSCLC in cohort 2 was subsequently
analyzed (Table IV). MAGE-A3 protein
expression was identified to be significantly correlated with lymph
node metastasis (P=0.044) and tumor stage (P=0.038), as 48% (19/40)
of non-lymph node metastasis and 70% (25/36) of lymph node
metastasis cancer tissue samples were MAGE-A3 positive. In
addition, 48% (20/42) of stage I–II and 71% (24/34) of stage III–IV
tumors were MAGE-A3 positive. There was no statistically
significant association between MAGE-C2 protein expression and
tumor stage (P=0.271). Coexpression of MAGE-A3 and MAGE-C2 protein
was identified in 26% (28/76) of NSCLC samples and was
significantly correlated with tumor stage (P=0.030).
 | Table IV.Association between MAGE-A3/C2
protein expression and the clinicopathological characteristics of
patients with non-small cell lung cancer in cohort 2. |
Table IV.
Association between MAGE-A3/C2
protein expression and the clinicopathological characteristics of
patients with non-small cell lung cancer in cohort 2.
|
| Number of samples
(%) |
|---|
|
|
|
|---|
| Clinicopathological
characteristic | MAGE-A3 | P-value | MAGE-C2 | P-value | MAGE-A3/C2
co-expression | P-value |
|---|
| Gender |
|
|
|
|
|
|
|
Male | 52 | 0.115 | 58 | 0.145 | 36 | 0.513 |
|
Female | 69 |
| 42 |
| 39 |
|
| Age (years) |
|
|
|
|
|
|
|
<65 | 62 | 0.223 | 56 | 0.535 | 40 | 0.296 |
|
≥65 | 50 |
| 46 |
| 31 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
Present | 69 | 0.044a | 61 | 0.120 | 53 | 0.143 |
|
Absent | 46 |
| 53 |
| 40 |
|
| Histological
grade |
|
|
|
|
|
|
| G1 | 67 | 0.503 | 50 | 0.292 | 39 | 0.172 |
| G2 | 52 |
| 48 |
| 30 |
|
| G3 | 64 |
| 71 |
| 57 |
|
| Histological
type |
|
|
|
|
|
|
|
Squamous cell carcinoma | 59 | 0.535 | 62 | 0.144 | 41 | 0.320 |
|
Adenocarcinoma | 57 |
| 45 |
| 35 |
|
| Tumor stage |
|
|
|
|
|
|
| I | 45 | 0.038a | 42 | 0.271 | 24 | 0.030a |
| II | 56 |
| 67 |
| 33 |
|
|
III–IV | 71 |
| 59 |
| 50 |
|
The association between MAGE-A3/C2 expression and
the overall survival of patients was analyzed. Regarding MAGE-A3
expression, 32/76 patients who did not express MAGE-A3 were
identified and 9 succumbed to mortality (median survival time, 48
months; data not shown). A total of 44/76 patients who expressed
MAGE-A3 were identified and 31 succumbed to mortality (median
survival time, 24 months; data not shown). Overall survival was
significantly negatively correlated with MAGE-A3 expression
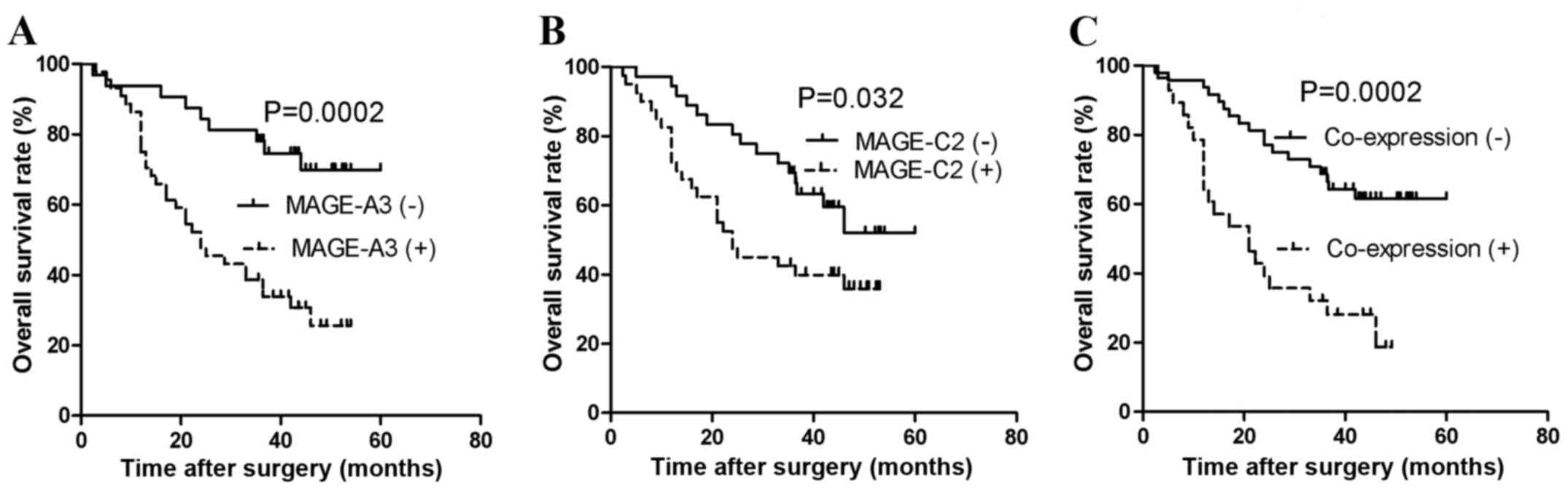
(P=0.0002; Fig. 2A). For MAGE-C2
expression, 36/76 patients who did not express MAGE-C2 were
identified and 15 succumbed to mortality (median survival time, 49
months; data not shown). A total of 40/76 patients with MAGE-C2
expression were identified and 25 succumbed to mortality (median
survival time, 24 months; data not shown). In addition, overall
survival was significantly negatively correlated with MAGE-C2
expression (P=0.032; Fig. 2B).
Coexpression of MAGE-A3 and MAGE-C2 was also correlated with a
poorer overall survival of patients with NSCLC (P=0.0002; Fig. 2C). In addition, multivariate analysis
demonstrated that MAGE-A3 expression was an independent predictor
of poor prognosis (hazard ratio, 3.226; 95% confidence interval,
1.446–7.918; P=0.004; Table V). This
indicates that MAGE-A3 is a marker of poor prognosis in patients
with NSCLC.
 | Table V.Univariate and multivariate analyses
of clinicopathological characteristics associated with overall
survival in cohort 2 patients with non-small cell lung cancer. |
Table V.
Univariate and multivariate analyses
of clinicopathological characteristics associated with overall
survival in cohort 2 patients with non-small cell lung cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
characteristic | HR | CI (95%) | P-value | HR | CI (95%) | P-value |
|---|
| Age | 1.158 | 0.576–2.328 | 0.679 | 1.342 | 0.617–2.919 | 0.458 |
| Gender | 0.865 | 0.453–1.652 | 0.660 | 0.962 | 0.425–2.177 | 0.926 |
| Histological
type | 1.879 | 0.995–3.545 | 0.052 | 1.057 | 0.416–3.879 | 0.364 |
| Lymph node
metastasis | 3.077 | 1.575–6.011 | 0.001a | 0.343 | 0.083–1.418 | 0.140 |
| Tumor stage | 2.159 | 1.475–3.162 | 0.000a | 3.505 | 1.582–7.766 | 0.002a |
| Histological
grade | 0.971 | 0.568–1.659 | 0.914 | 0.733 | 0.416–1.290 | 0.281 |
| MAGE-A3 | 4.129 | 1.888–9.030 | 0.000a | 3.226 | 1.446–7.918 | 0.004a |
| MAGE-C2 | 2.143 | 1.111–4.133 | 0.032a | 1.909 | 0.954–3.821 | 0.068 |
MAGE-A3 siRNA knockdown
Clinicopathological data analysis demonstrated that
MAGE-A3 expression was significantly correlated with advanced tumor
stage (P=0.006; Table III) and poor
patient prognosis (P=0.0002; Fig.
2A), indicating that MAGE-A3 expression contributes to tumor
metastasis and invasion. Thus, siRNA knockdown was performed to
identify the function of MAGE-A3 in NSCLC cells. High-level MAGE-A3
mRNA expression in the NSCLC cell line A549 cells was confirmed,
and siRNA specific to MAGE-A3 was purchased and tested (data not
shown). A total of 48 h post-transfection, si-MAGE-A3 downregulated
MAGE-A3 expression by 80–90% compared with cells transfected with
scrambled siRNA (P=0.0018; Fig.
3A).
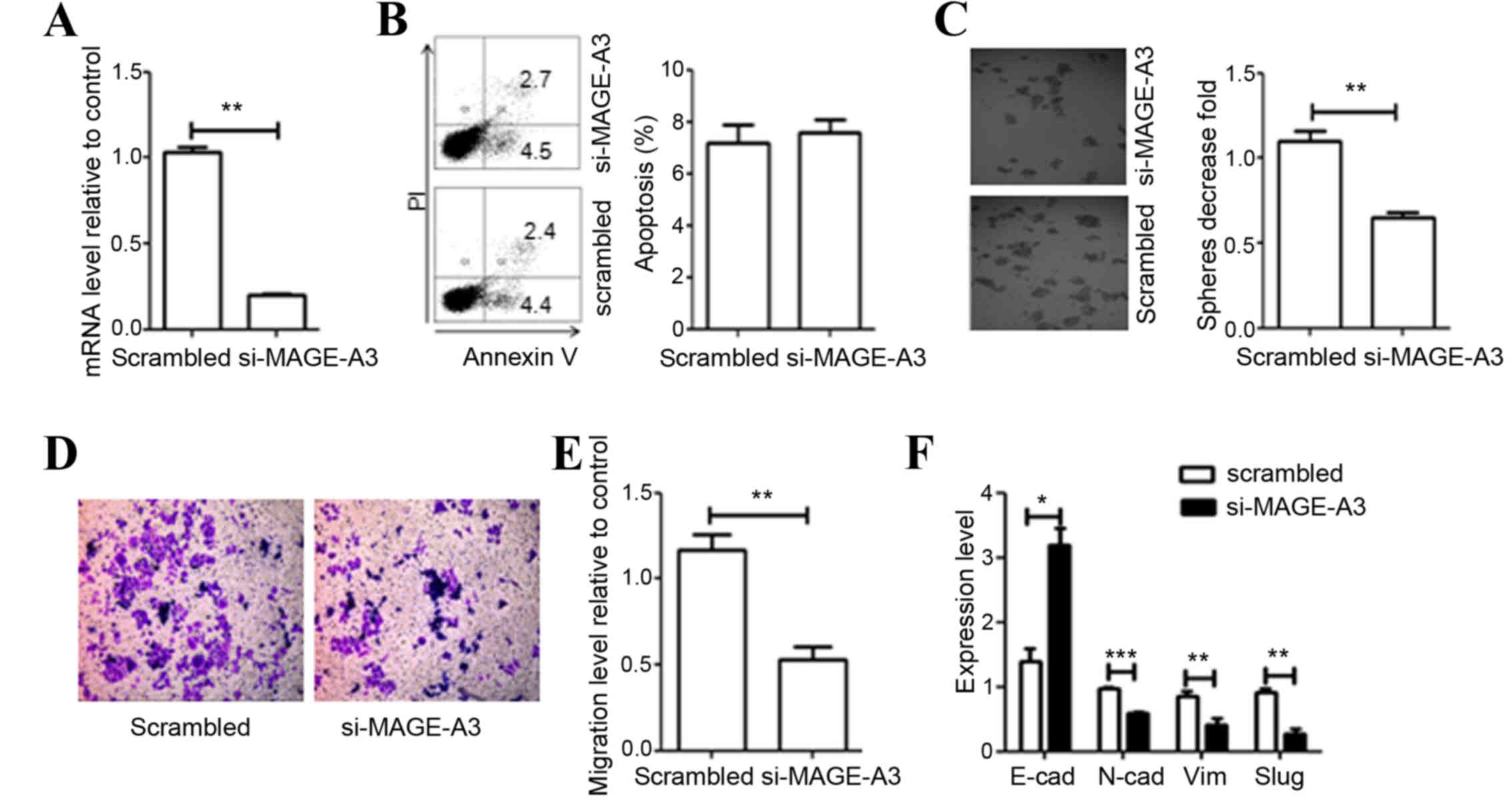 | Figure 3.Effect of MAGE-A3 knockdown on A549
cells. (A) Evaluation of efficiency of si-mediated MAGE-A3
knockdown via qRT-PCR analysis. (B) Effect of MAGE-A3 knockdown on
cell apoptosis was detected through flow cytometry and quantified.
(C) Depletion of MAGE-A3 inhibited cell colony formation. (D)
Representative distinction of migration in A549 cells treated by
scrambled and specific MAGE-A3 siRNA. (E) Downregulation of MAGE-A3
in A549 cells resulted in reduced cell migration. (F) Effect of
MAGE-A3 knockdown on the expression of epithelial-mesenchymal
transition markers was detected by qRT-PCR. All experiments were
repeated ≥3 times. Data were compared using paired two-tailed
t-tests. *P<0.05, **P<0.01, ***P<0.001 vs. the
scrambled control group. MAGE, melanoma-associated antigen; si,
small interfering RNA; EMT, epithelial-mesenchymal-transition
marker; E-cad, epithelial-cadherin; N-cad, neural-cadherin; SLUG,
snail family transcriptional repressor 2; Vim, vimentin; bp, base
pairs; qPCR, quantitative polymerase chain reaction. |
Effect of MAGE-A3 knockdown on A549
cell apoptosis and clonogenic survival
To investigate the biological effect of MAGE-A3
downregulation, siRNA knockdown of MAGE-A3 was performed and the
growth phenotypes of A549 cells were examined. The effect of
MAGE-A3 knockdown on cell apoptosis was determined through a
PI-Annexin V assay. The knockdown of MAGE-A3 expression did not
affect cell apoptosis (P=0.3592; Fig.
3B). The ability of si-MAGE-A3-treated cells to form colonies 7
days following transfection was subsequently analyzed. The
clonogenic cell survival assay assesses the ability of single cells
to proliferate, and form independent and viable colonies. Knockdown
of MAGE-A3 significantly reduced the colony-forming ability of A549
cells by 50% compared with control levels (P=0.0041; Fig. 3C).
Effect of MAGE-A3 knockdown on A549
cell migration and EMT
Clinicopathological data analysis indicated that
MAGE-A3 was involved in tumor metastasis. To confirm whether
MAGE-A3 serves a role in the migration of A549 cells a Transwell
migration assay was performed. The assay demonstrated that
treatment with siRNA targeting MAGE-A3 significantly inhibited the
migration of A549 cells (P=0.0098; Fig.
3D and E). EMT influences the migration ability of tumor cells.
Therefore, expression levels of several well-known EMT-associated
factors [epithelial-cadherin (E-cadherin), neural-cadherin,
vimentin and snail family transcriptional repressor 2] were
measured. As expected, following si-MAGE-A3 treatment of A549
cells, the expression of E-cadherin was significantly increased
compared with the control cells (P=0.0212; Fig. 3F). In contrast, the expression of
mesenchymal cell markers decreased significantly (P<0.05;
Fig. 3F). These results indicate that
the regulation of expression of MAGE-A3 is associated with the EMT
of tumor cells.
Discussion
Cancer-germline genes were first discovered and
described in 1991 (34). Antigens
encoded by cancer-germline genes are exclusively expressed within
germ cells and a number of malignancies, making them attractive
targets for cancer immunotherapy (9,35,36). When selecting appropriate targets for
cancer immunotherapy, it is important to consider the frequency of
expression of the target within the cancer cells of interest. To
improve the application and efficiency of immunotherapy for
patients with NSCLC, it is necessary to determine the pattern and
frequency of cancer-germline gene expression, in addition to the
corresponding antigens encoded by these genes, in NSCLC samples.
Numerous studies have investigated the expression of
cancer-germline genes in NSCLC using IHC or RT-PCR (15–17,19,37).
However, to the best of our knowledge, the expression pattern of
MAGE-C2 has not yet been investigated. In the present study, the
expression of MAGE-A3, MAGE-C2 and their coexpression was
investigated.
MAGE-A3 is one of the most commonly expressed
cancer-germline genes in malignancies. It has been suggested that
MAGE-A3 is expressed in 35–75% of patients with NSCLC, and is
associated with advanced tumor stage and poor patient prognosis
(6,9,38,39). In the present study, the frequency of
MAGE-A3 protein expression was decreased compared with that of
MAGE-A3 mRNA in the tumor tissue of patients with NSCLC. Similar
discrepancies between the expression of other MAGE mRNAs and
proteins have been demonstrated in other types of cancer (40,41). The
lower frequency of protein expression may be due to the lower
sensitivity of IHC compared with RT-PCR and variation between tumor
samples. Thus, further studies are warranted to determine whether
MAGE-A3 expression at the mRNA or protein level is a better
predictor of the patients with NSCLC in which vaccination therapy
will be most effective.
MAGE-A3 mRNA and protein expression was
significantly correlated with lymph node metastasis in the present
study, indicating that MAGE-A3 influences the ability of tumor
cells to metastasize to lymph nodes. The results of the current
study suggest that downregulation of MAGE-A3 expression reduces the
ability of cells migrate through repressing mesenchymal and
inducing epithelial cell phenotype. Knockdown of cancer-germline
genes could alter cell migration, proliferation and EMT, which was
consistent with the results of previous studies (10,42).
MAGE-A3 expression was significantly positively correlated with
smoking history in patients with NSCLC, indicating that smoking may
serve a role in DNA demethylation and the subsequent induction of
MAGE-A3 expression. These results are similar to those of several
previous studies (9,43–45).
Smoking is known to be involved in cancer promotion and progression
(46,47). In addition, MAGE-A3-positive patients
had a significantly poorer overall survival compared with
MAGE-A3-negative patients. Multivariate analysis revealed that
MAGE-A3 expression is an independent marker of poor prognosis in
patients with NSCLC. MAGE-A3 is considered a potential target for
NSCLC immunotherapy, particularly in advanced stage tumors, due to
its relatively high level of expression in NSCLC. MAGE-A3-positive
patients typically require further treatment following surgery and
could benefit from immunotherapy targeting MAGE-A3.
MAGE-C2 belongs to the MAGE family of
cancer-germline genes and has been proposed as a suitable candidate
for immunotherapy in patients with hepatocellular carcinoma
(29). MAGE-C2 has also been
associated with tumor metastasis in breast cancer through inducing
EMT (48). Previous studies have
suggested a correlation between MAGE-C2 expression and advanced
pathological tumor stage, in addition to poor prognosis, in
different tumor types, including multiple myeloma and
hepatocellular carcinoma (24,29). In
the present study, the frequency of MAGE-C2 expression was higher
in male patients with NSCLC and in patients with an advanced tumor
stage; however, no correlation was identified between MAGE-C2
expression and clinicopathological characteristics of NSCLC. The
overall survival of MAGE-C2-positive patients was significantly
decreased compared with MAGE-C2-negative patients. These results
indicate that MAGE-C2 is a target for immunotherapy and a potential
prognostic marker in patients with NSCLC.
Coexpression of MAGE-A3 and MAGE-C2 was
significantly positively correlated with advanced tumor stage and
poor patient prognosis. Several previous studies have indicated
that the coexpression of MAGE-A3 and MAGE-C2 is correlated with
advanced tumor stage and poor patient prognosis in a number of
tumor types (49,50). Furthermore, combined polyvalent or
sequential single vaccinations targeting multiple antigens encoded
by cancer-germline genes could improve antitumor activity in
NSCLC.
In conclusion, the present study identified a
significant positive correlation between MAGE-A3 expression and
tumor progression, in addition to poor patient prognosis. These
results suggest that MAGE-A3 is a potential target for cancer
immunotherapy. In addition, MAGE-A3 was identified to be an
independent marker of poor prognosis in patients with NSCLC. A
large proportion of patients with NSCLC, particularly patients with
an advanced tumor stage who express MAGE-A3/C2, could benefit from
vaccination with MAGE-A3/C2 antigens or antigen-specific TCR
modified T cell therapy.
Acknowledgements
The present study was supported by the China-US
Program for Biomedical Collaborative Research (grant no.
812111102), the National Natural Science Foundation of China (grant
no. 81171986), the Ministry of Public Health (grant no.
20110110001), the Basic and Advanced Technology Research Foundation
of the Science and Technology Department of Henan Province (grant
nos. 112300410153 and 122300410155).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quoix E, Lena H, Losonczy G, Forget F,
Chouaid C, Papai Z, Gervais R, Ottensmeier C, Szczesna A and
Kazarnowicz A: TG4010 immunotherapy and first-line chemotherapy for
advanced non-small-cell lung cancer (TIME): Results from the phase
2b part of a randomised, double-blind, placebo-controlled, phase
2b/3 trial. Lancet Oncol. 17:212–223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pinato DJ, Shiner RJ, White SD, Black JR,
Trivedi P, Stebbing J, Sharma R and Mauri FA: Intra-tumoral
heterogeneity in the expression of programmed-death (PD) ligands in
isogeneic primary and metastatic lung cancer: Implications for
immunotherapy. Oncoimmunology. 5:e12139342016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coulie PG, Van den Eynde BJ, van der
Bruggen P and Boon T: Tumour antigens recognized by T lymphocytes:
At the core of cancer immunotherapy. Nat Rev Cancer. 14:135–146.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ulloa-Montoya F, Louahed J, Dizier B,
Gruselle O, Spiessens B, Lehmann FF, Suciu S, Kruit WH, Eggermont
AM, Vansteenkiste J and Brichard VG: Predictive gene signature in
MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol.
31:2388–2395. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rao M, Chinnasamy N, Hong JA, Zhang Y,
Zhang M, Xi S, Liu F, Marquez VE, Morgan RA and Schrump DS:
Inhibition of histone lysine methylation enhances cancer-testis
antigen expression in lung cancer cells: Implications for adoptive
immunotherapy of cancer. Cancer Res. 71:4192–4204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dobrynin P, Matyunina E, Malov SV and
Kozlov AP: The novelty of human cancer/testis antigen encoding
genes in evolution. Int J Genomics. 2013:1051082013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gure AO, Chua R, Williamson B, Gonen M,
Ferrera CA, Gnjatic S, Ritter G, Simpson AJ, Chen YT, Old LJ and
Altorki NK: Cancer-testis genes are coordinately expressed and are
markers of poor outcome in non-small cell lung cancer. Clin Cancer
Res. 11:8055–8062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koop A, Sellami N, Adam-Klages S, Lettau
M, Kabelitz D, Janssen O and Heidebrecht HJ: Down-regulation of the
cancer/testis antigen 45 (CT45) is associated with altered tumor
cell morphology, adhesion and migration. Cell Commun Signal.
11:412013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Caballero OL, Cohen T, Gurung S, Chua R,
Lee P, Chen YT, Jat P and Simpson AJ: Effects of CT-Xp gene knock
down in melanoma cell lines. Oncotarget. 4:531–541. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laban S, Atanackovic D, Luetkens T, Knecht
R, Busch CJ, Freytag M, Spagnoli G, Ritter G, Hoffmann TK, Knuth A,
et al: Simultaneous cytoplasmic and nuclear protein expression of
melanoma antigen-A family and NY-ESO-1 cancer-testis antigens
represents an independent marker for poor survival in head and neck
cancer. Int J Cancer. 135:1142–1152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andrade VC, Vettore AL, Silva MR Regis,
Felix RS, Almeida MS, de Carvalho F, Zago MA, Caballero OL, Simpson
AJ and Colleoni GW: Frequency and prognostic relevance of cancer
testis antigen 45 expression in multiple myeloma. Exp Hematol.
37:446–449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gotoh K, Yatabe Y, Sugiura T, Takagi K,
Ogawa M, Takahashi T, Takahashi T and Mitsudomi T: Frequency of
MAGE-3 gene expression in HLA-A2 positive patients with non-small
cell lung cancer. Lung Cancer. 20:117–125. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yanagawa N, Tamura G, Oizumi H, Endoh M
and Motoyama T: MAGE mediated by demethylation of MAGE promoters
induce progression of non-small cell lung cancer. Anticancer Res.
31:171–175. 2011.PubMed/NCBI
|
|
16
|
Sienel W, Varwerk C, Linder A, Kaiser D,
Teschner M, Delire M, Stamatis G and Passlick B: Melanoma
associated antigen (MAGE)-A3 expression in Stages I and II
non-small cell lung cancer: Results of a multi-center study. Eur J
Cardiothorac Surg. 25:131–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tajima K, Obata Y, Tamaki H, Yoshida M,
Chen YT, Scanlan MJ, Old LJ, Kuwano H, Takahashi T, Takahashi T and
Mitsudomi T: Expression of cancer/testis (CT) antigens in lung
cancer. Lung Cancer. 42:23–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fourcade J, Sun Z, Pagliano O, Chauvin JM,
Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S,
et al: PD-1 and Tim-3 regulate the expansion of tumor
antigen-specific CD8+ T cells induced by melanoma
vaccines. Cancer Res. 74:1045–1055. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eikawa S, Kakimi K, Isobe M, Kuzushima K,
Luescher I, Ohue Y, Ikeuchi K, Uenaka A, Nishikawa H, Udono H, et
al: Induction of CD8 T-cell responses restricted to multiple HLA
class I alleles in a cancer patient by immunization with a 20-mer
NY-ESO-1f (NY-ESO-1 91–110) peptide. Int J Cancer. 132:345–354.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cerfolio RJ and Bryant AS: Optimal care of
patients with non-small cell lung cancer reduces perioperative
morbidity. J Thorac Cardiovasc Surg. 141:22–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Smet C and Loriot A: DNA
hypomethylation and activation of germline-specific genes in
cancer. Adv Exp Med Biol. 754:149–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Carvalho F, Alves VL, Braga WM, Xavier
CV Jr and Colleoni GW: MAGE-C1/CT7 and MAGE-C2/CT10 are frequently
expressed in multiple myeloma and can be explored in combined
immunotherapy for this malignancy. Cancer Immunol Immunother.
62:191–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brichard VG and Godechal Q:
MAGE-A3-specific anticancer immunotherapy in the clinical practice.
Oncoimmunology. 2:e259952013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pabst C, Zustin J, Jacobsen F, Luetkens T,
Kröger N, Schilling G, Bokemeyer C, Sauter G, Atanackovic D and
Marx A: Expression and prognostic relevance of MAGE-C1/CT7 and
MAGE-C2/CT10 in osteolytic lesions of patients with multiple
myeloma. Exp Mol Pathol. 89:175–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Straetemans T, van Brakel M, van
Steenbergen S, Broertjes M, Drexhage J, Hegmans J, Lambrecht BN,
Lamers C, van Der Bruggen P, Coulie PG and Debets R: TCR gene
transfer: MAGE-C2/HLA-A2 and MAGE-A3/HLA-DP4 epitopes as
melanoma-specific immune targets. Clin Dev Immunol.
2012:5863142012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma W, Germeau C, Vigneron N, Maernoudt AS,
Morel S, Boon T, Coulie PG and Van den Eynde BJ: Two new
tumor-specific antigenic peptides encoded by gene MAGE-C2 and
presented to cytolytic T lymphocytes by HLA-A2. Int J Cancer.
109:698–702. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Sun Z, Nicolay H, Meyer RG,
Renkvist N, Stroobant V, Corthals J, Carrasco J, Eggermont AM,
Marchand M, et al: Monitoring of anti-vaccine CD4 T cell
frequencies in melanoma patients vaccinated with a MAGE-3 protein.
J Immunol. 174:2404–2411. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Olarte I, Martinez A, Ramos-Peñafiel C,
Castellanos-Sinco H, Zamora J, Collazo-Jaloma J, Gutiérrez M,
Gutiérrez-Kobeh L, Chavez-Olmos P, Manzanilla H, et al: MAGE-A3
expression is an adverse prognostic factor in diffuse large B-cell
lymphoma. Hematology. 16:368–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riener MO, Wild PJ, Soll C, Knuth A, Jin
B, Jungbluth A, Hellerbrand C, Clavien PA, Moch H and Jochum W:
Frequent expression of the novel cancer testis antigen
MAGE-C2/CT-10 in hepatocellular carcinoma. Int J Cancer.
124:352–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Detterbeck FC, Postmus PE and Tanoue LT:
The stage classification of lung cancer: Diagnosis and management
of lung cancer, 3rd ed: American college of chest physicians
evidence-based clinical practice guidelines. Chest. 143(5): Suppl.
e191S–e210S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sobin LH and Compton CC: TNM seventh
edition: What's new, what's changed: Communication from the
international union against cancer and the American joint committee
on cancer. Cancer. 116:5336–5339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rapoport AP, Stadtmauer EA, Binder-Scholl
GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B,
Finklestein J, et al: NY-ESO-1-specific TCR-engineered T cells
mediate sustained antigen-specific antitumor effects in myeloma.
Nat Med. 21:914–921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van der Bruggen P, Traversari C, Chomez P,
Lurquin C, De Plaen E, Van den Eynde B, Knuth A and Boon T: A gene
encoding an antigen recognized by cytolytic T lymphocytes on a
human melanoma. Science. 254:1643–1647. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hofmann O, Caballero OL, Stevenson BJ,
Chen YT, Cohen T, Chua R, Maher CA, Panji S, Schaefer U, Kruger A,
et al: Genome-wide analysis of cancer/testis gene expression. Proc
Natl Acad Sci USA. 105:20422–20427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Caballero OL and Chen YT: Cancer/testis
(CT) antigens: Potential targets for immunotherapy. Cancer Sci.
100:2014–2021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoshida N, Abe H, Ohkuri T, Wakita D, Sato
M, Noguchi D, Miyamoto M, Morikawa T, Kondo S, Ikeda H and
Nishimura T: Expression of the MAGE-A4 and NY-ESO-1 cancer-testis
antigens and T cell infiltration in non-small cell lung carcinoma
and their prognostic significance. Int J Oncol. 28:1089–1098.
2006.PubMed/NCBI
|
|
38
|
Kim YD, Park HR, Song MH, Shin DH, Lee CH,
Lee MK and Lee SY: Pattern of cancer/testis antigen expression in
lung cancer patients. Int J Mol Med. 29:656–662. 2012.PubMed/NCBI
|
|
39
|
Pineda CT, Ramanathan S, Fon Tacer K, Weon
JL, Potts MB, Ou YH, White MA and Potts PR: Degradation of AMPK by
a cancer-specific ubiquitin ligase. Cell. 160:715–728. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vaughan HA, Svobodova S, Macgregor D,
Sturrock S, Jungbluth AA, Browning J, Davis ID, Parente P, Chen YT,
Stockert E, et al: Immunohistochemical and molecular analysis of
human melanomas for expression of the human cancer-testis antigens
NY-ESO-1 and LAGE-1. Clin Cancer Res. 10:8396–8404. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li M, Yuan YH, Han Y, Liu YX, Yan L, Wang
Y and Gu J: Expression profile of cancer-testis genes in 121 human
colorectal cancer tissue and adjacent normal tissue. Clin Cancer
Res. 11:1809–1814. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baia GS, Caballero OL, Ho JS, Zhao Q,
Cohen T, Binder ZA, Salmasi V, Gallia GL, Quinones-Hinojosa A,
Olivi A, et al: NY-ESO-1 expression in meningioma suggests a
rationale for new immunotherapeutic approaches. Cancer Immunol Res.
1:296–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Grah J, Samija M, Juretić A, Sarcević B
and Sobat H: Immunohystochemical expression of cancer/testis
antigens (MAGE-A3/4, NY-ESO-1) in non-small cell lung cancer: The
relationship with clinical-pathological features. Coll Antropol.
32:731–736. 2008.PubMed/NCBI
|
|
44
|
Tan Q, Wang G, Huang J, Ding Z, Luo Q, Mok
T, Tao Q and Lu S: Epigenomic analysis of lung adenocarcinoma
reveals novel DNA methylation patterns associated with smoking.
Onco Targets Ther. 6:1471–1479. 2013.PubMed/NCBI
|
|
45
|
Bhutani M, Pathak AK, Tang H, Fan YH, Liu
DD, Lee JJ, Kurie J, Morice RC, Hong WK and Mao L: Frequent
expression of MAGE1 tumor antigens in bronchial epithelium of
smokers without lung cancer. Exp Ther Med. 2:137–142.
2011.PubMed/NCBI
|
|
46
|
Chen RJ, Chang LW, Lin P and Wang YJ:
Epigenetic effects and molecular mechanisms of tumorigenesis
induced by cigarette smoke: An overview. J Oncol. 2011:6549312011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Park SY, Lee JG, Kim J, Bae MK, Lee CY,
Kim DJ and Chung KY: The influence of smoking intensity on the
clinicopathologic features and survival of patients with surgically
treated non-small cell lung cancer. Lung Cancer. 81:480–486. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang F, Zhou X, Miao X, Zhang T, Hang X,
Tie R, Liu N, Tian F, Wang F and Yuan J: MAGEC2, an
epithelial-mesenchymal transition inducer, is associated with
breast cancer metastasis. Breast Cancer Res Treat. 145:23–32. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen X, Wang L, Yue D, Liu J, Huang L,
Yang L, Cao L, Qin G, Li A, Wang D, et al: Correlation between the
high expression levels of cancer-germline genes with clinical
characteristics in esophageal squamous cell carcinoma. Histol
Histopathol. 118472016.PubMed/NCBI
|
|
50
|
Andrade VC, Vettore AL, Felix RS, Almeida
MS, Carvalho F, Oliveira JS, Chauffaille ML, Andriolo A, Caballero
OL, Zago MA and Colleoni GW: Prognostic impact of cancer/testis
antigen expression in advanced stage multiple myeloma patients.
Cancer Immun. 8:22008.PubMed/NCBI
|