Introduction
As the most common mesenchymal sarcoma in bone,
osteosarcoma (OS) mainly arises from the metaphysis of the long
bones (1). Although great efforts
have been made in OS diagnosis and therapy, chemoresistance remains
a major problem that causes low 5-year survival rates (1). Furthermore, the understanding of the
mechanism underlying chemoresistance is limited. It has been
demonstrated that various oncogenes and tumor suppressors are
involved in chemoresistance (2–4). Thus,
investigation into the molecular targets involved in OS cell
chemoresistance is urgently required.
Autophagy, an evolutionarily conserved function, is
a cellular self-catabolic degradation process, responsible for the
lysosomal degradation of long-lived proteins as well as aged or
damaged organelles (5). The amino
acids and fatty acids generated during autophagy may be reused and
thus autophagy may be beneficial for sustainable cell survival
(6). Accumulating evidence has been
reported that autophagy is rapidly activated when cancer cells are
treated with certain chemotherapy drugs (7,8), and
functions as a protective mechanism to promote cancer cell survival
(5). Therefore, autophagy frequently
contributes to chemoresistance, and inhibition of autophagy has
been observed to promote chemotherapy efficacy (9).
Stathmin 1 (STMN1), a cytosolic phosphoprotein, has
been reported to be abundantly expressed in various cell types
(10,11). Furthermore, STMN1 is able to regulate
microtubule dynamics by promoting depolymerization of microtubules
and/or preventing polymerization of tubulin heterodimers (12). Therefore, anti-STMN1 therapy has been
reported to improve sensitivity to antimicrotubule drugs in
esophageal squamous cell carcinoma (12). Zhang et al (13) reported that the expression of STMN1
was significantly increased in two human OS cell lines (SOSP-9607
and SOSP-9901) and 45 OS tissue specimens, compared with normal
tissues. It was also demonstrated that knockdown of STMN1 inhibited
OS cell proliferation and cell cycle progression, while inducing
apoptosis (13), suggesting that
STMN1 may act as an oncogene in OS. Phadke et al (14) evaluated the in vivo safety and
antitumor efficacy of bifunctional small hairpin RNAs specific for
STMN1. The results of this previous study confirmed the systemic
safety of the therapeutic dose, and thus supported the early-phase
assessments of clinical safety and preliminary efficacy (14). However, to the best of our knowledge,
there have been no reports of the role of STMN1 in the regulation
of chemosensitivity in OS.
The present study aimed to investigate the effect of
STMN1 on paclitaxel-induced chemoresistance, as well as the
underlying mechanism of action.
Materials and methods
Cell culture
OS cell lines HOS, Saos-2, U-2OS and MG-63, and
normal osteoblast cell line hFOB1.19, were obtained from American
Type Culture Collection (Manassas, VA, USA). All cell lines were
cultured in Dulbecco's modified Eagle's medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum
(Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator
containing 5% CO2.
Cell transfection or treatment
The recombinant lentivirus anti-STMN1 (GeneChem Co.,
Ltd., Shanghai, China), as well as the control anti-NC (negative
control; GeneChem Co., Ltd.) were transfected into U-2OS cells by
using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.). Stable
transfected cells were constructed using G418 (Thermo Fisher
Scientific, Inc.) selection. Cells in each group were treated with
3 µM paclitaxel (Sigma-Aldrich; KGaA, Darmstadt, Germany) for 3 h
at 37°C. The anti-STMN1 and anti-NC U-2OS cells were treated with 5
µM LY29054 (Selleck Chemicals, Houston, TX, USA).
Reverse transcription-quantitative
polymerase chain reaction (qPCR)
Total RNA was prepared using TRIzol reagent (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
For the analysis of mRNA expression, RevertAid™ H Minus First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) was used
to reverse transcribe RNA into cDNA, and qPCR was subsequently
performed using the Power SYBR Green PCR Master mix (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) on an ABI 7500 thermocycler
(Thermo Fisher Scientific, Inc.). The cycling conditions were as
follows: 95°C for 5 min, followed b7 40 cycles of 95°C for 10 sec
and 60°C for 30 sec. The primer sequences for STMN1 were as
follows: Sense, 5′-TCAGCCCTCGGTCAAAAGAAT-3′ and antisense,
5′-TTCTCGTGCTCTCGTTTCTCA-3′. The primer sequences for GAPDH were as
follows: Sense, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and antisense,
5′-GGCTGTTGTCATACTTCTCATGG-3′. GAPDH was used as an endogenous
control. The relative expression was analyzed by the
2−ΔΔCq method (15).
Western blot analysis
Protein was extracted from cells using
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Subsequently,
protein was quantified using the Pierce Protein Assay kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol
Proteins (50 µg) were separated by 12% SDS-PAGE, transferred to
polyvinylidene difluoride membranes and probed with primary
antibodies: Rabbit anti-STMN1 antibody (1:100; ab52630; Abcam,
Cambridge, MA, USA), rabbit anti-LC3B antibody (1:50; ab48394;
Abcam), rabbit anti-Beclin1 antibody (1:100; ab62557; Abcam),
rabbit anti-mammalian target of rapamycin (mTOR) antibody (1:100;
ab2732; Abcam), rabbit anti-phosphorylated (p)-mTOR (1:100;
ab109268; Abcam) or rabbit anti-GAPDH antibody (1:50; ab9485;
Abcam) at 4°C overnight. Membranes were subsequently incubated with
mouse anti-rabbit secondary antibody (1:10,000; ab99697; Abcam) at
room temperature for 40 min. The protein bands were visualized by
the Amersham enhanced chemiluminescence system (RPN998; GE
Healthcare Life Sciences, Chalfont, UK). Data was analyzed by
densitometry using Image-Pro plus software version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA) and were normalized to GAPDH
expression.
Cell survival assay
U-2OS cells in each group were seeded into 10 mm
dishes, and incubated for 14 days. Subsequently, cells were fixed
in methanol for 15 min, stained with Giemsa (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for 10 min and dried in air. The number
of colonies was counted under a microscope (CX23; Olympus
Corporation, Tokyo, Japan).
Statistical analysis
Data in the figures are expressed as the mean ±
standard deviation. Analysis of data was performed using SPSS
version 17 (SPSS, Inc., Chicago, IL, USA). Student's t-test or
one-way analysis of variance were used depending on the
experimental conditions. P<0.05 was considered to indicate a
statistically significant difference.
Results
Treatment with paclitaxel leads to
activation of autophagy, as well as upregulation of STMN1 in OS
cells
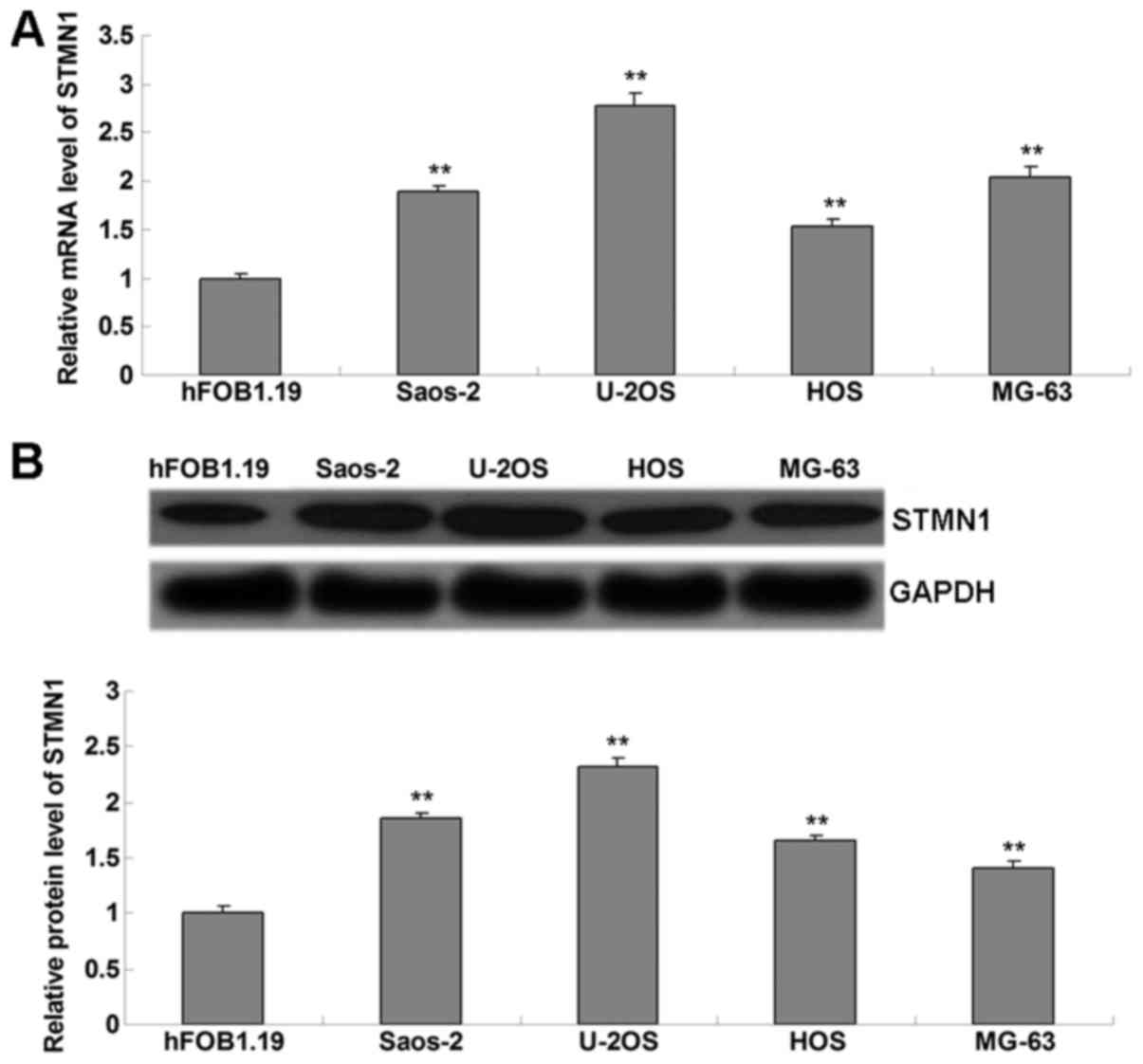
The present study performed western blot analysis to
examine the mRNA and protein levels of STMN1 in OS cell lines. As
presented in Fig. 1A and B, STMN1 was
significantly upregulated in OS cell lines (Saos-2, U-2OS, HOS and
MG-63), when compared to normal osteoblast hFOB1.19 cells. As U-2OS
cells demonstrated the highest level of STMN1, this cell line was
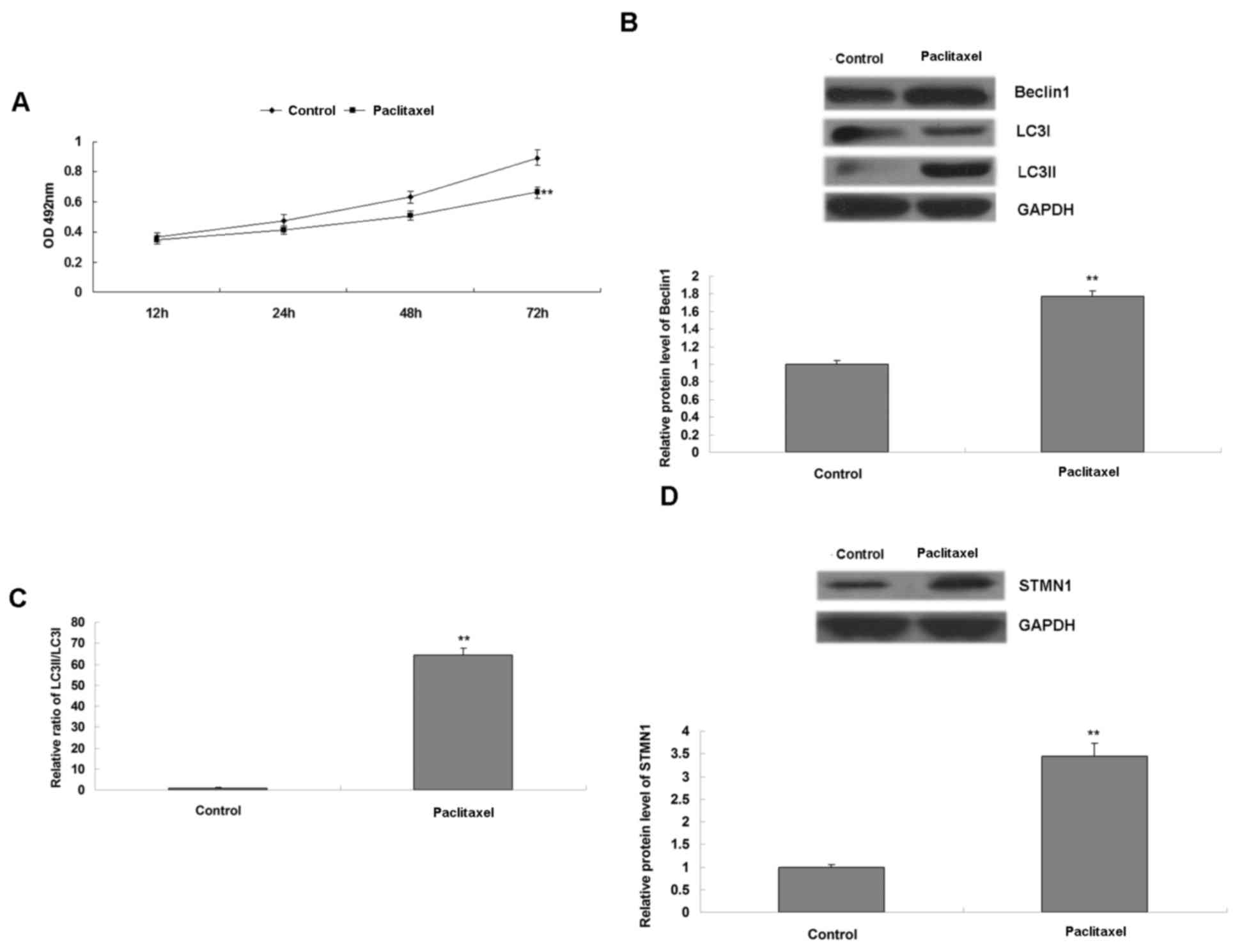
used in the subsequent experiments. U-2OS cells were treated with
paclitaxel for 3 h, and the cell survival and autophagy were
examined. It was observed that treatment with paclitaxel inhibited
cell survival (Fig. 2A). However, it
also led to activation of autophagy, demonstrated by the
upregulated protein levels of Beclin1 and LC3II, as well as the
increased radio of LC3II to LC3I (Fig. 2B
and C). In addition, it was also observed that STMN1 was
significantly upregulated in U-2OS cells following exposure to
paclitaxel (Fig. 2D), suggesting that
STMN1 may be associated with paclitaxel-induced autophagy in U-2OS
cells.
Knockdown of STMN1 expression
sensitizes U-2OS cells to paclitaxel
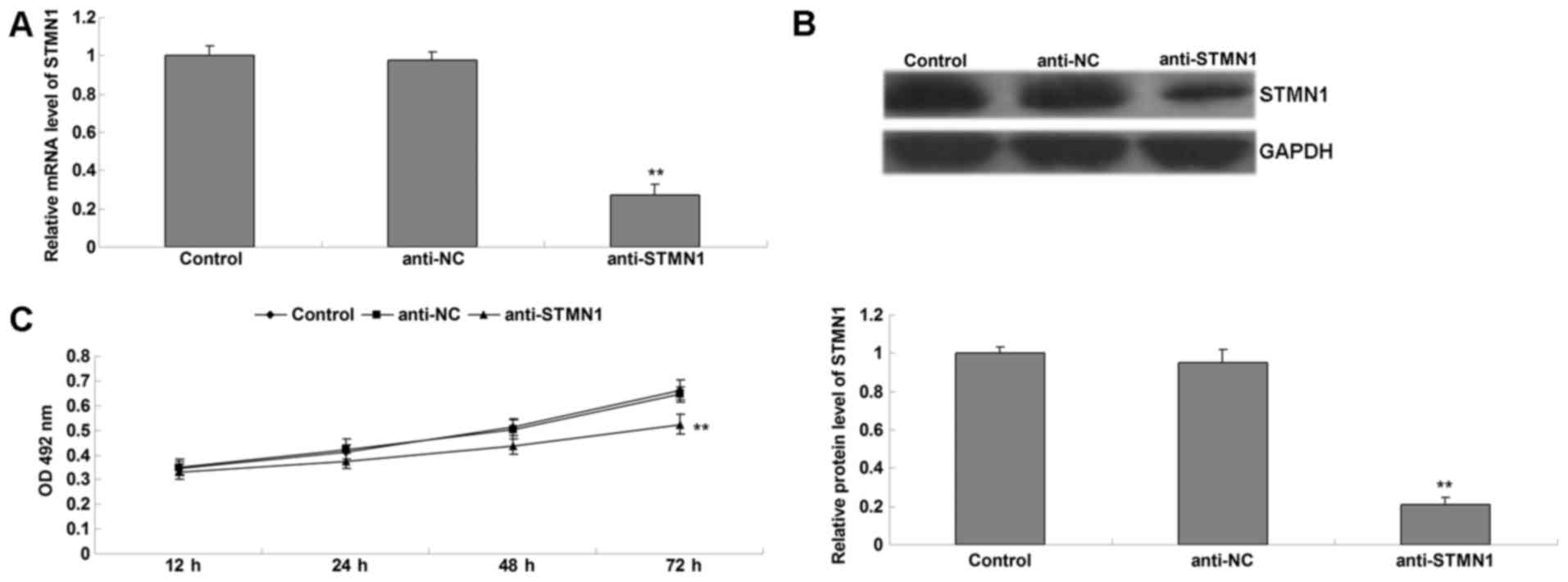
To confirm that STMN1 was involved in
paclitaxel-induced autophagy in U-2OS cells, U-2OS cells with
stably decreased expression of STMN1 were induced (Fig. 3A and B). The present study also
investigated the role of STMN1 in the sensitivity of OS to
paclitaxel. Cells in each group were treated with paclitaxel for 3
h, and the cell survival was examined. As shown in Fig. 3C, the cell survival in the anti-STMN1
group was significantly decreased, when compared with that in the
anti-NC group and the control group. Furthermore, there was no
significant change between the anti-NC group and control group.
These data suggest that knockdown of STMN1 expression enhanced the
sensitivity of U-2OS cells to paclitaxel.
Knockdown of STMN1 expression inhibits
paclitaxel-induced autophagy in OS cells
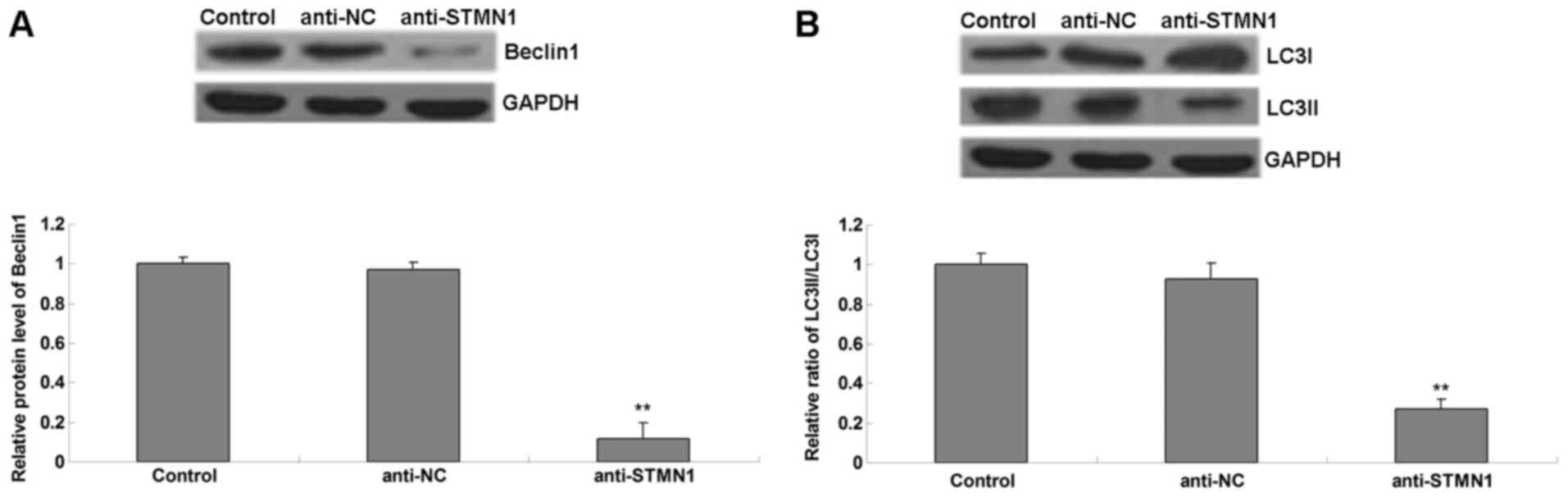
Western bolt analysis was conducted to determine the
levels of autophagy-associated proteins in each group. It was
observed that the protein levels of Beclin1 and LC3II (Fig. 4A), as well as the ratio of LC3II to
LC3I (Fig. 4B), were reduced in the
anti-STMN1 group, when compared with those in the anti-NC group and
the control group. These data suggest that knockdown of STMN1
inhibited paclitaxel-induced autophagy in OS cells.
Blockade of mTOR signaling attenuates
the inhibitory effect of STMN1 knockdown on autophagy in OS cells
treated with paclitaxel
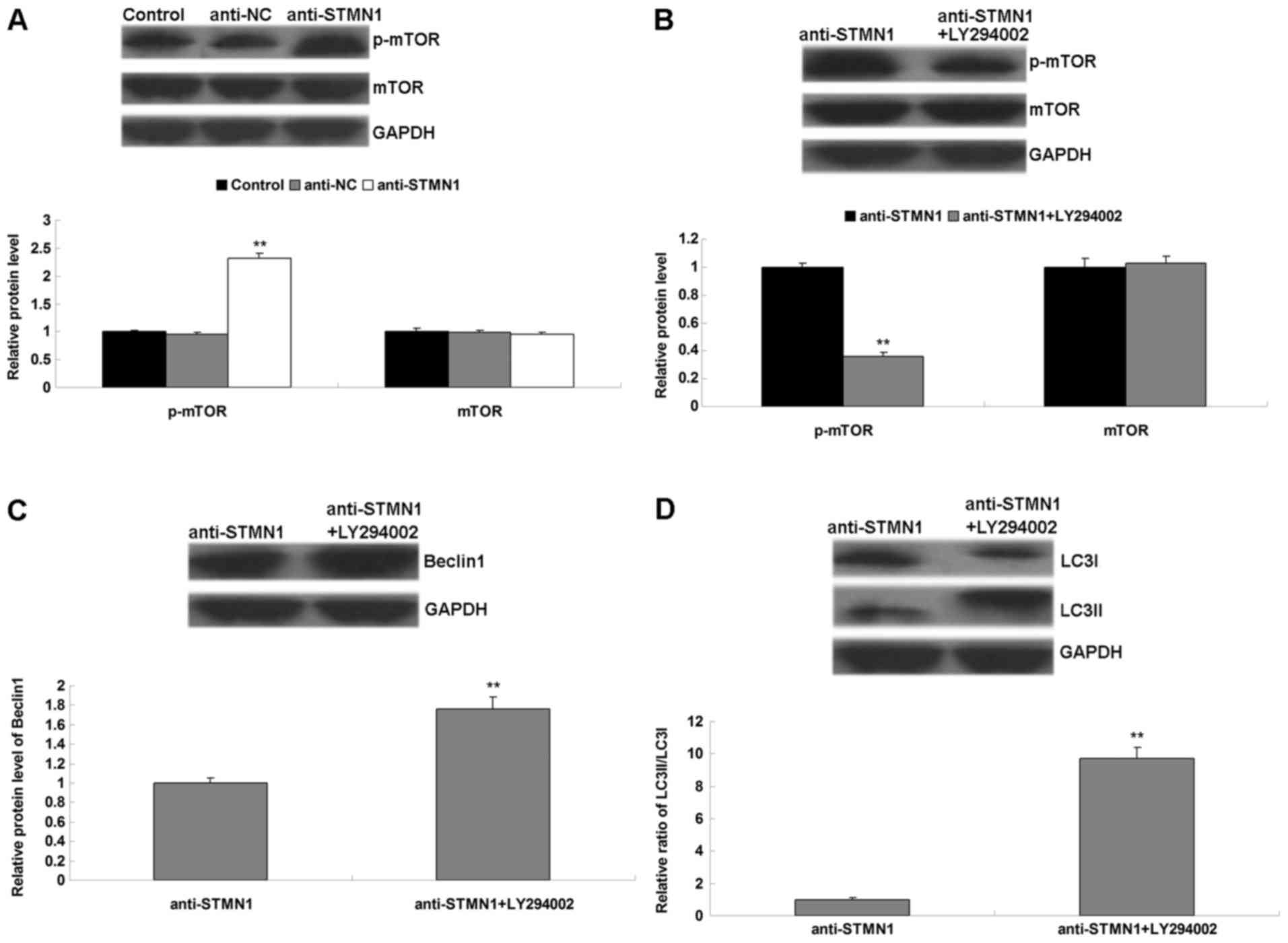
The molecular mechanisms underlying the role of
STMN1 in autophagy were investigated. The activity of mTOR
signaling was evaluated by using western blot analysis. It was
observed that knockdown of STMN1 expression increased the levels of
p-mTOR in OS cells treated with paclitaxel (Fig. 5A), suggesting that the activation of
mTOR signaling may be responsible for the inhibitory effect of
STMN1 knockdown on paclitaxel-induced autophagy in OS cells. To
additionally confirm this hypothesis, LY294002 was applied to
inhibit the activity of mTOR signaling. STMN1 silenced U-2OS cells
were treated with or without LY294002, followed by paclitaxel, and
the levels of mTOR signaling- and autophagy-associated proteins
were examined. As presented in Fig.
5B, STMN1 silenced U-2OS cells treated with LY294002 and
paclitaxel demonstrated a reduced p-mTOR level, when compared with
that in STMN1 silenced U-2OS cells treated with paclitaxel alone,
indicating that the activity of mTOR signaling was reduced.
Furthermore, STMN1 silenced U-2OS cells treated with LY294002 and
paclitaxel demonstrated increased levels of Beclin1 and LC3II, as
well as the ratio of LC3II/LC3I, when compared with those in STMN1
silenced U-2OS cells treated with paclitaxel alone, indicating that
the autophagy level was increased (Fig.
5C and D). Therefore, inhibition of mTOR activity attenuated
the inhibitory effect of STMN1 knockdown on paclitaxel-induced
autophagy in U-2OS cells, indicating that STMN1 participates in
paclitaxel-induced autophagy through mediation of mTOR
signaling.
Discussion
Investigation of the molecular mechanisms associated
with chemoresistance is important for chemotherapeutic treatment of
OS. In the present study, it was observed that STMN1 was
significantly upregulated in OS cell lines compared with normal
osteoblast hFOB1.19 cells. Treatment with paclitaxel enhanced the
expression of STMN1 in U-2OS cells, accompanied by activation of
autophagy. Knockdown of STMN1 expression suppressed
paclitaxel-induced autophagy and increased the cytotoxicity of
paclitaxel to U-2OS cells. Molecular mechanism investigation
revealed that knockdown of STMN1 expression activated mTOR
signaling in OS cells, while blockade of mTOR signaling attenuated
the inhibitory effect of STMN1 knockdown on autophagy in OS cells.
Therefore, the present study demonstrated that knockdown of STMN1
enhances osteosarcoma cell chemosensitivity through inhibition of
autophagy, and mTOR signaling is involved in this process.
STMN1 has been reported to be an oncogene, which is
expressed at high levels in a wide variety of human malignancies,
and serves important roles in maintenance of malignant phenotypes
(16). For instance, Hemdan et
al (17) reported that the
expression of STMN1 was significantly increased in bladder cancer
tissues, was correlated with reduced disease-specific survival and
70% of patients were STMN1-positive in primary tumor and matched
metastases groups, suggesting that STMN1 expression has prognostic
significance, and may be a potential treatment target in bladder
cancer. Furthermore, STMN1 is also upregulated in esophageal
carcinoma, and increased expression levels of STMN1 are associated
with low 5-year survival rates of patients with esophageal
carcinoma (18). In addition, STMN1
was observed to be upregulated in OS tissues compared with normal
tissues (13). The present study also
observed that expression levels of STMN1 were significantly
increased in four OS cell lines (HOS, Saos-2, U-2OS and MG-63)
compared with normal osteoblast hFOB1.19 cells. Therefore,
upregulation of STMN1 may be also involved in the development of
OS. However, to the best of our knowledge, the exact role of STMN1
in OS cell chemoresistance has not previously been studied. The
present study observed that treatment with paclitaxel in OS cells
led to a significant upregulation of STMN1 expression, as well as
autophagy, which has been reported to be associated with
chemoresistance in multiple types of human cancers including OS.
Guo et al also reported that paclitaxel induced apoptosis
accompanied by protective autophagy in OS cells via the
hypoxia-inducible factor-1α signaling pathway (19).
Furthermore, inhibition of autophagy has been
reported to have negative effects on OS. Knockdown of
autophagy-associated Beclin1 decreased cell proliferation, invasion
and metastasis, and had a positive effect on chemotherapy-induced
cytotoxicity in OS cells (20). In
the present study, it was observed that knockdown of STMN1 led to a
significant decrease in the expression of Beclin1, as well as the
ratio of LC3II to LC3I, and paclitaxel-induced cytotoxicity in OS
cells was enhanced. Wu et al (21) reported that inhibition of Beclin1
enhanced the chemotherapeutic sensitivity of osteosarcoma cells to
cisplatin.
The mTOR signaling pathway has been demonstrated to
serve a suppressive role in autophagy, and inhibition of mTOR
signaling causes upregulation of autophagy (22,23). Chang
et al (24) reported that dual
phosphoinositide 3-kinase/mTOR inhibitor NVP-BEZ235-induced
apoptosis of hepatocellular carcinoma cell lines was enhanced by
inhibition of autophagy. In the present study, it was observed that
knockdown of STMN1 led to upregulation of mTOR signaling, which
additionally inhibited autophagy. Therefore, LY294002-induced
blockade of mTOR signaling attenuated the inhibitory effect of
STMN1 knockdown on autophagy in OS cells treated with
paclitaxel.
In addition, STMN1 has been demonstrated to be a
direct target of microRNA (miR)-101, and is involved in
miR-101-mediated inhibition of autophagy in several types of human
cancer (25–27). Frankel et al (28) reported that miR-101 was able to
inhibit etoposide- and rapamycin-induced autophagy, while
overexpression of STMN1 partially rescued cells from
miR-101-mediated inhibition of autophagy. Xu et al (29) reported that miR-101 inhibited
autophagy and enhanced cisplatin-induced apoptosis in
hepatocellular carcinoma cells, partly at least, via direct
inhibition of STMN1 expression. In addition, STMN1 was observed to
be associated with radioresistance in human cancer. Sun et
al (30) reported that miR-101
sensitizes human nasopharyngeal carcinoma cells to radiation by
targeting STMN1.
In conclusion, the present study demonstrated that
knockdown of STMN1 enhances osteosarcoma cell chemosensitivity to
paclitaxel via inhibition of autophagy. Therefore, STMN1 may be a
potential target for the treatment of chemoresistant OS.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Hunan Proince, China (grant no.,
13JJ2013).
References
|
1
|
Thompson LD: Osteosarcoma. Ear Nose Throat
J. 92:288–290. 2013.PubMed/NCBI
|
|
2
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang Z, Huo L, Li K, Wu Y and Hu Z:
Blocked autophagy by miR-101 enhances osteosarcoma cell
chemosensitivity in vitro. ScientificWorldJournal. 2014:7947562014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou Y, Huang Z, Wu S, Zang X, Liu M and
Shi J: miR-33a is up-regulated in chemoresistant osteosarcoma and
promotes osteosarcoma cell resistance to cisplatin by
down-regulating TWIST. J Exp Clin Cancer Res. 33:122014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hippert MM, O'Toole PS and Thorburn A:
Autophagy in cancer: Good, bad, or both? Cancer Res. 66:9349–9351.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gonzalez CD, Alvarez S, Ropolo A,
Rosenzvit C, Gonzalez Bagnes MF and Vaccaro MI: Autophagy, Warburg
and Warburg reverse effects in human cancer. Biomed Res Int.
2014:9267292014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang R, Wang R, Chen Q and Chang H:
Inhibition of autophagy using 3-methyladenine increases
cisplatin-induced apoptosis by increasing endoplasmic reticulum
stress in U251 human glioma cells. Mol Med Rep. 12:1727–1732.
2015.PubMed/NCBI
|
|
8
|
Zheng B, Zhu H, Gu D, Pan X, Qian L, Xue
B, Yang D, Zhou J and Shan Y: MiRNA-30a-mediated autophagy
inhibition sensitizes renal cell carcinoma cells to sorafenib.
Biochem Biophys Res Commun. 459:234–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rebecca VW and Amaravadi RK: Emerging
strategies to effectively target autophagy in cancer. Oncogene.
35:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rana S, Maples PB, Senzer N and Nemunaitis
J: Stathmin 1: A novel therapeutic target for anticancer activity.
Expert Rev Anticancer Ther. 8:1461–1470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mistry SJ and Atweh GF: Role of stathmin
in the regulation of the mitotic spindle: Potential applications in
cancer therapy. Mt Sinai J Med. 69:299–304. 2002.PubMed/NCBI
|
|
12
|
Wang S, Akhtar J and Wang Z: Anti-STMN1
therapy improves sensitivity to antimicrotubule drugs in esophageal
squamous cell carcinoma. Tumour Biol. 36:7797–7806. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang HZ, Gao P, Yan L and Lin F:
Significance of stathmin gene overexpression in osteosarcoma cells.
Ai Zheng. 23:493–496. 2004.(In Chinies). PubMed/NCBI
|
|
14
|
Phadke AP, Jay CM, Wang Z, Chen S, Liu S,
Haddock C, Kumar P, Pappen BO, Rao DD, Templeton NS, et al: In vivo
safety and antitumor efficacy of bifunctional small hairpin RNAs
specific for the human Stathmin 1 oncoprotein. DNA Cell Biol.
30:715–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Shen J, Li X, Wang X, Long M, Lin
F, Wei J, Yang L, Yang C, Dong K and Zhang H: Rlim, an E3 ubiquitin
ligase, influences the stability of Stathmin protein in human
osteosarcoma cells. Cell Signal. 26:1532–1538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hemdan T, Lindén M, Lind SB, Namuduri AV,
Sjöstedt E, de Ståhl TD, Asplund A, Malmström PU and Segersten U:
The prognostic value and therapeutic target role of stathmin-1 in
urinary bladder cancer. Br J Cancer. 111:1180–1187. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang F, Xuan XY, Yang X, Cao L, Pang LN,
Zhou R, Fan QX and Wang LX: Stathmin is a marker of progression and
poor prognosis in esophageal carcinoma. Asian Pac J Cancer Prev.
15:3613–3618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo Y, Huang C, Li G, Chen T, Li J and
Huang Z: Paxilitaxel induces apoptosis accompanied by protective
autophagy in osteosarcoma cells through hypoxiainducible factor-1α
pathway. Mol Med Rep. 12:3681–3687. 2015.PubMed/NCBI
|
|
20
|
Zhang W, Li Q, Song C and Lao L: Knockdown
of autophagy-related protein 6, Beclin-1, decreases cell growth,
invasion, and metastasis and has a positive effect on
chemotherapy-induced cytotoxicity in osteosarcoma cells. Tumour
Biol. 36:2531–2539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu W, Li W, Zhou Y and Zhang C: Inhibition
of beclin1 affects the chemotherapeutic sensitivity of
osteosarcoma. Int J Clin Exp Pathol. 7:7114–7122. 2014.PubMed/NCBI
|
|
22
|
Choi J, Jo M, Lee E, Lee DY and Choi D:
Dienogest enhances autophagy induction in endometriotic cells by
impairing activation of AKT, ERK1/2, and mTOR. Fertil Steril.
104:655–664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuzawa Y, Oshima S, Takahara M,
Maeyashiki C, Nemoto Y, Kobayashi M, Nibe Y, Nozaki K, Nagaishi T,
Okamoto R, et al: TNFAIP3 promotes survival of CD4 T cells by
restricting MTOR and promoting autophagy. Autophagy. 11:1052–1062.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang Z, Shi G, Jin J, Guo H, Guo X, Luo
F, Song Y and Jia X: Dual PI3K/mTOR inhibitor NVP-BEZ235-induced
apoptosis of hepatocellular carcinoma cell lines is enhanced by
inhibitors of autophagy. Int J Mol Med. 31:1449–1456.
2013.PubMed/NCBI
|
|
25
|
Zheng F, Liao YJ, Cai MY, Liu TH, Chen SP,
Wu PH, Wu L, Bian XW, Guan XY, Zeng YX, et al: Systemic delivery of
microRNA-101 potently inhibits hepatocellular carcinoma in vivo by
repressing multiple targets. PLoS Genet. 11:e10048732015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang R, Wang HB, Hao CJ, Cui Y, Han XC, Hu
Y, Li FF, Xia HF and Ma X: MiR-101 is involved in human breast
carcinogenesis by targeting Stathmin1. PLoS One. 7:e461732012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Zhang X, Jia LT, Hu SJ, Zhao J,
Yang JD, Wen WH, Wang Z, Wang T, Zhao J, et al: c-Myc-mediated
epigenetic silencing of MicroRNA-101 contributes to dysregulation
of multiple pathways in hepatocellular carcinoma. Hepatology.
59:1850–1863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frankel LB, Wen J, Lees M, Høyer-Hansen M,
Farkas T, Krogh A, Jäättelä M and Lund AH: microRNA-101 is a potent
inhibitor of autophagy. EMBO J. 30:4628–4641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Y, An Y, Wang Y, Zhang C, Zhang H,
Huang C, Jiang H, Wang X and Li X: miR-101 inhibits autophagy and
enhances cisplatin-induced apoptosis in hepatocellular carcinoma
cells. Oncol Rep. 29:2019–2024. 2013.PubMed/NCBI
|
|
30
|
Sun Q, Liu T, Zhang T, Du S, Xie GX, Lin
X, Chen L and Yuan Y: MiR-101 sensitizes human nasopharyngeal
carcinoma cells to radiation by targeting stathmin 1. Mol Med Rep.
11:3330–3336. 2015.PubMed/NCBI
|