Introduction
Papillary thyroid carcinoma (PTC) is the most
prevalent form of thyroid cancer, with an incidence that has
continued to increase globally in recent decades (1). This tumor consists of a number of
histological variants, the most common of which are conventional
(classical) PTC, follicular variant PTC and tall cell variant (TCV)
PTC (2). TCV PTC was initially
described in 1976 by Hawk and Hazard (3) and is now an established variant composed
of cells that are at least three times taller than they are wide,
and which occasionally exhibit an abundant eosinophilic cytoplasm
and basally oriented nuclei (4,5). The 2004
World Health Organization classification of tumors defined a PTC as
TCV if it is composed predominantly of tall cells (6), and the majority of experts consider that
a tumor may contain ≥50% tall-cell features to be classified as the
TCV of PTC (4,5,7). However,
there remains no general consensus on this classification (8–10).
TCV PTC has a relatively higher predominance in
males than classical PTC, occurs at an older age and is considered
an aggressive variant (5,10). Patients with TCV exhibit poorer
survival than those with classical PTC (5,10). The
tall-cell feature alone remains a significant prognostic factor for
disease-specific mortality when the major prognostic factors for
thyroid cancer are controlled for, including age and extrathyroidal
extension (11). In a study of 12
cases of TCV PTC, this tumor type had an aggressive clinical course
and a poorer prognosis, compared with classical PTC, in patient
populations with a similar age and sex distribution, duration of
follow-up and tumor size (12). The
patient prognosis also remains less favorable in cases without
extrathyroidal extension (13). A
large multicenter study demonstrated varied prognostic risk for the
three major PTC variants, establishing a risk order for PTC as
follows: TCV PTC > classical PTC > follicular variant PTC
(14). This previous study
demonstrated clinical implications for the management of PTC based
on the specific variant (14).
In the present study, the main clinicopathological
features and the B-Raf proto-oncogene (BRAF) mutational
status of a series of TCV PTC with classical and follicular
variants (CaFVs) of PTC (matched in size and age) were compared in
order to determine if, regardless of patient age at diagnosis and
the tumor size, TCV is more aggressive than its classical and
follicular counterparts.
Materials and methods
Patient selection
The hospital database was searched for all cases
diagnosed as PTC and treated at the Clinical University Hospital
(Santiago de Compostela, Galicia, Spain) between January 1st, 1990
and December 31st, 2010. PTCs were re-reviewed according to the
criteria of the World Health Organization international
classification for thyroid tumors (6)
by one of the co-authors (J.M.C.-T.), a pathologist with special
expertise in thyroid tumors who was blinded to the clinical
outcomes of all patients involved. The tall cells were defined as
tall columnar cells whose height was at least three times their
width. A tumor was classified as TCV PTC if it contained ≥50% tall
cells (5–7). To avoid selection bias, and due to their
highly indolent behavior (15), cases
of encapsulated follicular variant of PTC were excluded from the
current study. For the same reasons, all tumors with ≥3 mitoses/10
high-power fields or necrosis with poorly differentiated-like
behavior (6,16) were also excluded. Written informed
consent was obtained from all patients. The Independent Ethics
Committee of Galicia (Galician Healthcare Service; SERGAS) approved
the study protocol, which was conducted in accordance with the
Declaration of Helsinki and applicable Spanish laws.
Clinical, histopathological,
immunohistochemical and molecular parameters
The following histopathological parameters were
assessed: Carcinoma size, multifocality, infiltration of the two
thyroid lobes (bilaterality), vascular invasion, perineural
infiltration, status of the resection margins, presence of tumor
cells invading beyond the thyroid capsule into perithyroid soft
tissue or organs (extrathyroid tumor extension) and the presence of
metastatic lymph nodes. The largest dimension of the carcinoma was
determined by a review of the gross pathology report and direct
measurement of the tumor on the microscopic slides. The patient's
electronic medical records were reviewed for the age at diagnosis,
type of surgery (including re-interventions) and administration of
radioactive iodine therapy. Staging was performed according to the
American Joint Committee on Cancer staging manual 7th edition
(17). The mean follow-up for all
patients was 59.6 months [standard deviation (SD), 44.7
months].
Immunohistochemical studies were performed on 4
µm-thick paraffin-embedded representative tumor sections from
thyroidectomy specimens. A peroxidase-conjugated labeled dextran
polymer (EnVision FLEX/HRP; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA) was used with 3,3′-diaminobenzidine as the
chromogen in the Autostainer Link 48 (Dako; Agilent Technologies,
Inc.) according to the manufacturer's protocol. Deparaffinization,
rehydration and target retrieval (pH 6.0) were performed on a PT
Link pretreatment module (Dako; Agilent Technologies, Inc.).
Subsequently, samples were incubated with primary antibodies
against thyroglobulin (catalog no. IR509; rabbit polyclonal; ready
to use; pH 6.0; Dako; Agilent Technologies, Inc.) and calcitonin
(catalog no. IR515; polyclonal; ready to use; pH 9.0; Dako; Agilent
Technologies, Inc.) at room temperature for 20 and 15 min,
respectively. An immunoglobulin fraction of normal rabbit serum
supplied in 0.05 mol/l NaCl, 15 and mmol/l NaN3 (pH 7.2), and
containing stabilizing protein (catalog no. IR600; Dako; Agilent
Technologies, Inc.) was used as the negative control. Tissue
sections containing a medullary carcinoma (calcitonin) and normal
thyroid (thyroglobulin) obtained from the Carlos III Health
Institute (Madrid, Spain; TIROCHUS collection no. 0003960), were
simultaneously evaluated as positive controls for thyroglobulin and
calcitonin. The slides were counterstained with hematoxylin. The
staining for thyroglobulin and for calcitonin was considered
positive only if the cytoplasm of tumor cells had been stained
brown with the chromogen following microscopic examination (Olympus
BX41TF; Olympus Corporation, Tokyo, Japan).
For BRAF molecular genetic analysis, tumor
tissue was identified and marked on the hematoxylin and
eosin-stained glass slides by the pathologist. Genomic DNA was
extracted from the paraffin-embedded tumor tissue using a QIAamp
DNA Mini kit (Qiagen Inc., Valencia, CA, USA) according to the
manufacturer's protocol. DNA samples were screened for mutations in
exons 11 and 15 of the BRAF gene (National Center for
Biotechnology Information reference sequence, NM_004333). Exons 11
and 15 of the BRAF gene were amplified by polymerase chain
reaction (PCR) using 50–70 ng of genomic DNA. Each 12.5-µl reaction
consisted of 1X PCR buffer (catalog no. M7801; Promega Corporation,
Madison, WI, USA), 3 mM MgCl2, 200 µM deoxynucleotides,
0.5 µM of each primer and 0.6 U GoTaq Flexi DNA Polymerase (catalog
no. M7801; Promega Corporation). Primer sequences were as follows:
Exon 11, forward 5′-GCATAAGGTAATGTACTTAGGGTGAA-3′ and reverse
5′-AACAGTGAATATTTCCTTTGATGAT-3′; and exon 15, forward
5′-TCATAATGCTTGCTCTGATAGG-3′ and reverse
5′-GGCCAAAAATTTAATCAGTGGA-3′ (18).
The thermocycling conditions consisted of an initial denaturation
at 94°C for 3 min; 35 cycles at 94°C for 30 sec, 30 sec of
annealing at 60°C for exon 15 and at 55°C for exon 11, and 72°C for
45 sec; and a final extension at 72°C for 7 min. The PCR products
were bidirectionally sequenced using capillary electrophoresis
(3730 DNA Analyzer; Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with the aforementioned primers.
Statistical analysis
Statistical analysis was conducted using SPSS
version 18.0 (SPSS Inc., Chicago, IL, USA). The TCV PTC cohort
(n=16) was compared with the matched series of classical (n=18) and
follicular (n=16) variants of PTC. Data are presented as the mean ±
SD or as a percentage. Categorical variables were compared using χ2
analysis, whereas continuous variables were compared using
student's t-tests or Mann Whitney U tests, as appropriate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological features
In total, 16 (3.66%) patients with TCV PTC were
identified in a series of 437 patients with PTC. The TCV series
included 11 females and 5 males aged 15–74 years (median, 57
years). Table I presents the
clinicopathological features of the TCV PTC series in comparison
with the control group of CaFVs of PTC. The clinicopathological
features and BRAFV600E mutational status of the TCV PTC
series were compared with the 34 cases of CaFVs of PTC matched for
tumor size and age at diagnosis (Table
II).
 | Table I.Clinicopathological features at
presentation according to the subtype of PTC. |
Table I.
Clinicopathological features at
presentation according to the subtype of PTC.
| Variable | TCV of PTC
(n=16) | CaFVs of PTC
(n=34) | P-value |
|---|
| Female gender
(%) | 11 (68.8) | 28 (82.4) | 0.400 |
| Age, years
(SD) | 57 (18.5) | 50.8 (15.7) | 0.100 |
| Follow-up time,
months (%) | 15 (93.8) | 34 (100.0) | 0.100 |
| Type of surgery, n
(%) |
|
| 0.100 |
|
obectomy | 1 (6.2) | 1 (2.9) |
|
|
Subtotal thyroidectomy | 2 (12.5) | 0 (0.0) |
|
| Total
thyroidectomy | 13 (81.3) | 33 (97.1) |
|
|
Lymphadenectomy | 5
(31.3) | 9
(26.4) | 0.900 |
| Surgical
re-intervention, n (%) | 2
(12.5) | 2
(5.9) | 0.800 |
| Received RAI
treatment, n (%) | 15 (93.8) | 33 (97.1) | 0.500 |
| Tumor size, cm
(SD) | 2.1
(0.9) | 2.6
(1.7) | 0.600 |
| Multifocal tumor, n
(%) | 8
(50.0) | 9
(26.4) | 0.090 |
| Bilateral tumor, n
(%) | 5
(31.3) | 5
(14.7) | 0.200 |
| Positive margin
status, n (%) | 13 (81.3) | 24 (70.6) | 0.300 |
| Lymphovascular
invasion, n (%) | 6
(37.5) | 10 (29.4) | 0.500 |
| Perineural
invasion, n (%) | 0 (0.0) | 1 (2.9) | 1.000 |
| Extrathyroid
extension, n (%) | 9
(56.3) | 5
(14.7) | 0.007b |
| Lymph node
metastases, n (%) | 9
(56.3) | 9
(26.4) | 0.040b |
| Distant metastases,
n (%) | 1
(6.2) | 0
(0.0) | 0.300 |
| TNM staging, n
(%)a |
|
| 0.010b |
| I | 5 (31.3) | 19 (55.9) |
|
| II | 1 (6.2) | 8 (23.5) |
|
|
III | 2 (12.5) | 6 (17.6) |
|
| IV | 8
(50.0) | 1
(2.9) |
|
|
| Tumor stage III/IV,
n (%) | 10 (62.5) | 7
(20.5) | 0.009b |
 | Table II.Clinicopathological features in a PTC
series according to the BRAF mutation status. |
Table II.
Clinicopathological features in a PTC
series according to the BRAF mutation status.
| Variable |
BRAFV600E (n=19) | BRAF wild
type (n=25) | P-value |
|---|
| Age, years
(SD) | 54.6 (17.9) | 51.1 (15.0) | 0.100 |
| Tumor size, cm
(SD) | 2.1
(1.1) | 2.6
(1.7) | 0.100 |
| Multifocal tumor, n
(%) | 7
(41.1) | 8
(38.0) | 0.800 |
| Bilateral tumor, n
(%) | 4
(23.5) | 5
(23.8) | 1.000 |
| Positive margin
status, n (%) | 15 (83.3) | 13 (61.9) | 0.200 |
| Lymphovascular
invasion, n (%) | 5
(26.3) | 9
(42.8) | 0.200 |
| Perineural
invasion, n (%) | 1 (5.2) | 0 (0.0) | 0.400 |
| Extrathyroid
extension, n (%) | 10 (52.6) | 2 (9.5) | 0.009a |
| Lymph nodes
metastases, n (%) | 11 (57.8) | 5 (23.8) | 0.028a |
| Advanced stage
(III/IV), n (%) | 14 (73.6) | 9 (42.8) | 0.049a |
| Persistent.disease,
n (%) | 6 (31.5) | 3 (14.2) | 0.300 |
| Disease.free
survival, n (%) | 11 (57.8) | 18 (85.7) | 0.100 |
In the TCV PTC series, the tumor size ranged from
5–45 mm (median, 19 mm). Extrathyroidal extension was present in 9
(56.3%) cases and lymph node metastases were detected in 9 (56.3%)
cases. In total, 8 patients (50.0%) had multifocal papillary
carcinomas, with lymphovascular invasion in 6 (37.5%) cases and
distant metastases in 1 (6.2%) case. A total of 10 patients (62.5%)
presented at stage III/IV. At presentation, one patient in the TCV
PTC group had distant metastases, whereas no distant metastasis was
detected in the control group. During follow-up, two patients with
TCV PTC developed metastases (cervical lymph node metastases in one
case and lung metastases in one case), and two other patients with
TCV PTC succumbed to the disease. No metastases or
disease-associated mortalities were observed in the control group.
At the end of the study, a lower percentage of disease-free
patients and slightly higher rate of recurrence was observed in the
TCV PTC group by comparison with the control group (50.0% vs. 85.3%
disease-free patients, P=0.02; 37.5% vs. 11.7% rate of recurrence,
P=0.08, respectively). The survival rate in patients with TCV PTC
was 87.5%, compared with 97.0% in the control group; however, this
difference was not statistically significant (P=0.1).
The control group exhibited less extrathyroidal
extension and lymph node metastases: 5 (14.7%; P=0.007) and 9
(26.5%; P=0.04) cases, respectively. In total, 9 (26.5%) tumors
were multifocal, with lymphovascular invasion in 10 (29.4%), no
distant metastases and 7 (20.5%) patients vs. 10 (62.5%) patients
presenting at stage III/IV (P=0.04).
Immunohistochemical and molecular
findings
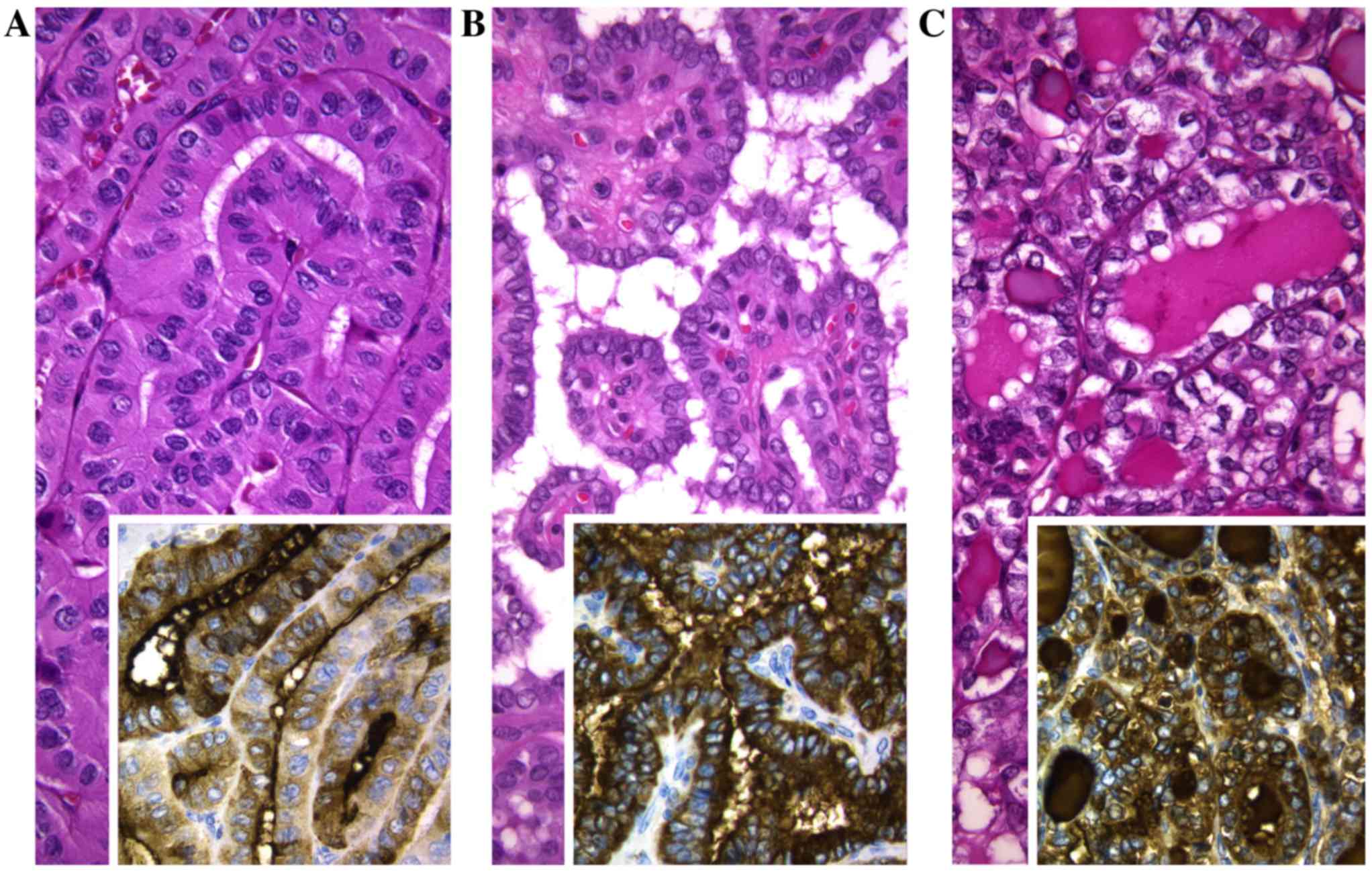
In accordance with their follicular cell derivation,
in the two series, all tumors were positive for thyroglobulin and
negative for calcitonin (Fig. 1). The
BRAF mutational analysis was performed in 40 cases. No
correlation was detected between age or tumor size and the
mutational status of the BRAF gene. The
BRAFV600E mutation was higher in the TCV PTC
series compared with the CaFVs of PTC series [12/15 (80.0%) vs.
7/25 (28.0%) cases; P=0.004). The BRAFV600E
mutation was associated with extrathyroidal extension (P=0.009),
lymph node metastases (P=0.028) and the advanced-stage of disease
(P=0.049). BRAFV600E mutation was present in the tumor of
the patient with distant metastases at diagnosis, in the two
patients developing metastases on follow-up, and in the two
patients that succumbed to the disease, all of whom belonged to the
TCV PTC series.
Discussion
In the current study, the main clinicopathological
features of the TCV of PTC were examined, the frequency of which
appears to be rising along with the increased incidence of PTC
worldwide (19,20). However, the causes of this increased
incidence of the TCV of PTC, and whether it is a true increase,
remain to be elucidated. The TCV made up ~10% of all cases of PTC
in the initial series studied by Hawk and Hazard (3); however, later studies on the incidence
of this entity detected the TCV in between 1.3 and 13% of all PTC
cases (21,22). Similar to the findings of Ito et
al (23), the current study
detected 3.6% of the TCV of PTC in a series of 437 PTC cases. This
variation in the reported prevalence of TCV PTC may occur as a
consequence of a poor definition of this variant due to the
following factors: i) A variance in the height of the neoplastic
cells depending on the plane of the section (6); ii) the presence of a significant
proportion of tall cells in various types of PTC (6); iii) the differing diagnostic criteria
proposed (for example, distinct threshold values in the percentage
of tall cells required to determine a particular case of PTC as
being a variant of tall cells) (3,4,6); and iv) the misdiagnosis of this variant
on routine pathological examination (7). Certain studies have revealed that 1–13%
of diagnosed cases of classical PTC were reclassified as TCV PTC
following revision by endocrine pathologists (13,24–27). The
TCV of PTC has a 6% prevalence (95% confidence interval, 4–7%) in
all PTC, following a pooling of the data from the literature
(10). Although there is no consensus
concerning the threshold of tall cells defining the TCV of PTC,
LiVolsi (4) proposed to specify the
presence of foci of tall cells in pathological reports irrespective
of the percentage of tall cell cytology. Despite the lower number
of cases in the current series, all cases of the TCV of PTC had
≥50% tall cells, which may indicate the increased reliability of
the study.
Due to the clinical differences between the patients
with the TCV of PTC and classical PTC, a control series with
exactly the same mean age could not be obtained in the current
study; however, no significant differences in patient age were
detected between the two groups (P=0.1). The female:male ratio in
the patients with TCV of PTC was 2.2, with females accounting for
69%, and males 31% of cases. In the control group of patients in
the present study, females accounted for 82%, and males for 18% of
cases. Certain studies have also observed that the TCV of PTC has a
higher prevalence in males compared with classical PTC (10,19,25). It is
established that, in differentiated thyroid carcinoma cases, male
patients tend to have more advanced disease diagnosed at an older
age, lower disease-free survival and higher mortality compared with
female patients (28,29). The mean age at presentation of the
patients in the present study with the TCV of PTC (57 years) was
similar that to observed in a large population-based cancer
registry (Surveillance, Epidemiology and End Results) from
1988–2008 (19). This data was also
concordant with the ranges and mean age pooled from the series that
has been previously reported (range, 41–66 years; mean, 50.1 years
for TCV PTC; range, 34–53 years; mean, 45.7 years for classical
PTC) (10). Age at diagnosis is also
an important prognostic factor in patients with differentiated
thyroid carcinoma (30) and,
particularly after 40–45 years, the adverse effect of age on
prognosis increases gradually with every decade (31).
The behavior of TCV is more aggressive than that of
CaFVs of PTC (3,8,10,12–14,23,24,27,30).
Although there is a lack of consensus on whether the TCV of PTC is
varied from the classical PTC in terms of tumor size at
presentation (10), a previous review
revealed that the mean tumor diameter in all cases of the TCV of
PTC was 20 mm, by comparison with 19 mm in classical PTC (10). The current series of the TCV of PTC
was compared with the two CaFVs to determine if, irrespective of
age and size, TCV is more aggressive than its classical and
follicular counterparts. The overall percentage of multifocality,
bilaterality, positive margins, vascular invasion, extrathyroidal
extension, lymph node metastasis, distant metastasis and stage
III/IV at presentation was greater in the TCV variant group, as
compared with the classical and follicular PTC groups (Table I). Similar figures have been reported
by previous studies, and the overall rates of multifocality,
extrathyroidal extension, lymph node metastasis, and distant
metastasis at the time of diagnosis in patients with TCV PTC were
45.7, 63.9, 59.0 and 8.6% respectively (10). The current study detected
significantly higher rates of extrathyroidal extension (56.3 vs.
14.7%; P=0.007), lymph node metastasis (56.3 vs. 26.4%; P=0.04) and
advanced-stage disease (62.5 vs. 20.5%; P=0.009) in the TCV series
in comparison with the CaFVs, and the tumor size was slightly
larger in the latter group [21 mm (SD, 0.9 mm) vs. 26 mm (SD, 1.7
mm), respectively]. A patient with distant metastases was present
in the TCV of PTC group in the present study, but no distant
metastases were detected in the control group. These findings were
consistent with previous studies concerning the increased
aggressiveness of TCV compared with the CaFVs of PTC, and support
such aggressiveness being independent of tumor size and patient
age.
BRAF gene encodes a serine/threonine kinase
that belongs to the RAS-RAF-mitogen-activated protein kinase (MAPK)
kinase-extracellular signal-regulated kinase-mitogen activated
protein kinase signaling pathway, the role of which is to mediate
the cellular responses to growth factors. The T1796A BRAF
mutation, leading to the substitution of a valine by a glutamic
acid at position 600 in exon 15, is the most prevalent mutation in
PTC. The BRAFV600E mutation increases BRAF kinase
activity, triggering the MAPK signaling pathway independent of the
activation of upstream factors. The BRAFV600E
mutation is the most frequent point mutation detected in PTC
(36–83%), with a strong genotype-phenotype association, and is
almost exclusively detected in cases of PTC that exhibit a
papillary or mixed papillary-follicular growth pattern (32,33).
Previous reports indicate that the BRAF mutation has the
highest prevalence in the TCV of PTC (80–100%) (10,34). In
the patient series of the current study, the percentage of cases
exhibiting the BRAFV600E mutation was also
significantly higher in the TCV of PTC group (80%; P=0.004).
Certain studies have reported significant
associations between BRAF-positive thyroid tumors and poor
prognostic indicators, including male gender, increased age, lymph
node metastases, extrathyroid extension, distant metastases, higher
tumor staging, tumor size and tumor recurrence (10,32). A
retrospective multicenter study demonstrated that the
BRAFV600E mutation was significantly correlated
with increased cancer-associated mortality in patients with PTC;
however, this association was not independent of a number of
clinicopathological features of aggressiveness, including older
patient age at diagnosis, lymph node metastases, extrathyroidal
extension, distant metastasis or advanced disease (stage IV)
(35). A recent meta-analysis
demonstrated that BRAFV600E-positive papillary
thyroid types of microcarcinoma are more likely to possess
aggressive clinicopathological characteristics (36). Subsequently, another previous study
revealed that the coexistence of BRAFV600E and
telomerase reverse transcriptase promoter mutations was
particularly associated with high-risk clinicopathological features
in PTC (37). Notably, in the current
study, the BRAFV600E mutation was associated with
extrathyroidal extension (P=0.009), lymph node metastases (P=0.028)
and advanced stage (P=0.049) in the TCV of PTC and the CaFVs of PTC
groups (Table II). In addition, all
the patients that presented with distant metastases at diagnosis,
that developed metastases on follow-up or that succumbed to the
disease, had BRAFV600E-positive TCV PTC. These
results suggest that the BRAFV600E mutation
functions in the aggressive biological behavior of the TCV of PTC.
The current hypothesis, based on this series of clinically relevant
PTC, is concordant with a previous study by Virk et al
(38) on papillary microcarcinoma. In
this previous study, Virk et al (38) proposed that the
BRAFV600E mutation is an early event in thyroid
carcinogenesis, and is associated with distinctive morphology and
aggressive features, even in papillary thyroid microcarcinomas.
In conclusion, the present study reports a series of
PTC strictly classified as the TCV (≥50% of tall cells) and reveals
that, irrespective of patient age and tumor size, this variant is
significantly associated with a higher rate of extrathyroidal
extension, lymph node metastasis, advanced stage and
BRAFV600E mutations, compared with the CaFVs of
PTC. The higher rate of BRAFV600E mutations in
the TCV of PTC compared with the classical and follicular
counterparts, also supports the hypothesis of a role for these
mutations in the aggressiveness of PTC.
Acknowledgements
The present study was supported by the Carlos III
Health Institute, Ministry of Economy and Competitiveness (Madrid,
Spain; grant no. PI15/01501-FEDER).
References
|
1
|
Davies L and Welch HG: Current thyroid
trends in the United States. JAMA Otolaryngol Head Neck Surg.
140:317–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lam AK, Lo CY and Lam KS: Papillary
carcinoma of thyroid: A 30-yr clinicopathological review of the
histological variants. Endocr Pathol. 16:323–330. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hawk WA and Hazard JB: The many
appearances of papillary carcinoma of the thyroid. Cleve Clin Q.
43:207–215. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
LiVolsi VA: Papillary carcinoma tall cell
variant (TCV): A review: Endocr Pathol. 21:12–15. 2010.
|
|
5
|
Lastra RR, LiVolsi VA and Baloch ZW:
Aggressive variants of follicular cell-derived thyroid carcinomas.
A cytopathologis's perspective. Cancer Cytopathol. 122:484–503.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
LiVolsi VA, Albores-Saavedra J, Asa SL,
Baloch ZW, Sobrinho-Simões M, Wenig B, DeLellis RA, Cady B,
Mazzaferri EL, Hay I, Fagin JA, et al: Papillary carcinomaDeLellis
RA, Heitz PU and Eng C: Pathology and Genetics: Tumours of
Endocrine Organs. 3rd. World Health Organization Classification of
Tumours. France. IARC Press; Lyon: pp. 57–66. 2004
|
|
7
|
Ghossein R and Livolsi VA: Papillary
thyroid carcinoma tall cell variant. Thyroid. 18:1179–1181. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ganly I, Ibrahimpasic T, Rivera M, Nixon
I, Palmer F, Patel SG, Tuttle RM, Shah JP and Ghossein R:
Prognostic implications of papillary thyroid carcinoma with
tall-cell features. Thyroid. 24:662–670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dettmer MS, Schmitt A, Steinert H, Capper
D, Moch H, Komminoth P and Perren A: Tall cell papillary thyroid
carcinoma: New diagnostic criteria and mutations in BRAF and TERT.
Endocr Relat Cancer. 22:419–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Cheng W, Liu C and Li J: Tall cell
variant of papillary thyroid carcinoma: Current evidence on
clinicopathologic features and molecular biology. Oncotarget.
7:40792–40799. 2016.PubMed/NCBI
|
|
11
|
Morris LG, Shaha AR, Tuttle RM, Sikora AG
and Ganly I: Tall-cell variant of papillary thyroid carcinoma: A
matched-pair analysis of survival. Thyroid. 20:153–158. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnson TL, Lloyd RV, Thompson NW,
Beierwaltes WH and Sisson JC: Prognostic implications of the tall
cell variant of papillary thyroid carcinoma. Am J Surg Pathol.
12:22–27. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghossein RA, Leboeuf R, Patel KN, Rivera
M, Katabi N, Carlson DL, Tallini G, Shaha A, Singh B and Tuttle RM:
Tall cell variant of papillary thyroid carcinoma without
extrathyroid extension: Biologic behavior and clinical
implications. Thyroid. 17:655–661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi X, Liu R, Basolo F, Giannini R, Shen
X, Teng D, Guan H, Shan Z, Teng W, Musholt TJ, et al: Differential
clinicopathological risk and prognosis of major papillary thyroid
cancer variants. J Clin Endocrinol Metab. 101:264–274. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nikiforov YE, Seethala RR, Tallini G,
Baloch ZW, Basolo F, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan
A, Kakudo K, et al: Nomenclature revision for encapsulated
follicular variant of papillary thyroid carcinoma: A paradigm shift
to reduce overtreatment of indolent tumors. JAMA Oncol.
2:1023–1029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gnemmi V, Renaud F, Do Cao C, Salleron J,
Lion G, Wemeau JL, Copin MC, Carnaille B, Leteurtre E, Pattou F and
Aubert S: Poorly differentiated thyroid carcinomas: Application of
the Turin proposal provides prognostic results similar to those
from the assessment of high-grade features. Histopathology.
64:263–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer staging handbook. 7th. NY,
Springer; New York: pp. 111–122. 2010
|
|
18
|
Cameselle-Teijeiro J, Abdulkader I,
Pérez-Becerra R, Vázquez-Boquete A, Alberte-Lista L, Ruiz-Ponte C,
Forteza J and Sobrinho-Simões M: BRAF mutation in solid cell nest
hyperplasia associated with papillary thyroid carcinoma. A
precursor lesion? Hum Pathol. 40:1029–1035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kazaure HS, Roman SA and Sosa JA:
Aggressive variants of papillary thyroid cancer: Incidence,
characteristics and predictors of survival among 43,738 patients.
Ann Surg Oncol. 19:1874–1880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ceresini G, Corcione L, Michiara M, Sgargi
P, Teresi G, Gilli A, Usberti E, Silini E and Ceda GP: Thyroid
cancer incidence by histological type and related variants in a
mildly iodine-deficient area of Northern Italy, 1998 to 2009.
Cancer. 118:5473–5480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guan H, Vandenbussche CJ, Erozan YS,
Rosenthal DL, Tatsas AD, Olson MT, Zheng R, Auger M and Ali SZ: Can
the tall cell variant of papillary thyroid carcinoma be
distinguished from the conventional type in fine needle aspirates?
A cytomorphologic study with assessment of diagnostic accuracy.
Acta Cytol. 57:534–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Axelsson TA, Hrafnkelsson J, Olafsdottir
EJ and Jonasson JG: Tall cell variant of papillary thyroid
carcinoma: A population-based study in Iceland. Thyroid.
25:216–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ito Y, Hirokawa M, Fukushima M, Inoue H,
Yabuta T, Uruno T, Kihara M, Higashiyama T, Takamura Y, Miya A, et
al: Prevalence and prognostic significance of poor differentiation
and tall cell variant in papillary carcinoma in Japan. World J
Surg. 32:1535–1545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terry JH, St John SA, Karkowski FJ, Suarez
JR, Yassa NH, Platica CD and Marti JR: Tall cell papillary thyroid
cancer: Incidence and prognosis. Am J Surg. 168:459–461. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rüter A, Nishiyama R and Lennquist S:
Tall-cell variant of papillary thyroid cancer: Disregarded entity?
World J Surg. 21:15–21. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Michels JJ, Jacques M, Henry-Amar M and
Bardet S: Prevalence and prognostic significance of tall cell
variant of papillary thyroid carcinoma. Hum Pathol. 38:212–219.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bernstein J, Virk RK, Hui P, Prasad A,
Westra WH, Tallini G, Adeniran AJ, Udelsman R, Sasaki CT, Roman SA,
et al: Tall cell variant of papillary thyroid microcarcinoma:
Clinicopathologic features with BRAF(V600E) mutational analysis.
Thyroid. 23:1525–1531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gilliland FD, Hunt WC, Morris DM and Key
CR: Prognostic factors for thyroid carcinoma. A population-based
study of 15,698 cases from the Surveillance, Epidemiology and End
Results (SEER) program 1973–1991. Cancer. 79:564–573. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kilfoy BA, Devesa SS, Ward MH, Zhang Y,
Rosenberg PS, Holford TR and Anderson WF: Gender is an age-specific
effect modifier for papillary cancers of the thyroid gland. Cancer
Epidemiol Biomarkers Prev. 18:1092–1100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soares P, Celestino R, Melo M, Fonseca E
and Sobrinho-Simões M: Prognostic biomarkers in thyroid cancer.
Virchows Arch. 464:333–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hay ID, Thompson GB, Grant CS, Bergstralh
EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL,
et al: Papillary thyroid carcinoma managed at the Mayo Clinic
during six decades (1940–1999): Temporal trends in initial therapy
and long-term outcome in 2444 consecutively treated patients. World
J Surg. 26:879–885. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Erler P, Keutgen XM, Crowley MJ, Zetoune
T, Kundel A, Kleiman D, Beninato T, Scognamiglio T, Elemento O,
Zarnegar R and Fahey TJ III: Dicer expression and microRNA
dysregulation associate with aggressive features in thyroid cancer.
Surgery. 156:1342–1350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sobrinho-Simões M, Máximo V, Rocha AS,
Trovisco V, Castro P, Preto A, Lima J and Soares P: Intragenic
mutations in thyroid cancer. Endocrinol Metab Clin North Am.
37:333–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tavares C, Melo M, Cameselle-Teijeiro JM,
Soares P and Sobrinho-Simões M: ENDOCRINE TUMOURS: Genetic
predictors of thyroid cancer outcome. Eur J Endocrinol.
174:R117–R126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xing M, Alzahrani AS, Carson KA, Viola D,
Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, et al:
Association between BRAF V600E mutation and mortality in patients
with papillary thyroid cancer. JAMA. 309:1493–1501. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li F, Chen G, Sheng C, Gusdon AM, Huang Y,
Lv Z, Xu H, Xing M and Qu S: BRAFV600E mutation in papillary
thyroid microcarcinoma: A meta-analysis. Endocr Relat Cancer.
22:159–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin L, Chen E, Dong S, Cai Y, Zhang X,
Zhou Y, Zeng R, Yang F, Pan C, Liu Y, et al: BRAF and TERT promoter
mutations in the aggressiveness of papillary thyroid carcinoma: A
study of 653 patients. Oncotarget. 7:18346–18355. 2016.PubMed/NCBI
|
|
38
|
Virk RK, Van Dyke AL, Finkelstein A,
Prasad A, Gibson J, Hui P, Theoharis CG, Carling T, Roman SA, Sosa
JA, et al: BRAFV600E mutation in papillary thyroid microcarcinoma:
A genotype-phenotype correlation. Mod Pathol. 26:62–70. 2013.
View Article : Google Scholar : PubMed/NCBI
|