Introduction
Non-small cell lung cancer (NSCLC) accounts for ~80%
of all lung cancers. Surgery remains the main treatment for
early-stage NSCLC patients (1).
Operable patients with stage IA-IIIA disease (2) are candidates for resection surgery with
curative intent; this group accounts for ~35% of all lung cancer
cases. However, in a large number of patients, tumors recur
following surgical resection (3).
Five-year survival rates are variable, at 57–67% and 39–55% for
stage I and II disease, respectively (3). Patients with completely resected stage
IIIA disease exhibit a 5-year overall survival (OS) rate of ~25%.
The most frequent cause of mortality in these patients is distant
metastases (4,5). Occult micrometastatic disease, which
remains undetected at the time of presurgical staging, may be the
cause of recurrence in distant sites following surgery. Therefore,
eradicating early metastatic disease using chemotherapy (CT) may
reduce the incidence of recurrence at distant sites, subsequently
improving survival (6). CT may be
administrated prior to [induction or neoadjuvant CT (NA-CT)] or
subsequent to (adjuvant CT) surgery.
At present, NA-CT is the standard treatment for
stage IIIA NSCLC. It is known to improve survival in patients who
are not candidates for surgery following induction CT; however,
response and survival rates remain low (7).
Theoretical advantages of induction CT include in
vivo evaluation of response to CT, which may identify patients
that would benefit from adjuvant treatment; early micrometastatic
treatment, which may prevent disease recurrence at distant sites;
reduced drug resistance due to early CT exposure; and increased
resectability and conservation of healthy pulmonary parenchyma
(6).
However, identification of patients that may benefit
from surgery following induction CT is controversial. A previous by
the Southwestern Oncology Group (8)
indicated that surgery should be avoided in cases where mediastinal
involvement persists subsequent to NA-CT. In this previous study,
patients with complete pathological response exhibited a median
survival time of 30 months compared to 10 months in patients with
residual tumor.
Novel chemotherapeutic drugs that have demonstrated
efficacy in the treatment of metastatic disease, including
gemcitabine (9), paclitaxel (10), vinorelbine (9) and docetaxel (11), have been added to neoadjuvant
treatment regimens, with response rates of 44–80%, and complete
resection rates of 67–79%. The aforementioned drugs are also strong
radiosensitizing agents.
In the current study, the effect of NA-CT treatment
with cisplatin plus vinorelbine on OS was analyzed in 21 N2
patients diagnosed with potentially resectable NSCLC.
Patients and methods
Patient cohort
A total of 21 patients were included and
retrospectively analyzed, meeting the following inclusion criteria:
Adults over 18 years, histologically diagnosed with stage IIIA
(T1-3 N1-2 and T4N0) NSCLC between March 2008 and December 2011.
Patients required available tissue remaining from biopsy for
analysis, had to have been treated with cisplatin and vinorelbine
NA-CT and were followed up at the Puerta de Hierro Hospital
(Madrid, Spain). All patients were followed up until April 2014.
The study adhered to the principles of the Declaration of Helsinki
and Good Clinical Practice guidelines (12), and was approved by the institutional
review board of Puerta de Hierro Hospital.
The clinical records of the patient cohort were
reviewed; this included the patient medical history and results of
physical examination, basic biochemical blood tests, blood count,
blood clotting tests, chest X-rays and biopsies, with a diagnosis
of NSCLC in all cases.
Patients underwent initial positron emission
tomography (PET)/computed tomography, as well as pathological
assessment of mediastinal nodes by biopsy or cytology. Staging was
determined according to the 7th edition of TNM Classification of
Malignant Tumours (13).
All cases were submitted to the thoracic tumor
committee, which includes radiation oncologists, pulmonologists,
thoracic surgeons, radiologists, nuclear medicine physicians,
pathologists and medical oncologists, where the neoadjuvant
treatment approach was selected.
All patients received three 21-day cycles of
induction treatment with 75 mg/m2 intravenous cisplatin
(day 1) and 25 mg/m2 vinorelbine (days 1 and 8).
Treatment response was assessed by PET/computed
tomography; if a response was observed, mediastinal node
involvement was re-evaluated. Cases that had been down-staged and
were suitable for surgery subsequently underwent lobectomy or
bilobectomy.
The following patient characteristics were
evaluated: Gender, smoking history, age at diagnosis, comorbidities
(including hypertension, chronic obstructive pulmonary disease,
heart disease, diabetes mellitus, transplant and coagulopathy),
personal history of cancer, Eastern Cooperative Oncology Group
performance status (ECOG PS) (14),
tumor histology, and tumor stage at diagnosis. Data relating to
induction treatment response and disease evolution were also
recorded.
Progression-free survival (PFS) was defined as the
time between diagnosis date and the date when the first recurrence
or progression was identified, and OS time was defined as the
period between diagnosis of lung cancer and patient mortality.
Statistical analysis
Qualitative variables were expressed as absolute
frequency and percentage. Normal distributions were tested using
the Shapiro-Wilk test. Mean comparisons between groups of
continuous variables with normal distribution were compared using
the Student's t-test for unpaired samples, while those with an
asymmetric distribution were compared using the Mann-Whitney U
test. The χ2 test and Fisher's exact test were used to
analyze qualitative variables. A hazard ratio (HR) and 95%
confidence interval (95% CI) were estimated for each variable.
P-values were two-sided, and P<0.05 was considered to indicate a
statistically significant difference. SPSS 14.0 software (SPSS,
Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Patient characteristics
The general characteristics of the patients at
diagnosis and patient response to NA-CT are shown in Table I. All patients exhibited an ECOG PS of
0 or 1, the mean age of patients was 62.57 years (range, 45–73
years) and 62% of patients exhibited >2 relevant comorbidities.
All patients exhibited stage IIIA NSCLC at diagnosis, and 14
patients exhibited N2, multistation, or bulky mediastinal node
involvement. Bulky disease was defined as mediastinal lymph nodes
measuring >2 cm at the longest axis. No cases of CT-induced
toxicity resulting in treatment delay occurred.
 | Table I.Clinicopathological characteristics
of 21 stage IIIA non-small cell lung cancer patients. |
Table I.
Clinicopathological characteristics
of 21 stage IIIA non-small cell lung cancer patients.
| Parameter | Patients |
|---|
| Median age at
diagnosis, years | 62.57 |
| Gender, n (%) |
|
|
Female | 4
(19) |
|
Male | 17 (81) |
| Age, years |
|
|
Mean | 62.57 |
|
Range | 45–73 |
| Smoking history, n
(%) |
|
|
Non-smokers | 3
(15) |
|
Smokers | 18 (85) |
| ECOG PS, n (%) |
|
| 0 | 16 (76) |
| 1 | 5
(24) |
| Comorbidities, n
(%) |
|
|
0–1 | 8
(38) |
|
2–3 | 9
(43) |
|
>3 | 4
(19) |
| Histology, n
(%) |
|
|
Adenocarcinoma | 10 (48) |
|
Squamous cell carcinoma | 8
(38) |
| Large
cell carcinoma | 3
(14) |
| TNM stage, n
(%) |
|
| T1-3,
N0-1 | 1 (5) |
| T1-3,
N2 | 17 (81) |
|
T4N0 | 3
(14) |
| Bulky or
multistation node involvement, n (%) |
|
|
Yes | 14 (67) |
| No | 7
(33) |
| Response to NA-CT,
n (%) |
|
|
Complete | 4
(19) |
|
Partial | 6
(29) |
|
Stable | 9
(43) |
|
Progression | 2 (9) |
| Down-staged, n
(%) |
|
|
Yes | 7
(33) |
| No | 14 (67) |
| Surgical treatment,
n (%) |
|
|
Yes | 7
(33) |
| No | 14 (67) |
Patient response to NA-CT
A total of 10 patients (48%) exhibited response to
induction treatment; a total of 4 patients (19%) exhibited a
complete response and 6 patients (29%) exhibited a partial response
according to the Response Evaluation Criteria In Solid Tumors
criteria (15). However, pathological
down-staging was only verified in 7 cases (33%). A total of 9
patients exhibited a stable response, and 2 progressed following
NA-CT. The characteristics of the patients that were successfully
down-staged following induction treatment are shown in Table II.
 | Table II.Comparison of patient characteristics
of down-staged (n=7) and non-down-staged patients (n=14). |
Table II.
Comparison of patient characteristics
of down-staged (n=7) and non-down-staged patients (n=14).
| A,
Clinicopathological parameters of patients. |
|---|
|
|---|
| Parameter | Down-staged
patients | Non-down-staged
patients |
|---|
| Median age (range),
years | 62 (50–70) | 61 (45–73) |
| Gender, n |
|
|
|
Female | 1 | 3 |
|
Male | 6 | 11 |
| ECOG PS, n |
|
|
| 0 | 6 | 10 |
| 1 | 1 | 4 |
| Smoking history,
n |
|
|
|
Smoker | 6 | 11 |
|
Non-Smoker | 0 | 3 |
|
Unknown | 1 | 0 |
| Histology, n |
|
|
|
Adenocarcinoma | 4 | 6 |
|
Squamous cell carcinoma | 1 | 7 |
| Large
cell carcinoma | 2 | 1 |
| Multistation/bulky
mediastinal node involvement, n |
|
|
Yes | 4 | 10 |
| No | 3 | 4 |
|
|---|
| B, TNM stages of
patients pre- and post-chemotherapy |
|
|---|
|
| TNM stage |
|
|
|
| Patients | preNA-CT | postNA-CT |
|
|---|
| Down-staged
patients | T1N2 | T0N0 |
|
| T2N2 | T0N0 |
|
| T2N2 | T0N0 |
|
| T3N2 | T0N0 |
|
| T3N2 | yT3N0 |
|
| T1N2 | yT2N0 |
|
| T3N1 | yT2N0 |
| Non-down-staged
patients | T2N2 | T2N2 |
|
| T3N2 | T3N2 |
|
| T2N2 | T2N2 |
|
| T4N0 | T4N0 |
|
| T3N2 | T3N2 |
|
| T2N2 | T2N2 |
|
| T3N2 | T3N2 |
|
| T2N2 | T2N2 |
|
| T4N0 | T4N0 |
|
| T2N2 | T2N2 |
|
| T2N2 | T2N2 |
|
| T4N0 | T4N0 |
|
| T2N2 | T2N2M1 |
|
| T3N2 | T3N2 |
Univariate analysis revealed a significant
association between multistation or bulky nodal involvement and
response to induction CT. This association indicated that the
response to NA-CT was worse in patients who exhibited this type of
nodal involvement, with an odds ratio of 15 (95% CI, 1.34–167.63;
P=0.0446) (Table III). However, no
significant difference was identified between response to NA-CT and
other clinicopathological factors, such as gender, smoking history,
ECOG PS, histology, primary lesion size and nodal involvement.
 | Table III.Response to chemotherapy and presence
of multistation or bulky lymph node involvement in non-small cell
lung cancer patients. |
Table III.
Response to chemotherapy and presence
of multistation or bulky lymph node involvement in non-small cell
lung cancer patients.
| Response to
neoadjuvant chemotherapy | Patients, n
(%a) | Patients with
multistation/bulky involvement, n (%b) |
|---|
| Complete | 4
(19) | 1 (25) |
| Partial | 6
(29) | 3 (50) |
| Stable | 9
(43) | 8 (89) |
| Progression | 2 (9) | 2
(100) |
Treatment following induction CT
A total of 7 (33%) patients underwent surgery
(lobectomy): 3 of the 4 patients who had exhibited a complete
response, and 4 out of 6 who had exhibited a partial response. Of
the 7 patients that underwent surgery, 6 underwent complete
resection (defined as tumor-free surgical margins and superior
mediastinal nodes in the surgical specimen with no infiltration of
tumor cells). The surgery-associated mortality rate was 0%, as no
patient mortalities occurred within the first 30 days following
surgery. Patients who had shown no response to NA-CT were treated
with radical radiotherapy at doses of ≤66 Gy (dose range from 45–66
Gy administered in a daily schedule at a fraction of 1.8 cGy per
day).
Follow-up
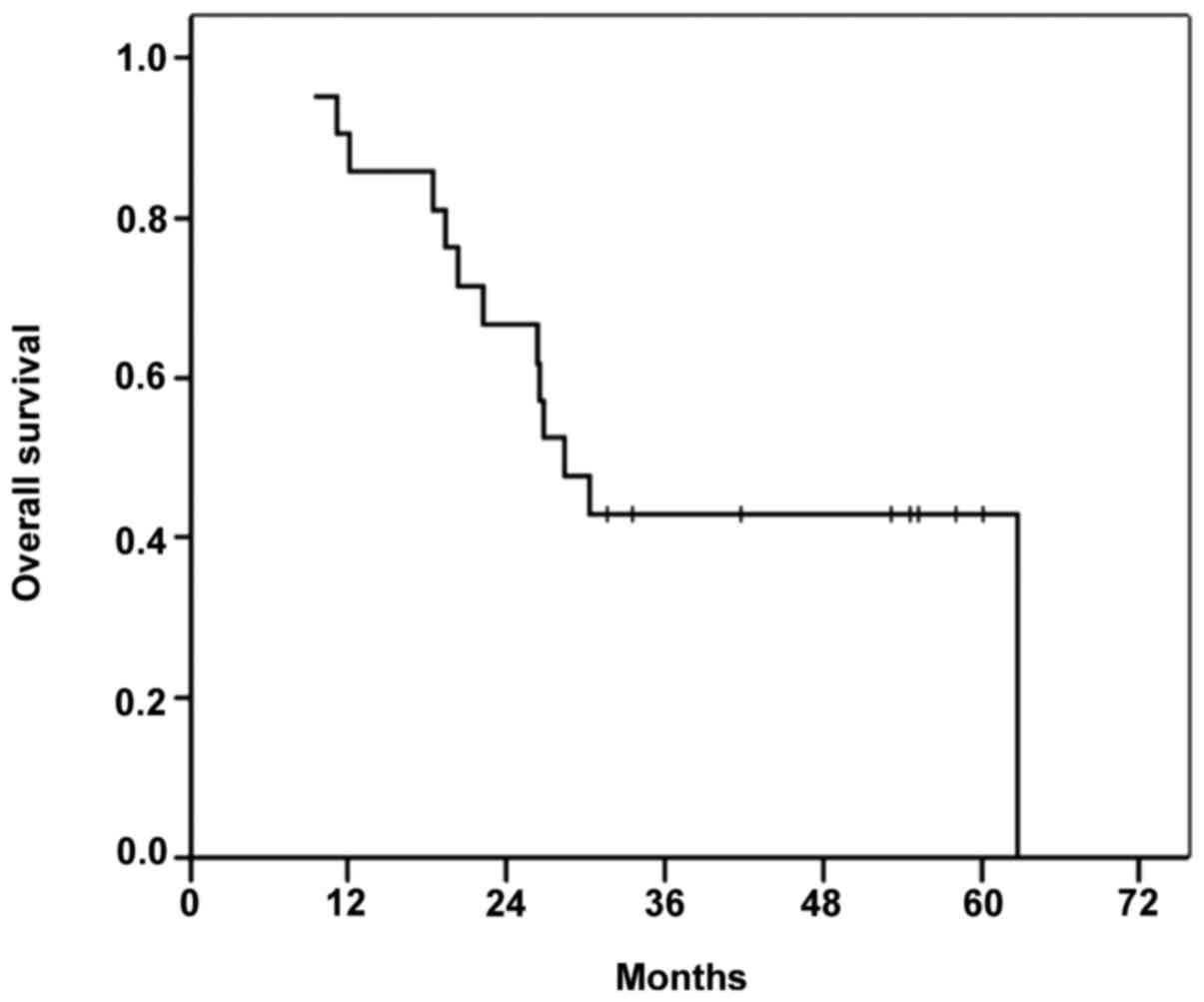
The median OS time in the cohort was 28.5 months
(range, 9–62 months), and the 3- and 5-year OS rates were 28.5 and
4.7%, respectively. By the end of follow up in April 2014, 13/21
patients included in the study had died, 92.3% of whom had
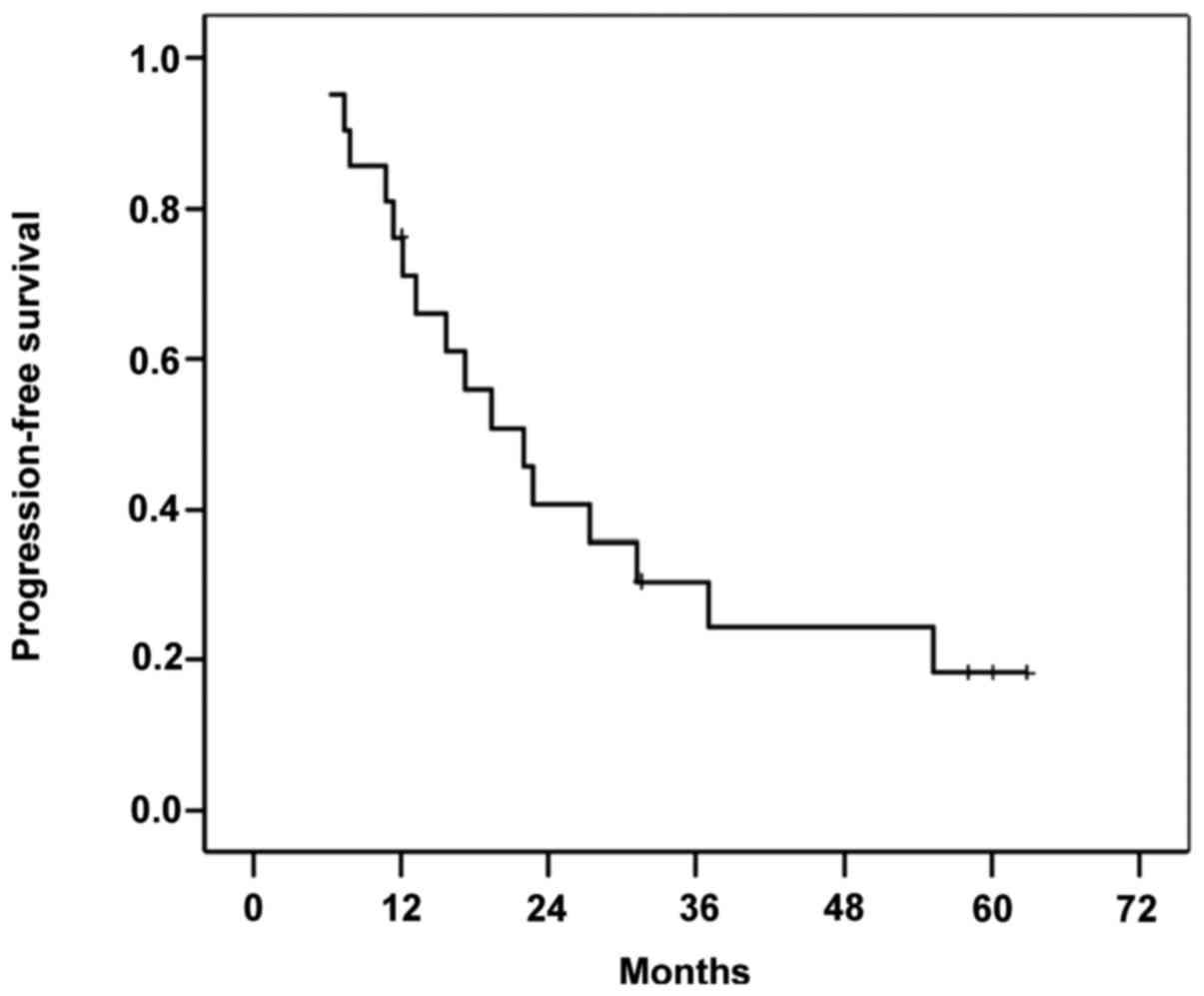
succumbed due to tumor progression. Figs.
1 and 2 show the PFS and OS of
the patient cohort, respectively. The 3-year survival rate of the
patients that underwent surgery was 42.8%, compared to 28.5% in
non-surgical patients. For the patients who remained alive upon
completion of the study, the follow-up period was 40 months.
Overall, the whole cohort exhibited a 3-year
disease-free survival rate of 23.8%, and a 5-year disease-free
survival rate of 4.7%. The median PFS time was 19.4 months (range,
6–62 months), and 76% of patients exhibited tumor recurrence.
In the group of patients who were successfully
down-staged, 4/7 (57.1%) patients exhibited recurrence, with a PFS
time of 18 months (range, 3–58 months). Among the non-down-staged
patients, 12 (85%) exhibited recurrence, with a PFS time of 14
months (range, 6–55 months).
Patients who underwent surgery exhibited a median
PFS time of 10 months (range, 3–18 months) and 5 (71%) patients
exhibited recurrence. No significant differences between any of the
clinicopathological patient characteristics analyzed and PFS were
identified.
Of the down-staged patient group, 4 (57%) patients
died, with an OS time of 58 months (range, 19–62 months). In the
non-down-staged group, 9/14 patients died (64.2%) with a median OS
time of 27 months (range, 8–58 months).
A significant association between tumor histology
and OS was identified; squamous cell carcinoma, which was diagnosed
in 8 patients (of whom 7 had died by the end of the study), was
associated with a shorter OS time (P=0.029).
No statistically significant differences were
identified between OS and gender, smoking history, ECOG PS, tumor
size, nodal involvement, multistation or bulky disease,
down-staging or surgery.
A total of 12 (57%) patients exhibited distant
metastasis at the end of the study. Distant metastasis to the lung
(7 cases) and central nervous system (5 cases) occurred most
frequently, whereas bone and mediastinal metastasis were less
common.
Discussion
Patients with stage IIIA N2 NSCLC exhibit 5-year OS
rates of 10–15%. In stage IIIA N2 patients with multistation or
bulky disease this rate is only 2–5%. The efficacy of surgical
treatment in these cases is controversial. In four previous
studies, which included a total of 1,180 patients undergoing
surgery, the 5-year OS rates ranged from 14 to 30% (16–19).
However, these studies used different inclusion criteria, included
patients with different prognoses, defined ‘resectable disease’ or
‘marginally resectable tumor’ differently, and used varying CT
regimens as induction or adjuvant treatment. Therefore, comparisons
must be considered with caution. Despite these limitations, other
studies suggest that treatment with cisplatin-based CT improves
survival in NSCLC patients (7,20–25).
Generally, patients treated with NA-CT exhibit a
median survival time of 20 months and a 3-year survival rate of 34%
(6,26). This is consistent with the results of
the present study, in which OS was 28 months and PFS was 19.5
months.
Complete resection, down-staging and complete
resection are predictors of long-term survival (27,28). There
is a variability in recurrence free survival following radical
treatment of stage III non-small cell lung cancer, and the
above-mentioned factors may assist with the selection of patients
who will show greater benefit from thoracic surgery. Patients who
undergo tumor resection have longer survival times than those who
do not (29,30).
Complete pathological response following NA-CT
typically varies from 0 to 9.5% (20,24,28). Two
previous studies reported rates of 16.7% (31) and 15% (32); however, this is rare. In the present
study, the complete response rate was 19%. This was a notable
results, although it did not correlate significantly with survival
due to the small sample size. The type of response to neoadjuvant
chemotherapy (complete, partial, stable or progressive disease)
correlated with the presence r not of bulky or multistation
mediastinal nodal involvement. In addition, the current study found
a median OS time of 58 months in patients achieving pathological
tumor response, which was significantly higher than that of
patients with no response, who exhibited an OS time of 27
months.
Andre et al (33) analyzed a cohort of 702 patients with
N2 NSCLC and identified four negative risk factors: clinical
evidence of N2 prior to surgery, multistation mediastinal lymph
node involvement, and pT3 or pT4 stage disease. Choi et al
(34), found that, among the 19
clinical pathological prognostic factors studied in patients with
pathological evidence of N2 NSCLC, incomplete resection and
persistent N2 disease after induction CT were negative prognostic
factors in univariate analysis. Clinical evidence of N2 disease,
multistation mediastinal lymph node involvement and adenocarcinoma
histology indicated a poorer prognosis; however, no statistical
significance was identified. Furthermore, adjuvant CT
administration did not significantly improve prognosis.
Univariate analysis indicated that complete
resection and adjuvant CT were favorable prognostic factors in the
present study in 6/7 patients who underwent surgery and complete
resection. Complete resection is an established prognostic factor
in several previous studies (27,28,34,35).
In these previous studies, overall survival and progression free
survival were increased in those patients who achieved complete
resection. Adjuvant chemotherapy demonstrated an improvement in
survival when compared with patients treated with surgery or
radiotherapy only (4,20).
In the present study, squamous cell carcinoma was
significantly associated with a shorter OS time and thus is
considered a negative prognostic factor. Clinicopathological
variables, including gender, smoking history, ECOG, primary tumor
size, nodal involvement, multistation lymph nodes and bulky
disease, were not statistically associated with OS.
Clinical trials specifically designed for patients
with stage IIIA NSCLC are listed in Table IV; five of the studies included did
not reach recruitment targets, mainly due to differences identified
in the treatment arms. Only the Spanish Lung Cancer Group 9901 and
the NCT0000262 trials were completed.
 | Table IV.Previous clinical trials involving
stage IIIA non-small cell lung cancer patients. |
Table IV.
Previous clinical trials involving
stage IIIA non-small cell lung cancer patients.
| Trial (ref.) | Recruitment period
(years) | Patients, n | Arms compared | NA-CT regimen | Adjuvant
chemotherapy | Adjuvant RT | Recruitment
completed | Reason for study
interruption | Median follow-up
(years) | Median OS time
(months) |
|---|
| MD Anderson 1994
(24) | 1987–1993 | 60 | NeoQT + Qx vs. Qx
alone operative QT | Cyclophosphamide +
etoposide + cisplatin; 3 cycles every 4 weeks | 3, to
respondersa | Yes, if surgery
incomplete or unresectable | No | Benefit of
preoperative chemotherapy | 6.7 | 64 for pre- vs. 11
for surgery alone |
| Spain 1994
(21) | 1989–1991 | 59 | NeoQT + Qx vs. Qx
alone | Mitomycin +
ifosfamide + cisplatin; 3 cycles every 3 weeks | 0 | Yes | No | Benefit of
preoperative chemotherapy | 6.3 | 26 for preoperative
QT vs. 8 for surgery alone |
| JCOG 9209 2003
(38) | 1993–1998 | 62 | NeoQT + Qx. vs. Qx
alone | Vindesine +
cisplatin; 3 cycles every 4 weeks | 0 | Yes, if surgery
incomplete | No | Poor accrual | 5.7 | 17 for preoperative
QT vs. 16 for surgery alone group |
| China 2002
(39) | 1999–2004 | 55 | NeoQT + Qx vs. Qx
alone | Docetaxel +
carboplatin; 2 cycles every 3 weeks | 0 a | Yes, if surgery
incomplete | No | Positive results of
adjuvant chemotherapy trials/poor accrual | 7.8 | – |
| China 2005
(40) | 1999–2004 | 40 | NeoQT + Qx vs. Qx
alone | Gemcitabine +
cisplatin; gemcitabine + carboplatin; 2 cycles every 3 weeks | 2, to
responders | No | No | Poor accrual | 3.3 | – |
| SLCG 9901 2007
(33) | 1999–2003 | 136 | NeoQT + surgery vs.
NeoQT + no surgery | Cisplatin +
gemcitabine + docetaxel; 3 cycles every 3 weeks | 2, if pathological
N2 | Yes if incomplete
resection or not resectable | Yes | N/A | 4.2 | 48.5 for completely
resected patients and 16.8 for non-resected patients |
| NCT0000262 2007
(29) | 1994–2002 | 579 | NeoQT+ surgery vs.
RT | Platin-based
doublet for 3 cycles | N/A | Yes | Yes | N/A |
| 16.4 for surgery
group vs. 17.5 for RT group |
The Spanish Lung Cancer Group (36) study included 136 patients with locally
advanced NSCLC. Due to the homogeneity of patients enrolled and the
geographical location, this is a good reference trial, despite the
clear differences in scientific evidence obtained from clinical
trials and patient series. The overall complete resection rate was
68.9% among patients eligible for surgery (72% of stage IIIA
patients and 66% of stage IIIB patients) and 48% of all assessable
patients. In the present study, the overall resection rate for all
assessable patients was 85.7%. In the aforementioned trial
(36), the rate of complete
pathological response was 12.9% of 62 completely resected patients,
compared with 42.85% in the present study (of 7 patients undergoing
surgery, 3 showed complete pathological response in the surgical
specimen). However, the fact that the results may have been
strongly influenced by the sample size must be considered.
With regard to CT and surgery-related toxicities, in
the Spanish group trial (36), 6/136
patients withdrew from the study due to CT toxicity, and 7 patients
(7.8%) died during the postoperative period. The trial used a
platin-based regimen with three drugs, which differs from the
current standard treatment, a platin-based doublet, as used in the
current study. No NA-CT, surgery or radiotherapy treatment was
delayed due to secondary effects in the present study cohort, and
the surgical death rate was 0%. The safety of this regimen has been
evaluated previously in clinical trials; Krzakowski et al
(37) investigated the use of this
doublet in combination with radiotherapy for the treatment of stage
III NSCLC. The median OS time of patients was 15.9 months, and
3-year survival rate was 36.8% (37).
In the present study, OS time was 28.5 months, and the 3-year
survival rate was 28.5%. In the study by Krzakowski et al
(37), the median survival time was
48.5 months in 62 completely resected patients, 12.9 months in 13
incompletely resected patients, and 16.8 months in 15 non-resected
patients (P=0.005). In the present study non-resected patients, the
OS time was 27 months. However, the higher median OS times observed
in the current study may be due to the small sample size.
In the Spanish group trial (36), the 3-year survival rate was 60.1% in
completely resected patients and 31.1% in non-resected patients. In
the present study, it was 42.8% in surgical patients, and 28.5% in
non-surgical patients. In the Spanish group trial study, clinical
response and age (<60 years) were the most significant
prognostic factors (HR, 0.35; P<0.0001; and HR,0.64; P=0.027,
respectively) (36).
In conclusion, cisplatin plus vinorelbine is a
feasible regimen in the neoadjuvant setting with good response
rates and acceptable toxicity. The most significant factor
associated with a poor response to induction treatment was
multistation or bulky N2 mediastinal lymph node involvement.
However, further studies are required, as long-term survival rates
in stage III NSCLC remain low.
Acknowledgements
This study was supported by the Instituto de
Investigación Carlos III-PI (grant no. 13/01806) (MP).
References
|
1
|
American Cancer Society, . Lung cancer
(Non-small cell). Atlanta, GA, USA: 2016
|
|
2
|
Mirsadraee S, Oswal D, Alizadeh Y, Caulo A
and van Beek E Jr: The 7th lung cancer TNM classification and
staging system: Review of the changes and implications. World J
Radiol. 4:128–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim E, Harris G, Patel A, Adachi I,
Edmonds L and Song F: Preoperative versus postoperative
chemotherapy in patients with resectable non-small cell lung
cancer: Systematic review and indirect comparison meta-analysis of
randomized trials. J Thorac Oncol. 4:1380–1388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arriagada R, Dunant A, Pignon JP, Bergman
B, Chabowski M, Grunenwald D, Kozlowski M, Le Péchoux C, Pirker R,
Pinel MI, et al: Long-term results of the international adjuvant
lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy
in resected lung cancer. J Clin Oncol. 28(1): 35–42. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
Mariotto A, et al: SEER Cancer Statistics Review. 1975–2013,
National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2013Accessed
September 1, 2016.
|
|
6
|
NSCLC Meta-analysis Collaborative Group, .
Preoperative chemotherapy for non-small-cell lung cancer: A
systematic review and meta-analysis of individual participant data.
Lancet. 383:1561–1571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Depierre A, Milleron B, Moro-Sibilot D,
Chevret S, Quoix E, Lebeau B, Braun D, Breton JL, Lemarié E, Gouva
S, et al: Preoperative chemotherapy followed by surgery compared
with primary surgery in resectable stage I (except T1N0), II and
IIIa non-small-cell lung cancer. J Clin Oncol. 20:247–253. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Albain KS, Rusch VW, Crowley JJ, Rice TW,
Turrisi AT III, Weick JK, Lonchyna VA, Presant CA, McKenna RJ,
Gandara DR, et al: Concurrent cisplatin/etoposide plus chest
radiotherapy followed by surgery for stages IIIA (N2) and IIIB
non-small-cell lung cancer: Mature results of southwest oncology
group phase II study 8805. J Clin Oncol. 13:1880–1892.
1995.PubMed/NCBI
|
|
9
|
Vokes EE, Herndon JE II, Crawford J,
Leopold KA, Perry MC, Miller AA and Green MR: Randomized phase II
study of cisplatin with gemcitabine or paclitaxel or vinorelbine as
induction chemotherapy followed by concomitant chemoradiotherapy
for stage IIIB non-small-cell lung cancer: Cancer and leukemia
group B study 9431. J Clin Oncol. 20:4191–4198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Brien ME, Splinter T, Smit EF, Biesma B,
Krzakowski M, Tjan-Heijnen VC, Van Bochove A, Stigt J,
Smid-Geirnaerdt MJ, Debruyne C, et al: Carboplatin and paclitaxol
(Taxol) as an induction regimen for patients with biopsy-proven
stage IIIA N2 non-small cell lung cancer. An EORTC phase II study
(EORTC 08958). Eur J Cancer. 39:1416–1422. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zarogoulidis K, Kontakiotis T,
Hatziapostolou P, Fachantidou E, Delis D, Goutsikas J,
Constantinidis TC and Athanasiadis A: A Phase II study of docetaxel
and carboplatin in the treatment of non-small cell lung cancer.
Lung Cancer. 32:281–287. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
World Medical Association, . Declaration
of Helsinki: WMA Declaration of Helsinki - Ethical Principles for
Medical Research Involving Human Subjects. http://www.wma.net/en/30publications/10policies/b3/Accessed.
September 1–2016.
|
|
13
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC lung cancer staging project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martini N and Flehinger BJ: The role of
surgery in N2 lung cancer. Surg Clin North Am. 67:1037–1049. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watanabe Y, Shimizu J, Oda M, Hayashi Y,
Watanabe S, Tatsuzawa Y, Iwa T, Suzuki M and Takashima T:
Aggressive surgical intervention in N2 non-small cell cancer of the
lung. Ann Thorac Surg. 51:253–261. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Naruke T, Goya T, Tsuchiya R and Suemasu
K: The importance of surgery to non-small cell carcinoma of lung
with mediastinal lymph node metastasis. Ann Thorac Surg.
46:603–610. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mountain CF: Expanded possibilities for
surgical treatment of lung cancer. Survival in stage IIIa disease.
Chest. 97:1045–1051. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chemotherapy in non-small cell lung
cancer, . A meta-analysis using updated data on individual patients
from 52 randomised clinical trials. Non-small cell lung cancer
collaborative group. BMJ. 311:899–909. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosell R, Gómez-Codina J, Camps C, Maestre
J, Padille J, Cantó A, Mate JL, Li S, Roig J, Olazábal A, et al: A
randomized trial comparing preoperative chemotherapy plus surgery
with surgery alone in patients with non-small-cell lung cancer. N
Engl J Med. 330:153–158. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosell R, Gómez-Codina J, Camps C, Sánchez
J Javier, Maestre J, Padilla J, Cantó A, Abad A and Roig J:
Preresectional chemotherapy in stage IIIA non-small-cell lung
cancer: A 7-year assessment of a randomized controlled trial. Lung
Cancer. 26:7–14. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roth JA, Atkinson EN, Fossella F, Komaki
R, Ryan M Bernadette, Putnam JB Jr, Lee JS, Dhingra H, De Caro L,
Chasen M and Hong WK: Long-term follow-up of patients enrolled in a
randomized trial comparing perioperative chemotherapy and surgery
with surgery alone in resectable stage IIIA non-small-cell lung
cancer. Lung Cancer. 21:1–6. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roth JA, Fossella F, Komaki R, Ryan MB,
Putnam JB Jr, Lee JS, Dhingra H, De Caro L, Chasen M, McGavran M,
et al: A randomized trial comparing perioperative chemotherapy and
surgery with surgery alone in resectable stage IIIA non-small-cell
lung cancer. J Natl Cancer Inst. 86:673–680. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pass HI, Pogrebniak HW, Steinberg SM,
Mulshine J and Minna J: Randomized trial of neoadjuvant therapy for
lung cancer: Interim analysis. Ann Thorac Surg. 53:992–998. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martin J, Ginsberg RJ, Venkatraman ES,
Bains MS, Downey RJ, Korst RJ, Kris MG and Rusch VW: Long-term
results of combined-modality therapy in resectable non-small-cell
lung cancer. J Clin Oncol. 20:1989–1995. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kirn DH, Lynch TJ, Mentzer SJ, Lee TH,
Strauss GM, Elias AD, Skarin AT and Sugarbaker DJ: Multimodality
therapy of patients with stage IIIA, N2 non-small-cell lung cancer.
Impact of preoperative chemotherapy on resectability and
downstaging. J Thorac Cardiovasc Surg. 106:696–702. 1993.PubMed/NCBI
|
|
28
|
Sugarbaker DJ, Herndon J, Kohman LJ,
Krasna MJ and Green MR: Results of cancer and leukemia group B
protocol 8935. A multiinstitutional phase II trimodality trial for
stage IIIA (N2) non-small-cell lung cancer. Cancer and leukemia
group B thoracic surgery group. J Thorac Cardiovasc Surg.
109:473–483; discussion 483–485. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Meerbeeck JP, Kramer GW, Van Schil PE,
Legrand C, Smit EF, Schramel F, Tjan-Heijnen VC, Biesma B, Debruyne
C, van Zandwijk N, et al: European Organisation for Research and
Treatment of Cancer-Lung Cancer Group: Randomized controlled trial
of resection versus radiotherapy after induction chemotherapy in
stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst.
99:442–450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garrido P, González-Larriba JL, Insa A,
Provencio M, Torres A, Isla D, Sanchez JM, Cardenal F, Domine M,
Barcelo JR, et al: Long-term survival associated with complete
resection after induction chemotherapy in stage IIIA (N2) and IIIB
(T4N0-1) non small-cell lung cancer patients: The Spanish Lung
Cancer Group Trial 9901. J Clin Oncol. 25:4736–4742. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martini N, Kris MG, Flehinger BJ, Gralla
RJ, Bains MS, Burt ME, Heelan R, McCormack PM, Pisters KM, Rigas
JR, et al: Preoperative chemotherapy for stage IIIa (N2) lung
cancer: The Sloan-Kettering experience with 136 patients. Ann
Thorac Surg. 55:1365–1373; discussion, 1373–1374. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kumar P, Herndon J, Elias AD, Sugarbaker
DJ and Green MR: Comparison of pre-operative thoracic radiation
therapy (TRT) to pre-operative chemotherapy (CT) in surgically
staged IIIA(N2) non-small cell lung cancer (NSCLC): Initial results
of cancer and leukemia group B (CALGB) phase III protocol 9134. Int
J Radiation Oncol Biol Phys. 39:1951997. View Article : Google Scholar
|
|
33
|
Andre F, Grunenwald D, Pignon JP, Dujon A,
Pujol JL, Brichon PY, Brouchet L, Quoix E, Westeel V and Le
Chevalier T: Survival of patients with resected N2 non-small-cell
lung cancer: Evidence for a subclassification and implications. J
Clin Oncol. 18:2981–2989. 2000.PubMed/NCBI
|
|
34
|
Choi YS, Shim YM, Kim J and Kim K:
Recurrence-free survival and prognostic factors in resected pN2
non-small cell lung cancer. Eur J Cardiothorac Surg. 22:695–700.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rami-Porta R, Mateu-Navarro M, Freixinet
J, de la Torre M, Torres-García AJ, Pun YW and Armengod AC:
Bronchogenic Carcinoma Cooperative Group of the Spanish Society of
Pneumology and Thoracic Surgery (GCCB-S): Type of resection and
prognosis in lung cancer. Experience of a multicentre study. Eur J
Cardiothorac Surg. 28:622–628. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Garrido P, González-Larriba JL, Insa A,
Provencio M, Torres A, Isla D, Sanchez JM, Cardenal F, Domine M,
Barcelo JR, et al: Long-term survival associated with complete
resection after induction chemotherapy in stage IIIA (N2) and IIIB
(T4N0-1) non small-cell lung cancer patients: The Spanish lung
cancer group trial 9901. J Clin Oncol. 25:4736–4742. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krzakowski M, Provencio M, Utracka-Hutka
B, Villa E, Codes M, Kuten A, Henke M, Lopez M, Bell D, Biti G, et
al: Oral vinorelbine and cisplatin as induction chemotherapy and
concomitant chemo-radiotherapy in stage III non-small cell lung
cancer: Final results of an international phase II trial. J Thorac
Oncol. 3:994–1002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nagai K, Tsuchiya R, Mori T, Tada H,
Ichinose Y, Koike T and Kato H: Lung Cancer Surgical Study Group of
the Japan Clinical Oncology Group: A randomized trial comparing
induction chemotherapy followed by surgery with surgery alone for
patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209).
J Thorac Cardiovasc Surg. 125:254–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu Y-L, Gu L-J, Weng Y-M, Feng W-N and
Cheng C: Neo-adjuvant chemotherapy with docetaxel plus carboplatin
for non-small cell lung cancer. Ann Oncol. 13:(Suppl 5).
1402002.PubMed/NCBI
|
|
40
|
Yang X, Wu Y and Gu L: A randomized trial
comparing neoadjuvant gemcitabine plus carboplatin or cisplatin
followed by surgery with surgery alone in Clinical Stage IIIA
non-small-cell lung cancer (NSCLC). Lung Cancer. 49:S2882005.
View Article : Google Scholar
|