Introduction
The importance of developing improved treatments for
non-small cell lung cancer (NSCLC) cannot be overestimated. Lung
cancer remains one of the major causes of mortality, with incidence
on the increase in numerous parts of the world (1); NSCLC is the most common type, accounting
for 80–85% of all lung cancer cases. Targeting the carcinoma with a
combination of several drug types and physical methods, including
radiotherapy and phototherapy, is proving to be an effective
strategy (2).
Metformin is a biguanidine and a hypoglycemic agent.
Since its introduction in Europe in 1957, metformin has been used
as a drug for lowering elevated blood glucose levels in diabetes
mellitus patients (3). In 2005, it
was suggested that metformin could reduce the incidence of cancer,
making it into the focus of tumor research (4) and potential applications for metformin
in oncology were investigated. It was reported that metformin is
able to perform its antitumor action by altering neoplastic
cellular energy metabolism (5). Since
2013, to the best of our knowledge there have been >173 ongoing
clinical trials on the use of metformin in cancer (6). Furthermore, in a recent study, Birsoy
et al (7) showed a link
between the glucose limitation activity of phenformin, another
biguanide, and its effects on the metabolic determinants of cancer
cell sensitivity. Due to the rapid growth of cancer cells and high
consumption of nutrients, particularly glucose, it was observed
that glucose concentration is lower in tumors than in normal
tissues (7). However, the majority of
cancer cells are able to develop and reproduce rapidly in spite of
the low-glucose conditions (7).
Biguanide drugs, e.g., phenformin and metformin, are inhibitors of
mitochondrial oxidative phosphorylation, and this is thought to
account for the observed antineoplastic activity of these diabetes
drugs (7).
Since 2004, the present authors have been focusing
on the antitumor effects of metformin in NSCLC. A multi-center
clinical trial confirmed that metformin could improve chemotherapy
survival outcomes for diabetes mellitus patients who have NSCLC
(8). The present review discusses how
metformin affects cellular energy metabolism in NSCLC, the
mechanism of its antitumor action and its synergy with other
therapies. The goal is to investigate the feasibility of adjunct
metformin for treating NSCLC and to stimulate further study.
Glucose metabolism in NSCLC and the effects
of metformin
Glucose metabolism in normal cells includes
glycolysis, aerobic oxidation and the pentose phosphate pathway.
The first step of glycolysis is the enzymatic conversion of glucose
to pyruvate, which is then reduced to lactate. In aerobic
oxidation, pyruvate enters the tricarboxylic acid cycle, and it is
eventually completely oxidized to water and CO2 with the
production of adenosine triphosphate (ATP) (Fig. 1) (9).
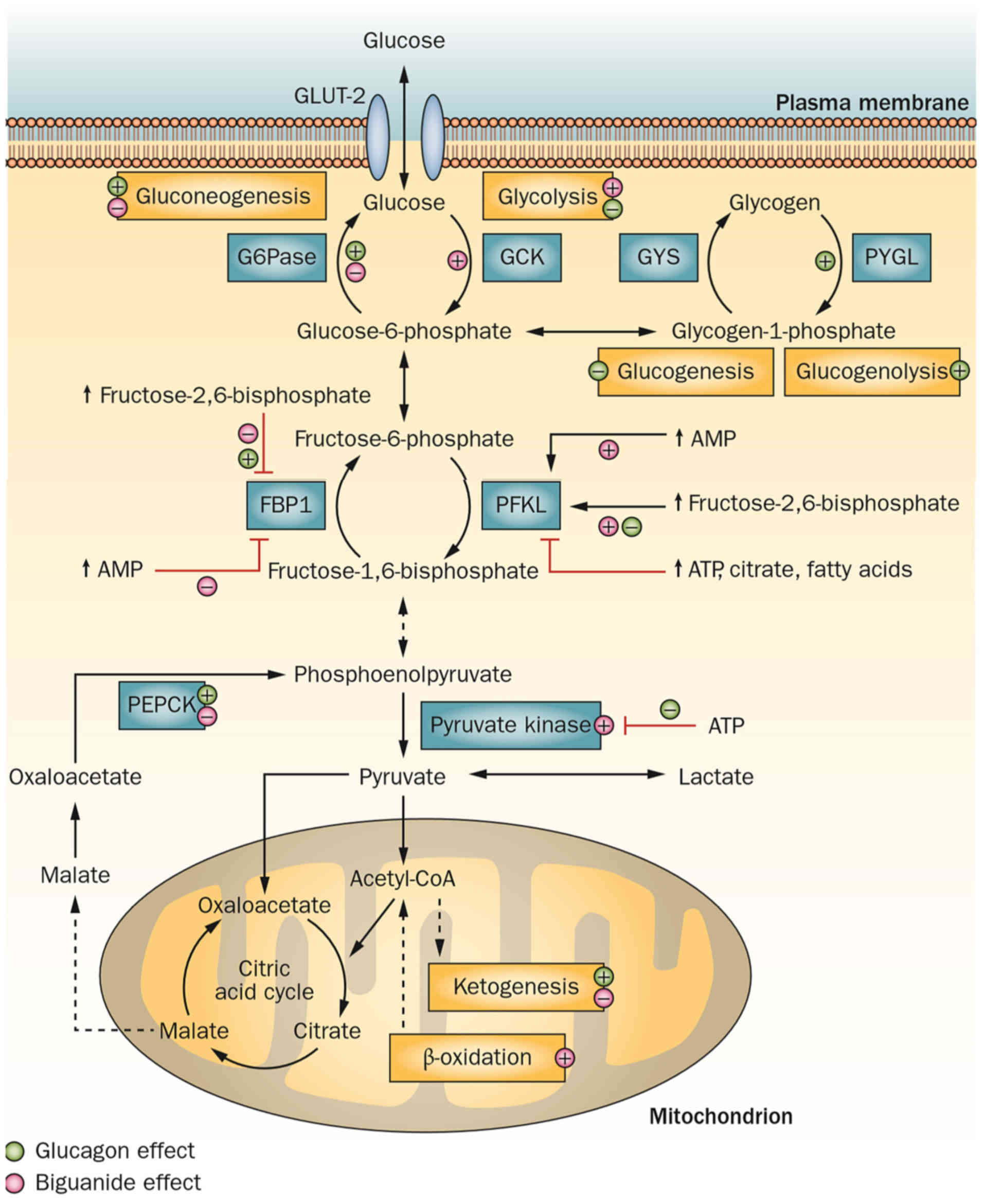 | Figure 1.Glucose metabolism (glycolysis and
aerobic oxidation) and the hypoglycemic mechanism of biguanides.
AMP, adenosine monophosphate; ATP, adenosine triphosphate; FBP1,
fructose-bisphosphatase 1; GLUT-2, solute carrier family 2 member
2; G6Pase, glucose-6-phosphatase; GCK, glucokinase; GYS, glycogen
synthase 1; PYGL, phosphorylase, glycogen, liver; PFKL,
phosphofructokinase, Liver Type; PEPCK, phosphoenolpyruvate
carboxykinase 2, mitochondrial. Reprinted by permission from
Macmillan Publishers Ltd.: Nature Reviews Endocrinology (9), copyright (2014). |
Compared with aerobic oxidation, glycolysis provides
less energy per mole of glucose, but it operates at a faster rate.
Even under aerobic conditions, cancer cells preferentially utilize
glycolysis as their main energy source rather than oxidative
phosphorylation and this is known as the ‘Warburg effect’ (10).
Under normoxic conditions, adenocarcinomas perform
glycolysis, whilst squamous cell carcinomas, which are subjected to
varying degrees of hypoxia, perform a high level of glycolysis even
in anaerobic environments to obtain sufficient energy for survival
(11). These findings prompted
further studies into how this finding can be utilized in developing
new treatments. In particular, the oral antidiabetic drug metformin
is able to stimulate glycolysis by altering the activity of
specific metabolic enzymes, including fructose-2, 6-bisphosphate
(9); therefore, it may promote the
switch to glycolysis in NSCLC cells as the main method of producing
energy. On the surface, this may have a stimulatory effect on the
growth of NSCLC cells, but in fact the switch in the energy
pathway, in the presence of metformin, is associated with its
primary antitumor mechanism (Fig. 1)
(9). As glycolysis provides less
energy per mole of glucose, a decrease in ATP generation results in
an increased level of adenosine monophosphate (AMP), which leads to
an increase in the ratio of intracellular AMP to ATP and an energy
metabolism imbalance. This is one of the mechanisms by which
metformin is able to achieve its antineoplastic activity. It is
likely to involve the active 5′-AMP-activated protein kinase (AMPK)
and its downstream signaling pathways (9).
Cellular transport of metformin in
NSCLC
The antitumor effect of metformin on energy
metabolism is dependent on whether metformin can be transported
into the mitochondria inside NSCLC cells. It has been observed in
tracking experiments that metformin is able to move across the cell
plasma membrane (9,12–15) and
the mitochondrial membranes (12,16,17).
Transport of metformin across the
NSCLC cell membrane
At physiological pH, metformin is positively charged
(12), meaning the movement of
metformin across NSCLC cell membranes may be mediated by organic
cation transporters (OCTs) (9).
Organic cation transporter 1 (OCT1) is primarily responsible for
metformin uptake in the liver (9).
Solute carrier family 22 member 18 (SLC22A18) and OCT1 share
certain homology (13). The Homo
sapiens gene SLC22A18 is located on chromosome 11 at 11p15.5
(13). It has been demonstrated that
microRNA-137 significantly inhibits NSCLC cell proliferation,
invasion and migration, as it targets SLC22A18 (14). Provided that SLC22A18 is highly
expressed in the NSCLC tissue, it may actively transport metformin
into the NSCLC cells, particularly in squamous cell carcinoma and
adenocarcinoma. An examination of NSCLC cells has observed that
SLC22A18 is primarily expressed in the cell membranes and cytoplasm
(13). Additionally, SLC22A18 is not
expressed in normal lung tissues, and it is upregulated in squamous
cell carcinoma and adenocarcinoma (15).
Transport of metformin across the
mitochondrial membrane of NSCLC cells
Mitochondria have both an outer and an inner
membrane. Compared with the inner membrane, the permeability of the
outer membrane is relatively high (16). Molecules with a molecular mass ≤5,000
kDa or less can freely travel through the outer membrane of
mitochondria, therefore the environment of the intermembrane space
and cytoplasm is similar (16). As
metformin is a simple molecule with a molecular mass of only 129
kDa, it is able to pass through the outer membrane and enter the
intermembrane space freely (16). At
physiological pH, metformin exists as a cation (>99.9%)
(12). The mitochondrial membrane
potential allows the metformin cation to accumulate in the matrix
of the mitochondria (17).
Antitumor mechanism of metformin and its
application in NSCLC
The mitochondrial electron transport chain is
composed of complexes I, II, III and IV, all of which have the
ability to transfer electrons (18).
The protons along the concentration gradient move back across the
inner membrane through the enzyme ATP synthase, with the aid of the
mitochondrial membrane potential (18). The flow of protons back into the
matrix of the mitochondrion via ATP synthase provides sufficient
energy for adenosine diphosphate to combine with inorganic
phosphate to form ATP (18).
Metformin is thought to target complex I, enabling
its antidiabetic and antitumor activity (9). Metformin limits respiration and citric
acid cycle activity in the mitochondria and alters cellular
bioenergetics (19). A reduction in
ATP generation results in increased levels of AMP, which in turn
leads to an increase in the ratio of intracellular AMP to ATP, as
well as an energy metabolism imbalance (9). This is likely to drive two major
signaling pathways: The inhibition of glucagon-induced cyclic AMP
synthesis in the liver, and the activation of 5′-AMPK and
downstream signaling pathways (9).
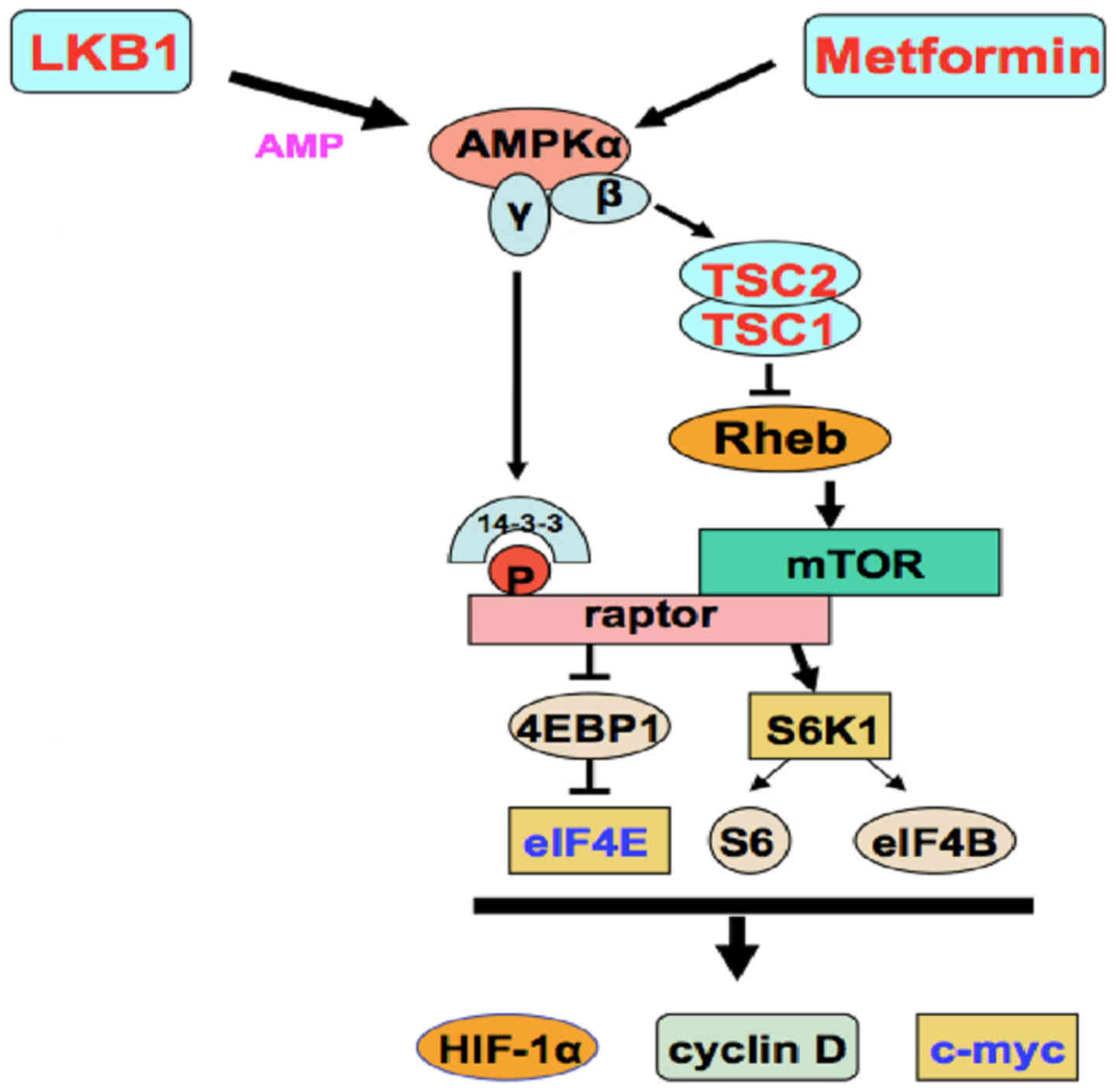
The antitumour activity of metformin may be direct or indirect
(systemic), and its direct effects involve the AMPK signaling
pathway (Fig. 2) (9).
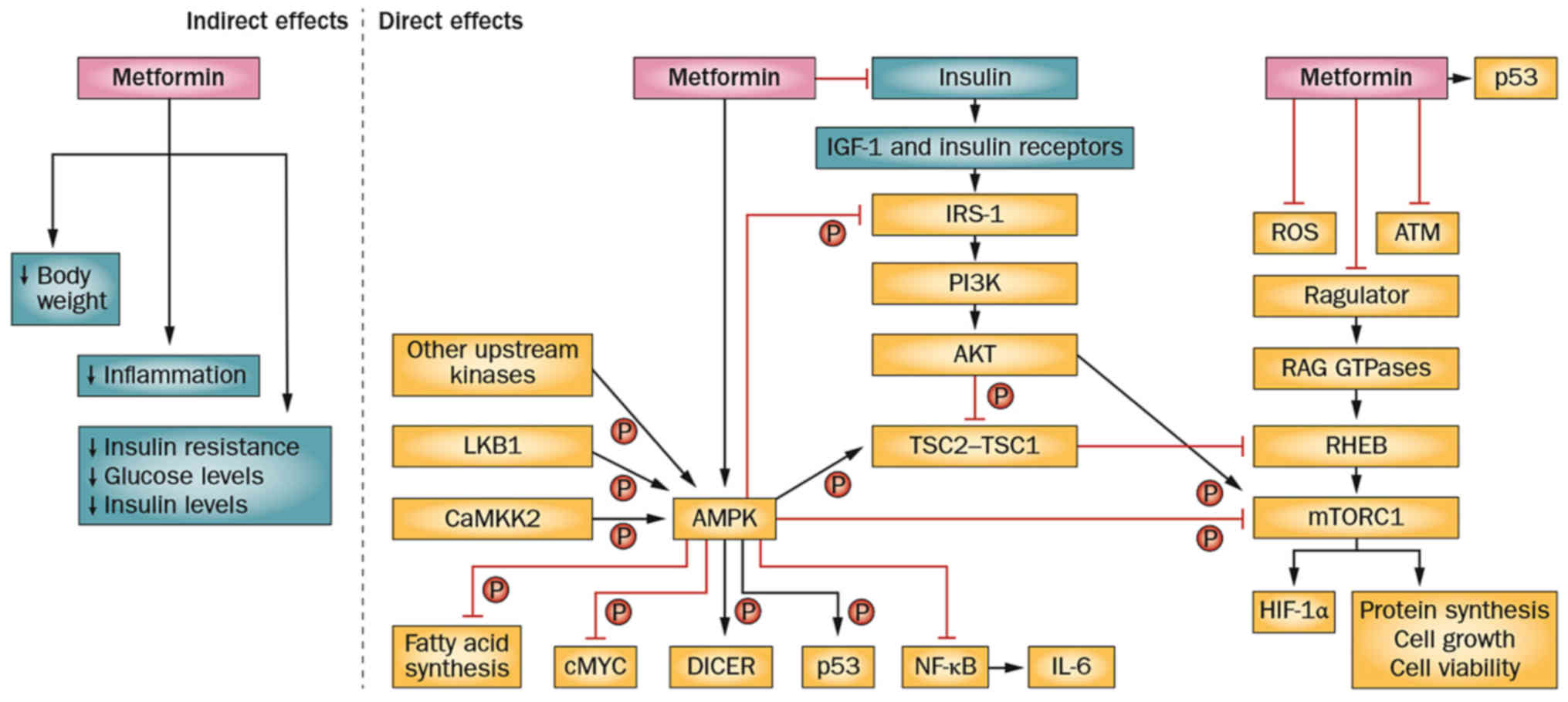 | Figure 2.Antitumor mechanisms of metformin.
AMPK, adenosine monophosphate-activated protein kinase; ATM,
ataxia-telangiectasia mutated; CaMKK2, calcium/calmodulin dependent
protein kinase kinase 2; DICER, dicer 1, ribonuclease III; HIF-1α,
hypoxia inducible factor 1 alpha subunit; IGF-1, insulin like
growth factor 1; IL-6, interleukin-6; IRS-1, insulin receptor
substrate 1; LKB1, serine/threonine kinase 11; mTORC1, mechanistic
target of rapamycin complex1; NF-κB, nuclear factor-κB; PI3K,
phosphoinositide 3-kinase; RHEB, ras homolog enriched in brain;
ROS, reactive oxygen species; TSC2-TSC1, tuberous sclerosis
1-tuberous sclerosis 2. Reprinted by permission from Macmillan
Publishers Ltd.: Nature Reviews Endocrinology (9), copyright (2014). |
The systemic influence is secondary and results in
several consequences, including a decrease in body weight,
anti-inflammatory actions, improvements in insulin-resistance, and
a reduction in systemic levels of glucose and insulin. The direct
effects include AMPK-dependent and AMPK-independent signaling
pathways (9).
Indirect antitumor effects of
metformin in NSCLC: Decreasing body weight
The primary mode of action of metformin in causing
body weight loss is via decreasing appetite (20). The secondary mechanisms include
improvements in gastrointestinal physiology and circadian rhythms,
and regulation of fat oxidation and storage of fat in liver,
skeletal muscle or adipose tissue (20). Dahlberg et al (21) evaluated the association between
body-mass index and clinical outcomes for 2,585 NSCLC patients. It
was reported that obese patients had improved outcomes earlier in
the study compared to normal or overweight patients, but
subsequently obesity was demonstrated to increase risk (21). Therefore, for obese NSCLC patients,
reducing weight can improve prognosis and metformin may aid in
accomplishing this.
Indirect antitumor effects of
metformin in NSCLC: Inflammation, tumor progression and
immunity
There is increasing evidence that certain
tumor-associated inflammatory markers (C-reactive protein,
Toll-like receptors 2 and 4, and tumor necrosis factor-α) are
associated with poorer prognosis of certain types of cancer,
including NSCLC (22–24). Antitumor immune responses involve CD8+
T lymphocytes and mature dendritic cells (mDCs) (22–28).
Alifano et al (25) reported
on the role of systemic inflammation, nutritional status and tumor
immune microenvironment in determining the outcome of resection in
NSCLC patients. It was suggested that the tumoral immune
microenvironment is associated with long-term outcome in primary
and metastatic tumors in resected NSCLC patients (25).
In NSCLC, a high intratumoral concentration of mDCs
and low intratumoral numbers of CD8+ T lymphocytes are associated
with an improved prognosis (25). The
intratumoral concentration of mDCs is inversely correlated with
age. It is lower in males, smokers, and patients with squamous cell
carcinoma or chronic obstructive pulmonary disease (25). Therefore, inhibiting inflammation may
improve the prognosis of patients with NSCLC. As well as glycemic
control, metformin reduces a number of inflammatory markers
(C-reactive protein, tumor necrosis factor-α, Toll-like receptors 2
and 4) and causes oxidative stress in obese type 2 diabetic
patients (24). Furthermore,
metformin is able to reduce the production of tumor necrosis
factor-α by inhibiting the extracellular signal-regulated
kinase-1/2-early-growth response-1 (ERK1/2-Egr-1) signaling pathway
in human monocytes (26).
Consequently, metformin is able to improve the prognosis of
NSCLC.
Indirect antitumor effects of
metformin in NSCLC: Improvements in insulin-resistance, and
reductions in insulin and glucose levels
An examination of Kirsten rat sarcoma viral oncogene
homolog (K-RAS)-induced effects revealed that hyperglycemia is able
to promote the expansion of tumor-initiating lung bronchoalveolar
stem cells (BASCs) in bronchoalveolar duct junctions (29). The active K-RAS oncogene is able to
increase the expression of glucose transporter 1, thereby promoting
glucose uptake and glycolysis in BASCs (29). Hyperglycemia is also able to increase
autonomous hyperplasia of BASCs, which leads to oxidative stress
and the production of reactive oxygen species (ROS), as well as a
reduction in mitochondrial function and inhibition of oxidative
phosphorylation (29). Therefore,
lung cancer patients may benefit from metformin treatment via the
reduction of blood glucose levels.
The ability of metformin to reduce circulating
glucose levels may be explained by multiple mechanisms including:
i) Reduction in glucose output by inhibiting gluconeogenesis in the
liver (9); ii) increase in
insulin-mediated glucose uptake in the skeletal muscle by elevating
circulating glucagon-like peptide-1 (GLP-1) (30); and iii) increase in expression of
GLP-1 receptors in the pancreas (31,32).
Metformin indirectly inhibits dipeptidyl peptidase-4 activity,
which reduces the breakdown of GLP-1, thereby increasing levels of
circulating GLP-1 (9). Metformin has
limited effects on glucose absorption in the digestive tract, and
marginally delays the absorption process (9). Metformin also improves insulin
resistance by reducing circulating insulin levels (9).
Direct antitumor effects of metformin
in NSCLC
Metformin is able to inhibit the mammalian target of
rapamycin complex 1 (mTORC1) through either AMP kinase-dependent or
independent signaling pathways to achieve antitumor effects
(Fig. 2) (9). Metformin may also suppress the
phosphorylation of cytokines, including translation initiation
factor 4E-binding protein 1 and S6 kinase-1, by inhibiting mTOR.
Metformin is able to decrease protein synthesis, tumor cell
proliferation and survival (33).
Solid tumors often exhibit a hypoxic microenvironment state
(34). Hypoxia-inducible factor-1
(HIF-1) is a nuclear transcription factor produced by cells under
hypoxic conditions. HIF-1α, a subunit of HIF-1, is responsible for
HIF-1 activity and is highly expressed in NSCLC cells. HIF-1α
serves an important role in NSCLC tumorigenesis, local invasion and
distant metastasis, by upregulating the expression of the nuclear
proliferation protein antigen Ki-67 and vascular endothelial growth
factor (VEGF) (34). By inhibiting
mTORC1, metformin inhibits HIF-1α and is able to reduce local
invasiveness and metastasis in NSCLC (33).
Metformin improves the prognosis of
NSCLC via the AMPK signaling pathway
Metformin may cause phosphorylation of the tumor
suppressor gene tuberous sclerosis 2 by phosphorylating AMPK, which
in turn results in the inhibition of the mTORC-1 activator, GTPase
and Ras homolog enriched in brain. The liver kinase B-1 (LKB1) gene
in Homo sapiens encodes a tumor suppressor that phosphorylates the
AMPK α subunit at Thr-172 to activate AMPK (33). Activated AMPK inhibits tumorigenesis
by inhibiting mTOR (Fig. 2) (9). The phosphorylation of AMPK may also
result in direct phosphorylation and inhibition of the positive
regulatory-associated protein of mTOR (Fig. 3) (33).
Ultimately, metformin may achieve its primary antitumor effect by
inhibiting mTORC1. The incidences of LKB1 mutations in
adenocarcinomas and squamous cell carcinomas are reported to be 13
and 5%, respectively (35). With
certain LKB1 gene mutations, the LKB1-AMPK axis remains mostly
functional and can be stimulated by metformin in NSCLC cells
(33). Metformin could serve an
effective role in the treatment of NSCLC via the LKB1-AMPK-mTOR
signaling pathway (33,35–37). NSCLC
patients with a high level of phosphorylated (p)-AMPK have higher
overall survival (OS) and recurrence free survival (RFS) rates,
particularly in adenocarcinoma. However, in squamous cell lung
cancer, the level of pAMPK does not affect OS and RFS. Taken
together, these data support the conclusion that NSCLC patients may
benefit from metformin adjunct therapy through its inhibition of
mTOR by activating the LKB1/AMPK signaling pathway (38).
Metformin improves NSCLC prognosis via
an AMPK-independent signaling pathway
Metformin was reported to target liver
tumor-initiating cells through the phosphoinositide 3-kinase
(PI3K)/AKT/mTOR survival pathway both in vivo and in
vitro (39). The PI3K/AKT/mTOR
signaling pathway was aberrantly activated in squamous cell lung
carcinoma (40), and the aberrant
activation of this signaling pathway is more common in squamous
cell lung carcinoma than in adenocarcinoma (41). In addition, in patients with
adenocarcinoma and epidermal growth factor receptor
(EGFR)-activating mutations, the aberrant activation of the
PI3K/AKT/mTOR signaling pathway is one of the mechanisms of
acquired resistance to EGFR-tyrosine kinase inhibitors (TKIs)
(41). A further study demonstrated
that activation of the PI3K/AKT/mTOR signaling pathway in NSCLC
leads to a more aggressive form of the disease and a poorer
prognosis (42). Inhibition of the
PI3K/AKT/mTOR signaling pathway may overcome radioresistance,
chemoresistance and immune evasion in NSCLC (42). Metformin can block the insulin-like
growth factor-1-insulin signaling pathway via phosphorylation of
insulin receptor substrate 1 (IRS-1), which inhibits the
IRS-1/PI3K/AKT signaling pathway to prevent mTOR activation
(Fig. 2) (9). Therefore, metformin may provide benefit
to NSCLC patients through its inhibition of the PI3K/AKT/mTOR
signaling pathway (9).
Use of metformin in the treatment of
NSCLC
Metformin and targeted therapy
The development of targeted therapies for lung
cancer has been centered on pharmaceutical interventions to block
the EGFR-TKI axis. A number of EGFR-TKI inhibitors are currently in
use, including gefitinib (Iressa) and erlotinib (Tarceva), which
are able to block EGFR-mediated proliferation and anti-apoptosis
signaling pathways. However, following ~10 months of treatment,
these agents may lose their effectiveness due to drug resistance
(43). The primary resistance of TKI
is associated with K-RAS mutations. The causes of secondary
resistance of TKI include: Second-site mutation of the EGFR kinase
domain (T790M), other kinase amplifications (such as MET), NSCLC
conversion into small cell lung cancer and epithelial-mesenchymal
transition (EMT).
As metformin is able to inhibit the PI3K/AKT/mTOR
signaling pathway, using metformin in combination with an EGFR-TKI
blocker could produce a synergistic, antiproliferative effect on
the tumor. As mentioned previously, the aberrant activation of the
PI3K/AKT/mTOR signaling pathway is one of the mechanisms by which
patients with adenocarcinoma and EGFR-activating mutations are able
to gain resistance to EGFR-TKI blockers. Metformin adjunct therapy
could reverse EGFR-TKI resistance by inhibiting the PI3K/AKT/mTOR
signaling pathway (41). Li et
al (43) reported that when
metformin was combined with an EGFR-TKI blocker (gefitinib or
erlotinib) in vivo and in vitro, the
interleukin-6/signal transducer and activator of transcription 3
(IL-6/STAT3) signaling pathway was inhibited, which reversed EMT
and eventually overcame resistance in NSCLC cells. In 2013, one
clinical trial involving combined metformin and gefitinib to treat
NSCLC patients resulted in one-year progression-free survival
(6).
Sorafenib is a novel, multi-target anticancer drug
that inhibits a number of kinases, including AMPK (44). Sorafenib has dual antitumor effects.
When it is used in combination with metformin to treat NSCLC, the
antitumor AMPK signaling pathway is activated through either
calcium/calmodulin-dependent-kinase-kinase-2 or LKB1. Sorafenib can
also cause cytosolic calcium mobilization and mitochondrial calcium
overload. Therefore, it can promote mitochondrial ROS production
and lead to tumor cell death (44).
Notably, the average tumor volume and growth rate were lower in
patients treated with the combination therapy vs. patients treated
with sorafenib alone (44).
Metformin and radiotherapy
Radiotherapy is a method of destroying tumors via
the biological effects of ionizing radiation (45). The ataxia-telangiectasia mutated (ATM)
gene product is part of a signal transduction cascade that is
important for repairing damaged DNA and, as a consequence when
mutated may lead to sensitivity to ionizing radiation. Therefore,
the degree of radiosensitivity or radioresistance of NSCLC cells is
dependent on ATM. Lung cancer radiotherapy can achieve antitumor
effects by causing G2-M arrest and cytotoxicity, by activating the
ATM-AMPK-tumor protein p53 (p53)/cyclin dependent kinase inhibitor
1A (p21cip1) signaling pathway, reducing the
phosphorylation of AKT and inhibiting AKT-mTOR-eukaryotic
translation initiation factor 4e binding protein 1 (4EBP1)
(46). Resistance to radiation is
associated with the AMPK and AKT-mTOR signaling pathways. Metformin
is able to activate AMPK and inhibit AKT-mTOR to sensitize lung
tumors to ionizing radiation (9).
Storozhuk et al (47) tested the hypothesis that metformin can
inhibit growth and enhance radiosensitivity of NSCLC through ATM
and AMPK. It was reported that combined treatment with metformin
and radiotherapy consistently activated the
ATM-AMPK-p53/p21cip1 signaling pathway and inhibited the
AKT-mTOR-4EBP1 signaling pathway (47). A constant concentration of 7.8 µM
metformin was achieved in patients who took a daily dosage of
850–1,700 mg. As a daily dosage of 2.5–3.0 g of metformin has no
notable toxicity, a recommended dosage of 850–1,700 mg metformin is
well within the safe range (47).
Since 2013, to the best of our knowledge there have been three
studies involving the use of metformin in combination with
radiotherapy to treat NSCLC (48–50). These
trials led to progression-free survival according to Response
Evaluation Criteria in Solid Tumors (51).
Metformin and chemotherapy
Cisplatin is the most commonly used chemotherapy
drug for the treatment of NSCLC, and it has been used as the
first-line treatment for NSCLC in patients without EGFR mutations
(52). Chemoresistance to cisplatin
is associated with ROS production, IL-6 secretion and STAT3
phosphorylation. In NSCLC, STAT3 is active and may facilitate tumor
proliferation, survival and angiogenesis by overexpressing
anti-apoptotic proteins (Bcl-2-like protein 1 and myeloid cell
leukemia 1), cell cycle regulators (cyclin D1 and c-Myc), and VEGF
(52). Cisplatin is able to promote
the generation of ROS and the phosphorylation of STAT3 (52).
Metformin is able to inhibit cisplatin-induced ROS
generation, STAT3 phosphorylation and autocrine IL-6 secretion,
thereby enhancing the chemosensitivity of NSCLC to cisplatin
(52). Furthermore, metformin is able
to improve the effect of cisplatin in A549/CDDP cells (53). Metformin also has a synergistic effect
with cisplatin or etoposide in large cell lung carcinoma cells
(NCI-H460) by increasing the antitumor effectiveness of the
chemotherapeutics (54). There are
several clinical trials underway to examine the effectiveness of
the combination of cisplatin or carboplatin with metformin. Using a
combination of cisplatin or carboplatin with metformin may be a
promising treatment for NSCLC patients (49,50,55,56).
Safety of metformin as an antitumor
adjuvant
The safety of metformin has been previously
investigated for the treatment of type 2 diabetes mellitus. A
number of major adverse effects have been reported concerning
gastrointestinal reactions, including nausea, abdominal discomfort
and diarrhea. These symptoms are usually mild and can be treated
(57). At present, large-scale
clinical trials and meta-analyses have yet to show evidence that
metformin increases the risk of lactic acidosis (57). Metformin has no reported renal
toxicity, but it is excreted by the kidneys (57). This means metformin may accumulate in
patients with renal impairments, thus increasing the risk of lactic
acidosis. However, metformin-induced liver toxicity is rare
(57). Considering that metformin has
been in use for >50 years and its side-effects (including low
incidence of hypoglycemia and transient gastrointestinal reactions)
have been thoroughly investigated and that the benefits outweigh
the side effects, the conclusion is that it is safe for use in
clinical trials for the treatment of cancer (57).
Future prospects
NSCLC is the most commonly occurring solid tumor
worldwide (1). Although progress in
improving chemotherapy, radiotherapy and targeted therapies has
increased patient survival, the five-year overall survival time
remains low (1). Cisplatin one of the
most commonly used first-line chemotherapeutic agents for NSCLC
(52), has several toxic side
effects. The aim is to find novel adjuvant drugs, in order to
enhance the antitumor effect of cisplatin, but without increasing
the dose and toxicity. There is compelling evidence from a number
of preclinical studies to suggest that the antidiabetic drug
metformin is a prime candidate as an adjunct to chemotherapy and
radiotherapy in the treatment of NSCLC (45–47,52–54,58).
It has been demonstrated that metformin is able to improve survival
time among diabetes mellitus patients with stage IV NSCLC (59). Furthermore, numerous NSCLC patients in
advanced stages are too weak to undergo chemotherapy, radiotherapy
or surgery, thus reducing their chance of survival. Combining
metformin with such treatments, may be a promising strategy to
improve patient survival.
Squamous cell lung carcinoma is insensitive to
chemotherapy, radiotherapy and targeted therapy (40). For the majority of patients, it is too
late to undergo surgical resection by the time they are diagnosed.
The US Food and Drug Administration has approved nivolumab and
ramucirumab for the treatment of NSCLC, but improvements in overall
survival and recurrence rates have yet to be reported. As described
previously, the PI3K/AKT/mTOR signaling pathway is aberrantly
activated in squamous cell lung carcinoma (40), which has been correlated with poor
prognosis for these patients (42).
As metformin is able to inhibit the PI3K/AKT/mTOR signaling pathway
(42), it may improve survival
outcomes when used in combination with everolimus or gemcitabine
and cisplatin chemotherapy.
At present, to the best of our knowledge there are
nine clinical trials involving the use of metformin in treating
NSCLC. The potential for the long-term use of metformin in
improving survival and recurrence is yet to be evaluated.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodin D, Grover S, Xu MJ, Hanna TP, Olson
R, Schreiner LJ, Munshi A, Mornex F, Palma D, Gaspar LE, et al:
Radiotherapeutic management of non-small cell lung cancer in the
minimal resource setting. J Thorac Oncol. 11:21–29. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mu YM, Ji LN, Ning G, Li GW, Li Y, Sun ZL,
Li YB, Zhao JJ, Wang WQ, Zhu DL, et al: Chinese experts consensus
statement on metformin in the clinical practice. Chin J Diabetes.
24:871–884. 2016.(In Chinese).
|
|
4
|
Evans JM, Donnelly LA, Emslie-Smith AM,
Alessi DR and Morris AD: Metformin and reduced risk of cancer in
diabetic patients. BMJ. 330:1304–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pollak M: Potential applications for
biguanides in oncology. J Clin Invest. 123:3693–3700. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
U.S. National Library of Medicine.
ClinicalTrials.gov: Metformin and cancer. http://www.clinicaltrials.gov/ct2/results?term=metformin+and+cancer&Search=SearchAccessed.
January 7–2014.
|
|
7
|
Birsoy K, Possemato R, Lorbeer FK,
Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB and
Sabatini DM: Metabolic determinants of cancer cell sensitivity to
glucose limitation and biguanides. Nature. 508:108–112. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan BX, Yao WX, Ge J, Peng XC, Du XB,
Zhang R, Yao B, Xie K, Li LH, Dong H, et al: Prognostic influence
of metformin as first-line chemotherapy for advanced nonsmall cell
lung cancer in patients with type 2 diabetes. Cancer.
117:5103–5111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pernicova I and Korbonits M:
Metformin-mode of action and clinical implications for diabetes and
cancer. Nat Rev Endocrinol. 10:143–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Warburg O: Metabolism of tumours. Biochem
Z. 142:317–333. 1923.
|
|
11
|
Schuurbiers OC, Meijer TW, Kaanders JH,
Looijen-Salamon MG, de Geus-Oei LF, van der Drift MA, van der
Heijden EH, Oyen WJ, Visser EP, Span PN and Bussink J: Glucose
Metabolism in NSCLC is histology-specific and diverges the
prognostic potential of 18FDG-PET for adenocarcinoma and squamous
cell carcinoma. J Thorac Oncol. 9:1485–1493. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Graham GG, Punt J, Arora M, Day RO, Doogue
MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick
CM, et al: Clinical pharmacokinetics of metformin. Clin
Pharmacokinet. 50:81–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lei M, Cheng Q, Zhao Y, Liu T, Wang X,
Deng Y, Yang J and Zhang Z: Expression and its clinical
significance of SLC22A18 in non-small cell lung cancer. Zhongguo
Fei Ai Za Zhi. 15:17–20. 2012.(In Chinese). PubMed/NCBI
|
|
14
|
Zhang B, Liu T, Wu T, Wang Z, Rao Z and
Gao J: microRNA-137 functions as a tumor suppressor in human
non-small cell lung cancer by targeting SLC22A18. Int J Biol
Macromol. 74:111–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lei M, Cheng QS and Zhang ZP, Li XF, Huang
Q and Zhang ZP: Expression of SLC22A18 in NSCLC and its correlation
with chemoresistance of NSCLC. Chin J Cancer Pre Treat. 21:368–371.
2014.(In Chinese).
|
|
16
|
Alberts B, Johnson A, Lewis J, Raff M,
Roberts K and Walter P: Molecular Biology of the Cell. 4th. Garland
Science; New York, NY: 2002
|
|
17
|
Wheaton WW, Weinberg SE, Hamanaka RB,
Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM,
Budigner GS and Chandel NS: Metformin inhibits mitochondrial
complex I of cancer cells to reduce tumorigenesis. Elife.
3:e022422014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mitchell P: Coupling of phosphorylation to
electron and hydrogen transfer by a chemi-osmotic type of
mechanism. Nature. 191:144–148. 1961. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Andrzejewski S, Gravel SP, Pollak M and
St-Pierre J: Metformin directly acts on mitochondria to alter
cellular bioenergetics. Cancer Metab. 2:122014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malin SK and Kashyap SR: Effects of
metformin on weight loss: Potential mechanisms. Curr Opin
Endocrinol Diabetes Obes. 21:323–329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dahlberg SE, Schiller JH, Bonomi PB,
Sandler AB, Brahmer JR, Ramalingam SS and Johnson DH: Body mass
index and its association with clinical outcomes for advanced
non-small-cell lung cancer patients enrolled on Eastern Cooperative
Oncology Group clinical trails. J Thorac Oncol. 8:1121–1127. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alifano M, Falcoz PE, Seegers V, Roche N,
Schussier O, Younes M, Antonacci F, Forgez P, Dechartres A, Massard
G, et al: Preresection serum C-reactive protein measurement and
survival among patients with resectable non-small cell lung cancer.
J Thorac Cardiovasc Surg. 142:1161–1167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li D, Jin Y, Sun Y, Lei J and Liu C:
Knockdown of toll-like receptor 4 inhibits human NSCLC cancer cell
growth and inflammation cytokine secretion in vitro and in vivo.
Int J Oncol. 45:813–821. 2014.PubMed/NCBI
|
|
24
|
Andrews M, Soto N and Arredondo M: Effect
of metformin on the expression of tumor necrosis factor-α, Toll
like receptors 2/4 and C reactive protein in obese type-2 diabetic
patients. Rev Med Chil. 140:1377–1382. 2012.(In Spanish).
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alifano M, Mansuet-Lupo A, Lococo F, Roche
N, Bobbio A, Canny E, Schussler O, Dermine H, Régnard JF, Burroni
B, et al: Systemic inflammation, nutritional status and tumor
immune microenvironment determine outcome of resected non-small
cell lung cancer. PLoS One. 9:e1069142014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arai M, Uchiba M, Komura H, Mizuochi Y,
Harada N and Okajima K: Metformin, an antidiabetic agent,
suppresses the production of tumor necrosis factor and tissue
factor by inhibiting early growth response factor-1 expression in
human monocytes in vitro. J Pharmacol Exp Ther. 334:206–213. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pearce EL, Walsh MC, Cejas PJ, Harms GM,
Shen H, Wang LS, Jones RG and Choi Y: Enhancing CD8 T-cell memory
by modulating fatty acid metabolism. Nature. 460:103–107. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Araki K and Ahmed R: AMPK: A metabolic
switch for CD8+ T-cell memory. Eur J Immunol. 43:878–881. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Micucci C, Orciari S and Catalano A:
Hyperglycemia promotes K-Ras-induced lung tumorigenesis through
BASCs amplification. PLoS One. 9:e1055502014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sjoberg KA, Rattigan S, Jeppesen JF,
Lundsgaard AM, Holst JJ and Kiens B: Differential effects of
glucagon-like peptide-1 on microvascular recruitment and glucose
metabolism in short-and long-term insulin resistance. J Physiol.
593:2185–2198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maida A, Lamont BJ, Cao X and Drucker DJ:
Metformin regulates the incretin receptor axis via a pathway
dependent on peroxisome proliferator-activated receptor-α in mice.
Diabetologia. 54:339–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lenhard JM, Croom DK and Minnick DT:
Reduced serum dipeptidyl peptidase-IV after metformin and
pioglitazone treatments. Biochem Biophys Res Commun. 324:92–97.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han D, Li SJ, Zhu YT, Liu L and Li MX:
LKB1/AMPK/mTOR signaling pathway in non-small-cell lung cancer.
Asian Pac J cancer Prev. 14:4033–4039. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu HY, Zhong H, Lu SW, Li J and Zhao H:
Correlation of expression of HIF-1α to proliferation and
neovascularization of tumor and prognosis of patients with
non-small cell lung cancer. Journal of Clinical Pulmonary Medicine.
19:73–76. 2014.(In Chinese).
|
|
35
|
Koivunen JP, Kim J, Lee J, Rogers AM, Park
JO, Zhao X, Naoki K, Okamoto I, Nakagawa K, Yeap BY, et al:
Mutation in LKB1 tumor suppressor are frequently detected in
tumours from Caucasian but not Asia lung cancer patients. Br J
Cancer. 99:245–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong LX, Sun LL, Zhang X, Pan L, Lian LJ,
Chen Z and Zhong DS: Negative regulation of mTOR activity by
LKB1-AMPK signaling in non-small cell lung cancer cells. Acta
Pharmacol Sin. 34:314–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marcus AI and Zhou W: LKB1 regulated
pathways in lung cancer invasion and metastasis. J Thorac Oncol.
5:1883–1886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
William WN, Kim JS, Liu DD, Solis L,
Behrens C, Lee JJ, Lippman SM, Kim ES, Hong WK, Wistuba II, et al:
The impact of phosphorylated AMP-activated protein kinase
expression on lung cancer survival. Ann Oncol. 23:78–85. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin F, Yan W, Song G, Ting W, Hu T and Wu
G: Metformin targets liver tumor-initiating cells through the
PI3K/Akt/mTOR survival pathway. Chin Sci Bull. 59:3585–3594. 2014.
View Article : Google Scholar
|
|
40
|
Beck JT, Ismail A and Tolomeo C: Targeting
the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of
rapamycin (mTOR) pathway: An emerging treatment strategy for
squamous cell lung carcinoma. Cancer Treat Rev. 40:980–989. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fumarola C, Bonelli MA, Petronini PG and
Alfieri RR: Targeting PI3K/AKT/mTOR pathway in non small cell
cancer. Biochem Pharmacol. 90:197–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heavey S, O'Byrne KJ and Gately K:
Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC.
Cancer Treat Rev. 40:445–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li L, Han R, Xiao H, Lin C, Wang Y, Liu H,
Li K, Chen H, Sun F, Yang Z, et al: Metformin sensitizes
EGFR-TKI-resistant human lung cancer cells in vitro and in vivo
through inhibition of IL-6 signaling and EMT reversal. Clin Cancer
Res. 20:2714–2726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Groenendijk FH, Mellema WW, van der Burg
E, Schut E, Hauptmann M, Horlings HM, Willems SM, van den Heuvel
MM, Jonkers J, Smit EF and Bernards R: Sorafenib synergizes with
metformin in NSCLC through AMPK pathway activation. Int J Cancer.
136:1434–1444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim BM and Hong Y, Lee S, Liu P, Lim JH,
Lee YH, Lee TH, Chang KT and Hong Y: Therapeutic implications for
overcoming radiation resistance in cancer therapy. Int J Mol Sci.
16:26880–26913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Storozhuk Y, Sanli T, Hopmans SN, Schultz
C, Farrell T, Cutz JC, Steinberg GR, Wright J, Singh G and
Tsakiridis T: Chronic modulation of AMP-Kinase, Akt and mTOR
pathways by ionizing radiation in human lung cancer xenografts.
Radiat Oncol. 7:712012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Storozhuk Y, Hopmans SN, Sanli T, Barron
C, Tsiani E, Cutz JC, Pond G, Wright J, Singh G and Tsakiridis T:
Metformin inhibits growth and enhances radiation response of
non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J
Cancer. 108:2021–2032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Heath Skinner: Metformin in Non small Cell
Lung Cancer (NSCLC). ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT02285855Accessed.
November 5–2014.
|
|
49
|
Theodoros Tsakiridis: Chemotherapy and
Radiation Therapy With or Without Metformin Hydrochloride in
Treating Patients With Stage III Non-small Cell Lung Cancer.
ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT02186847Accessed.
July 8–2014.
|
|
50
|
Theodoros Tsakiridis: Advanced Lung Cancer
Treatment With Metformin and Chemo-Radiotherapy (ALMERA).
ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT02115464Accessed.
April 14–2014.
|
|
51
|
Wolchok JD, Hoos A, O'Day S, Weber JS,
Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, et al:
Guidelines for the evaluation of immune therapy activity in solid
tumors: Immune-related response criteria. Clin Cancer Res.
15:7412–7420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lin CC, Yeh HH, Huang WL, Yan JJ, Lai WW,
Su WP, Chen HH and Su WC: Metformin enhances cisplatin cytotoxicity
by suppressing signal transducer and activator of transcription-3
activity independently of the liver kinase B1-AMP-activated protein
kinase pathway. Am J Respir cell Mol Biol. 49:241–250. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang Y, Lin B, Wu J, Zhang H and Wu B:
Metformin inhibits the proliferation of A549/CDDP cells by
activating P38 mitogen-activated protein kinase. Oncol Lett.
8:1269–1274. 2014.PubMed/NCBI
|
|
54
|
Teixeira SF, Idos Guimarães S, Madeira KP,
Daltoé RD, Silva IV and Rangel LB: Metformin synergistically
enhances antiproliferative effects of cisplatin and etoposide in
NCI-H460 human lung cancer cells. J Bras Pneumol. 39:644–649. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Benjamin Levy: Metformin and Carbohydrate
Restriction With Platinum Based Chemotherapy In Stage IIIB/IV
Non-Squamous Non-small Cell Lung Cancer (NS-NSCLC) (METRO).
ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT02019979Accessed.
December 13–2013.
|
|
56
|
Yumin Yeh: Metformin in Stage IV Lung
Adenocarcinoma. ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT01997775Accessed.
November 17–2013.
|
|
57
|
Li M and Ji LN: The safety of Metformin in
Type 2 diabetes. Chin J Diabetes. 22:289–292. 2014.(In
Chinese).
|
|
58
|
Morgillo F, Sasso FC, Corte CM Della,
Festino L, Manzo A, Martinelli E, Troiani T, Capuano A and
Ciardiello F: Metformin in lung cancer: Rationale for a combination
therapy. Expert Opin Investig Drugs. 22:1401–1409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lin JJ, Gallagher EJ, Sigel K, Mhango G,
Galsky MD, Smith CB, LeRoith D and Wisnivesky JP: Survival of
patients with stage IV lung cancer with diabetes treated with
metformin. Am J Respir Crit Care Med. 191:448–454. 2015. View Article : Google Scholar : PubMed/NCBI
|