Introduction
Liver cancer is the sixth most common type of cancer
and the second-leading cause of cancer-associated mortality
worldwide (1). As estimated by the
World Health Organization, 782,000 people are diagnosed with liver
cancer and 521,000 liver cancer-associated mortalities were
reported globally in 2012 (1).
Hepatocellular carcinoma (HCC) is the most common form of primary
liver cancer, accounting for 70–85% of cases (2–4). In
general, the majority of patients are diagnosed when HCC progresses
to the middle to late stages. Despite providing several effective
therapies, including transarterial chemoembolization,
radioembolization, percutaneous ethanol injection, ablation and
chemotherapy, the 5-year survival rate is only 17–34% in these
patients (5–9). In addition, HCC is associated with
chronic hepatitis infection, chronic alcohol consumption and
non-alcoholic fatty liver disease (3,4). However,
the underlying molecular pathogenesis has not yet been completely
elucidated.
MicroRNAs (miRNAs) are a group of small,
evolutionarily conserved, non-coding RNA molecules, which
negatively regulate the expression of genes by interacting with 3′
untranslated regions of targeted mRNA. miRNAs are involved in
numerous biological processes, including cell proliferation,
differentiation, apoptosis and metabolism (10,11). The
misregulation of miRNAs is often associated with various human
diseases, ranging from inflammatory disorders to cancers (12–14).
Presently, it is established that >80 miRNAs are involved in the
regulation of tumorigenesis and metastasis signaling networks that
cause HCC (3). In patients diagnosed
with HCC, miR-199a is downregulated (15–17), while
miR-21 and miR-221 are upregulated (16–19).
Presently, traditional gene transfection is
generally mediated by viral vectors or non-viral vectors. However,
due to the security of viral vectors and the low transfection
efficiency of non-viral vectors, additional application of these
vectors is limited (20). Ultrasound
microbubbles are nanobubbles with good biological compatibility and
stability (20). The ultrasound
images are enhanced using ultrasound contrast agents. Furthermore,
microbubbles are used in non-invasive gene/drug delivery systems
(20). Compared with traditional
transfection vectors, ultrasound microbubbles have the advantages
of high safety, stability and transfection efficiency (20). Ultrasound microbubbles have been
widely used to investigate the functions of genes and miRNA
(21–25).
The present study aimed to optimize the parameters
of ultrasound microbubbles, which mediate the transfection of miRNA
into the human hepatoma HepG2 cell. In addition, the effects of
anti-miR-21, anti-miR-221 and miR-199a on HepG2 were also
investigated. In the present study, anti-miR-21, anti-miR-221 and
miR-199a were transfected with ultrasound microbubbles.
Materials and methods
Plasmid construction
The experimental protocol was established according
to the ethical guidelines of the Helsinki Declaration and was
approved by the Human Ethics Committee of Guangzhou Red Cross
Hospital (Guangdong, China). Written informed consent was obtained
from individual patients.
Sequences of anti-hsa-miR-21-5p, anti-hsa-miR-221-3p
and hsa-mir-199a-1 were synthesized (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and inserted into BamHI
and HindIII sites of the GV249 vector [a vector containing
enhanced green fluorescent protein (EGFP); Shanghai Jikai
Communication Technology Co., Ltd., Shanghai, China]. The
recombinant plasmids were named EGFP-anti-miR21, EGFP-anti-miR221
and EGFP-miR199a. These plasmids were confirmed by commercial
sequencing (Invitrogen; Thermo Fisher Scientific, Inc.) using an
ABI3730XL capillary sequencer. The primers used for cloning were as
follows: miR-21 sense, 5′-AGCTAAAAATAGCTTATCAGACTGATGTTGAG-3′ and
antisense, 5′-GATCCTCAACATCAGTCTGATAAGCTATTTTT-3′; miR-221 sense,
5′-AGCTAAAAAAGCTACATTGTCTGCTGGGTTTCG-3′ and antisense,
5′-GATCCGAAACCCAGCAGACAATGTAGCTTTTTT-3′; and miR-199a sense,
5′-TGGGATCCGGAAGAGTGGTGGTTTCCTTG-3′ and antisense,
5′-ACCGAAGCTTAAAAAAAATCTTCTATGCGAGGCTCTG-3′.
Cells
The human hepatoma HepG2 cell line (Dongguang BioJet
Biotechnology Co., Ltd., Guangdong, China) was maintained in
Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) using 10% fetal bovine serum (FBS;
HyClone; GE Healthcare Life Sciences) at 37°C in an atmosphere
containing 5% CO2. The cells were routinely passaged
every 1–2 days.
Preparation and optimization of the
concentration of microbubbles
Two ultrasound microbubble contrast agents, sulfur
hexafluoride (SF6; Bracco Suisse SA, Manno, Switzerland) and
perfluoropropane (C3F8; Kanrun Technology Co., Ltd., Hunan, China),
were used in the present study. Microbubbles were prepared
according to the manufacturer's protocol. To prepare SF6, 5 ml of
saline solution was injected into a vial containing freeze-dried
powder. The vial was then agitated until the powder was completely
dissolved in the saline solution. The suspension was used within 6
h. To prepare C3F8, perfluoropropane-albumin microsphere injection
was performed.
A total of 10,000 cells were seeded onto a 96-well
plate 24 h prior to subjecting the cells to treatment. Prior to
conducting the treatment, cells were divided into 10 groups: Blank
control (no cells); negative control (cells without treatment); SF6
treatment group (four subgroups treated with 1, 5, 10 and 20% SF6);
and C3F8 treatment group (four subgroups treated with 1, 5, 10 and
20% C3F8). Contrast agents were suspended in DMEM containing 10%
FBS. The culture was maintained for 48 h at 37°C, and subsequently,
a MTT assay was performed. Each treatment was performed in 6 wells,
and the experiment was performed in triplicate.
MTT assay
MTT (20 µl; 5 mg/ml dissolved in PBS; Genview;
Sigma-Aldrich; EMD Millipore, Billerica, MA, USA) was added to the
well, and the cell culture was incubated for 4 h at 37°C. The
culture medium mixture was then discarded, and the cells were
dissolved in 150 µl dimethyl sulfoxide for 10 min. The absorbance
of the sample was determined at 490 nm on a Microplate
Spectrophotometer (BioTek Elx800; BioTek Instruments, Inc.,
Winooski, VT, USA). Controls were a blank control (no cells) and a
negative control (untreated cells).
Optimization of ultrasound
microbubble-mediated transfection
Cells were seeded on a 6-well plate and subjected to
transfection until 70–80% confluency was attained. Prior to
subjecting the cells to treatments, cells were divided into 6
groups: Empty control (no treatment); negative control (plasmid);
positive control [plasmid + liposome (Lipofectamine®
2000, Invitrogen; Thermo Fisher Scientific, Inc.)]; ultrasound
control (plasmid + ultrasound exposure); ultrasound SF6
microbubbles (plasmid + ultrasound exposure + SF6); and ultrasound
C3F8 microbubbles (plasmid + ultrasound exposure + C3F8). The
latter three groups, which were treated with ultrasound, were
divided into 4 subgroups according to their different ultrasound
parameters: 2.0 MHz and MI, 0.12; 2.0 MHz and MI, 0.20; 2.0 MHz and
MI, 0.28; and 2.0 MHz and MI, 0.35. Prior to treatment, cells were
rinsed with DMEM. Ultrasound treatment was then provided with
different parameters to the latter 3 groups for 30 sec. Following
transfection, cells were maintained in DMEM. To this medium, 10%
FBS was added 6–8 h post-transfection. Lipofectamine 2000-mediated
gene transfection was performed according to the manufacturer's
protocol, as described previously (23). Following 48 h transfection, cells were
subjected to fluorescence microscopy and flow cytometry. Each
treatment was performed in 3 wells, and the experiment was
performed in triplicate. While performing fluorescence microscopy,
EGFP-positive cells and total cells of each group were recorded in
9 fields under a magnification of ×200 (Zeiss GmbH, Jena, Germany).
Thereafter, transfection efficiency was calculated using the
following formula: Transfection efficiency (%) = (positive cells /
total cells) × 100.
Flow cytometry analysis was performed using
FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA). This
procedure was performed at an excitation wavelength of 488 nm and
an emission wavelength of 530±15 nm.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Cells were transfected with three recombinant
plasmids (EGFP-anti-miR21, EGFP-anti-miR221 and EGFP-miR199a) and
vector plasmid (GV249) through ultrasound microbubble SF6. Cells
were then collected 48 h post-transfection. Cells that were not
transfected were set as a blank control. Total RNA was extracted
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol.
RT-qPCR was performed using the reverse Tra Ace
RT-qPCR kit (Toyobo Co., Ltd., Osaka, Japan) according to the
manufacturer's protocol. The primers for miR-21, miR-221, miR-199
(as aforementioned for plasmid construction) and the internal U6
control (sense, 5′-TCGCTTCGGCAGCACA-3′ and antisense,
5′-AACGCTTCACGAATTTGCGT-3′) were obtained from Ruibo Bio-Technology
Co., Ltd. (Shanghai, China).
Cell cycle and apoptosis assay
Cells were transfected with three recombinant
plasmids (EGFP-anti-miR21, EGFP-anti-miR221 and EGFP-miR199a) and
vector plasmid (GV249) through ultrasound microbubble SF6. Cells
were then collected 48 h post-transfection. Cells that were not
subjected to transfection were set as the blank control. Cells were
stained with propidium iodide and subjected to cell cycle analysis
on FACSCalibur. The apoptosis of cells was determined using an
Annexin V-PE/7-AAD apoptosis detection kit (Beijing Bioco Laibo
Technology Co., Ltd., Beijing, China) according to the
manufacturer's protocol. Flow cytometry was performed using
FACSCalibur.
Statistical analysis
The data were analyzed using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA). Continuous data was expressed as the mean ±
standard deviation. For group comparisons, the homogeneity of
variance was first tested using the Levene test. To compare equal
variances, one-way analysis of variance (ANOVA) was performed,
followed by Fisher's least significant difference post-hoc test. To
compare unequal variances, one-way ANOVA modified with Welch or
Brown-Forsythe tests was performed, followed by Dunnett's T3 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Optimization of the concentration of
contrast agent microbubbles and ultrasound parameters
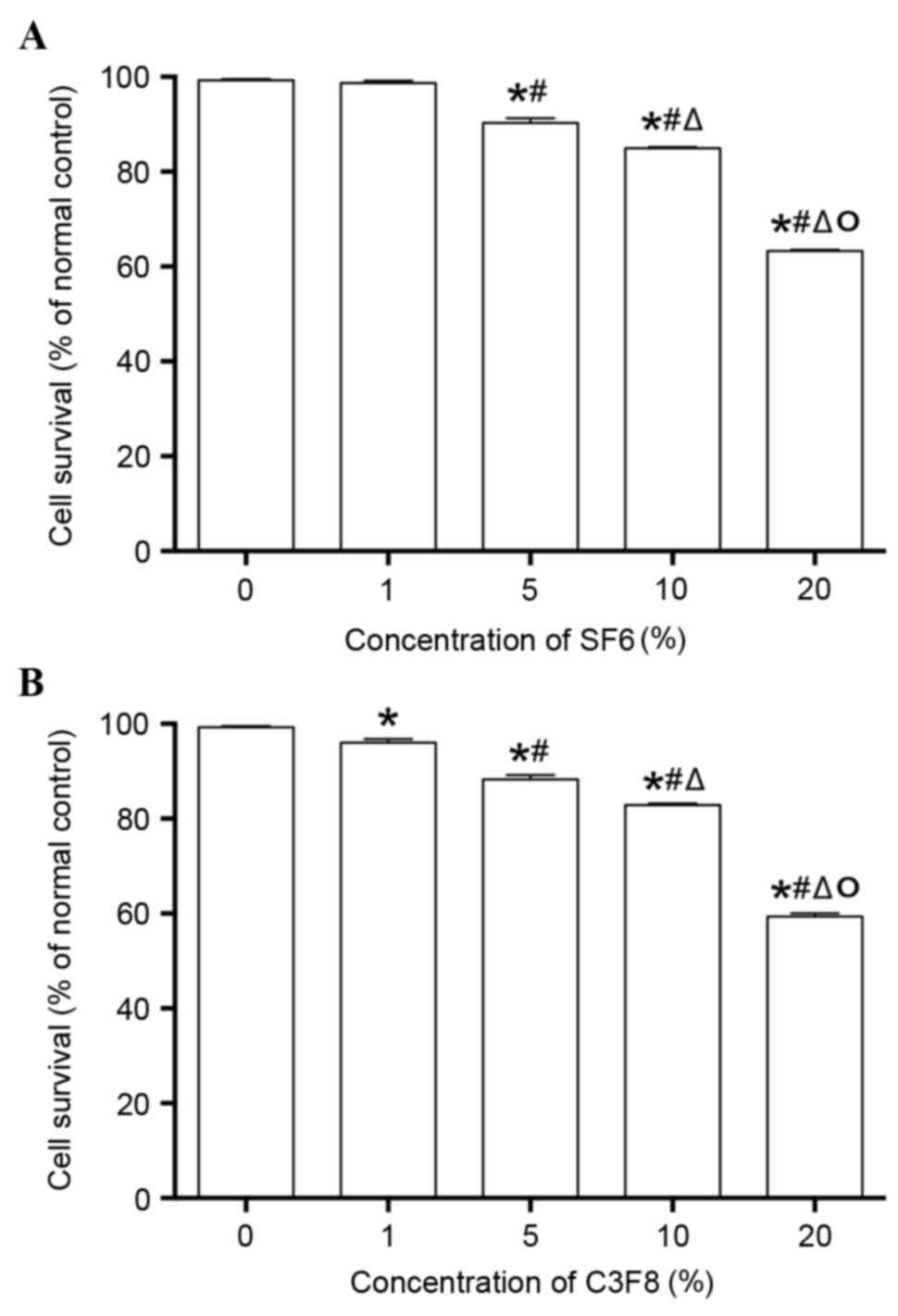
HepG2 cells were treated with different
concentrations of ultrasound contrast agent microbubbles, and the
cytotoxicity of microbubbles was detected. The results are shown in
Fig. 1. The two contrast agents
exhibited similar cytotoxicity. When the concentration of the two
contrast agents was increased, cell activity decreased
significantly (P<0.05). When the concentration of the contrast
agents was <10%, no evident cytotoxicity was observed in the two
agents as the cell viability was >80%. However, 20% of
microbubble agents exhibited evident cytotoxicity when the cell
viability was <65%. A previous study established that
transfection efficiency was positively associated with the
concentration of ultrasound microbubble agents when the
microbubbles did not affect the proliferation of cells (9). Therefore, it was ensured that the
concentration of contrast agents was 10% in the subsequent
experiments.
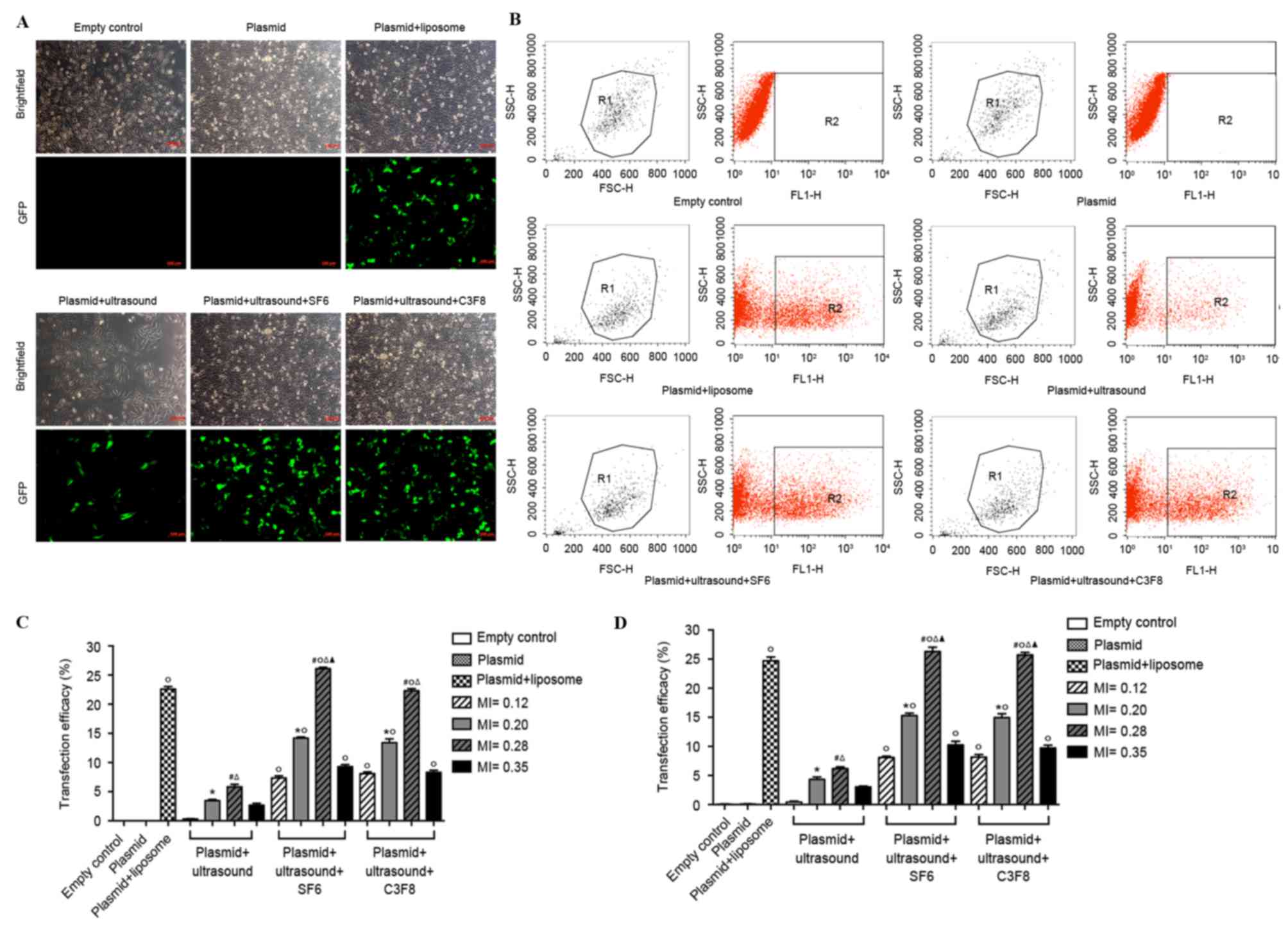
The cells were transfected with EGFP-miR-199a using
two ultrasound contrast agent microbubbles with different
mechanical indexes (MIs; 0.12, 0.2, 0.28 and 0.35). Thereafter,
transfection efficiency was determined using fluorescence
microscopy and flow cytometry. Similar trends were observed in the
data obtained from the two assays. Compared with ultrasound-treated
cells, the two ultrasound contrast agent microbubbles significantly
improved transfection efficiency (P<0.05; Fig. 2). When MI was increased, transfection
efficiency increased initially, and then decreased.
Transfection efficiency was the highest when MI was
0.28. The transfection efficiency in the ultrasound SF6 microbubble
group was a little higher than in the ultrasound C3F8 microbubble
group but this did not reach statistical significance. However, no
statistically significant difference was observed between the
transfection efficiency of the ultrasound SF6 microbubble group and
the C3F8 microbubble group (P>0.05). In the ultrasound SF6
microbubble group, transfection efficiency was highest when MI was
0.28, which was significantly increased compared with that of the
positive control group (plasmid + liposome; 26.31±0.72% vs.
24.70±0.67%; P<0.05). Therefore, in the remaining experiments,
ultrasound SF6 microbubbles with an MI of 0.28 were used.
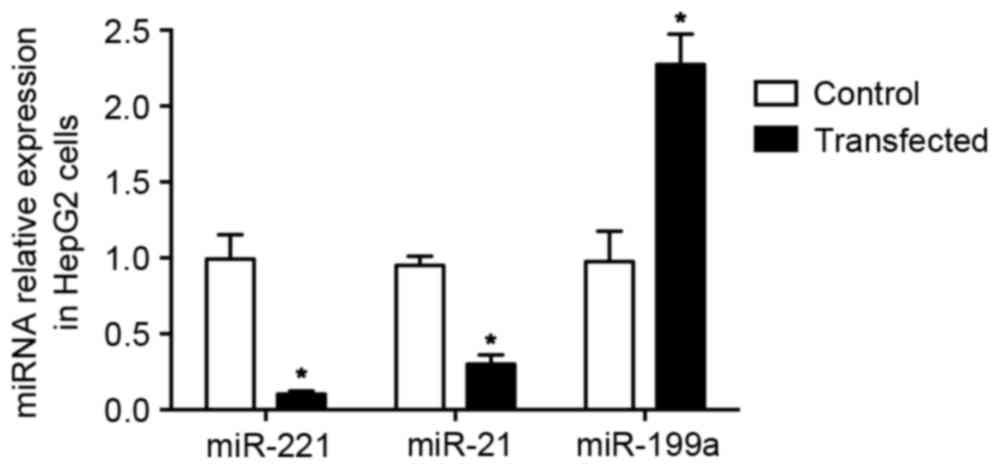
miRNA expression in cells transfected
with recombinant plasmids through ultrasound SF6 microbubbles
The three recombinant plasmids were transfected into
HepG2 cells using ultrasound SF6 microbubbles. The expression of
miR-21, miR-221 and miR-199a was determined using RT-qPCR.
Following transfection, miR-21 and miR-221 were significantly
downregulated, while miR-199a was significantly upregulated
compared with the negative control (P<0.05; Fig. 3).
Anti-miRNA-21/221 and miRNA-199a
induce apoptosis of HepG2 cells
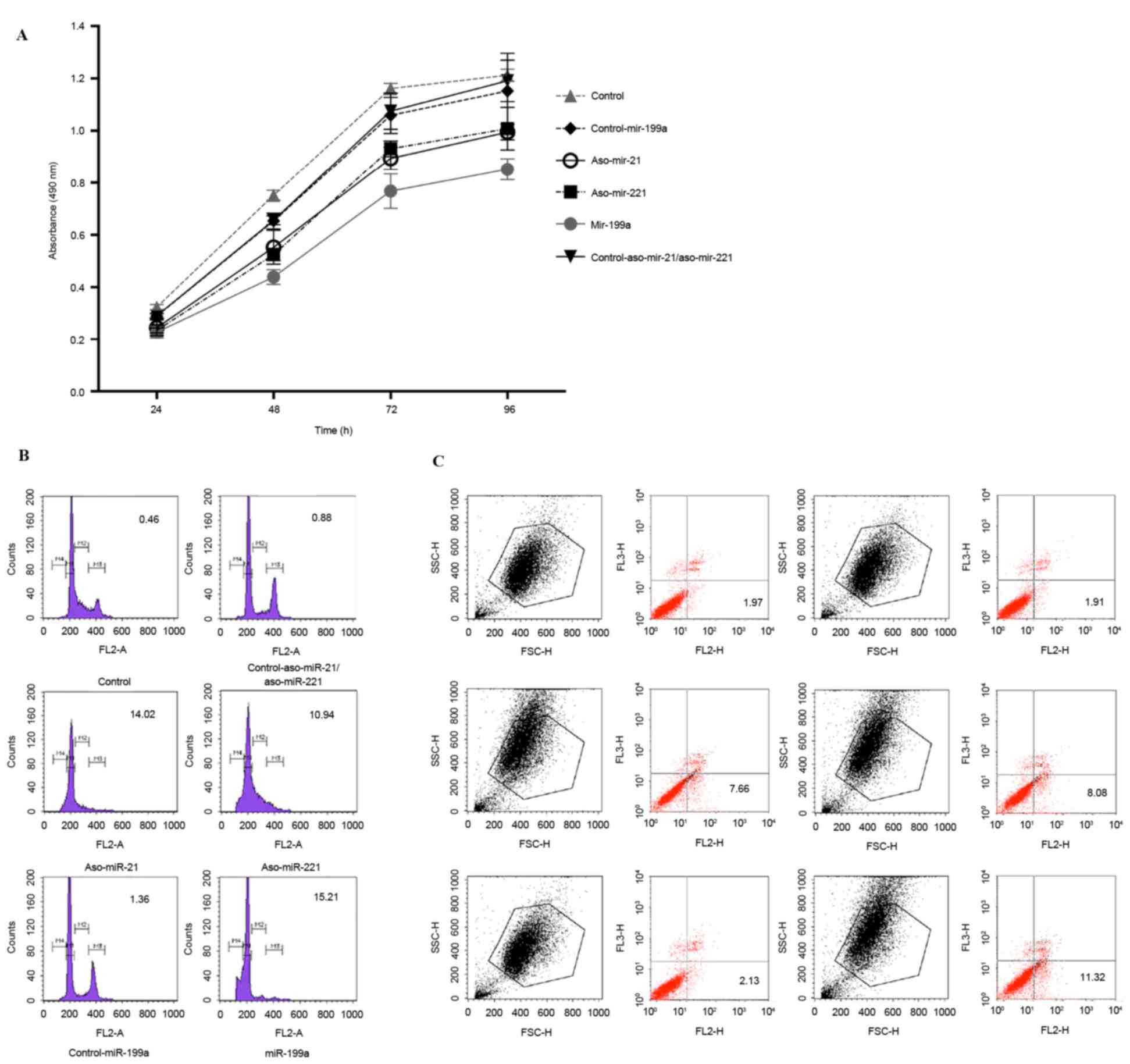
The impact of anti-miRNA-21/221 and miRNA-199a on
HepG2 cells was determined in the present study. First, cell
proliferation was detected using MTT at different time points (24,
48, 72 and 96 h). Compared with the negative control, the growth of
the transfected cells was significantly inhibited (P<0.05;
Fig. 4A). Compared with
anti-miR-21/miR-221, miR-199a exhibited the most significant
inhibition (P<0.05).
The cell cycle of transfected cells was analyzed.
While performing flow cytometry, the cell cycle was divided into
four stages: M1, cells in G0/G1 phase with diploid DNA; M2, cells
whose DNA lies between the diploid and tetraploid stages; M3, cells
in the S phase with tetraplid DNA; and M4, cells that have
undergone apoptosis. In the M4 stage, the cell percentage was 0.46%
in the blank group and 2% in the negative control. In comparative
terms, the cell percentage in the M4 stage was significantly
increased in anti-miR-21, anti-miR-221 and miR-199a-transfected
groups (all P<0.05; Fig. 4B). The
percentage in miR-199a-transfected cells was the highest. These
data revealed that treatment with anti-miR-21, anti-miR-221 and
miR-199a induces apoptosis of HepG2 cells.
Furthermore, the apoptosis of transfected cells was
confirmed by performing an Annexin V-PE/7-AAD double staining
assay. Compared with negative controls, the apoptotic rate was
significantly increased in anti-miR-21, anti-miR-221 and
miR-199a-transfected groups (P<0.05; Fig. 4C; all >7% vs. <3%). In
miR-199a-transfected cells, the apoptotic rate was highest at
11.10±0.46%. Thus, the apoptotic rate was statistically increased
compared with that observed in two anti-miRNA-transfected groups
(P<0.05).
Discussion
miRNAs are involved in the occurrence, development
and prognosis of cancers, making them a promising target for cancer
gene therapy (12,26). Previous studies demonstrated that
numerous miRNAs are involved in the pathogenesis of HCC
(3–4,15-17). Gene therapy, particularly miRNA-targeted therapy, is
a promising candidate in the treatment of cancer, including HCC.
However, this area of study is hampered by the shortage of
available delivery vectors (27,28). The
conventional viral vectors are marred by safety problems, while
non-viral vectors have a major drawback of low transfection
efficiency (27,28). Previous studies established that under
ultrasound exposure, contrast agent microbubbles improve
transfection efficiency and the expression of DNA in local tissue
or cells (25,29,30). The
present results also indicated that ultrasound contrast agent
microbubbles significantly improved the transfection efficiency of
DNA (P<0.05).
In general, transfection efficiency that is mediated
by ultrasound microbubbles is affected by the following parameters:
Ultrasound exposure condition; type and concentration of
microbubbles; and cell types (20).
Therefore, it is important to optimize the conditions of ultrasound
intensity and concentration of microbubbles in gene delivery
systems that are mediated by ultrasound microbubbles. In HepG2
cells, to the best of our knowledge, previous studies have not
identified DNA transfection that is mediated by ultrasound
microbubbles. Therefore, the present study first optimized the
concentration of contrast agent microbubbles and ultrasound
intensity. In the present study, two contrast agents, SF6 and C3F8,
were used. The present data indicated that the two agents did not
exhibit any marked cytotoxicity when the concentration was <10%.
When the ultrasound intensity was 2.0 MHz, the MI was 0.28, and the
transfection efficiency was significantly increased compared with
that of lipofection-mediated transfection. In addition, SF6 and
C3F8 exhibited high transfection efficiency. This indicated that
two ultrasound microbubbles were efficacious gene delivery vectors.
Since SF6 microbubbles exhibited the highest transfection
efficiency, 10% of SF6 microbubbles were applied at an ultrasound
frequency of 2.0 MHz (MI, 0.28) in order to determine the effect of
miR-21, miR-221 and miR-199a on HepG2 cells.
Using ultrasound microbubble-mediated transfection,
anti-miR-21/miR-221 and miR-199a were revealed to inhibit cell
proliferation and induce cell apoptosis in HepG2 cells. Previous
studies have established that miR-21 was overexpressed in numerous
cancers, including HCC, breast cancer, cervical cancer, lung
cancer, colon cancer, adenocarcinoma and glioma (31–33).
Furthermore, miR-21 was involved in the proliferation of tumor
cells, invasion of tumor vascular phase and tumor staging (31–33). These
previous studies indicated that miR-21 acts as an oncogene,
promoting the occurrence and progression of tumors. The present
study demonstrated that anti-miR-21 inhibited the proliferation and
induced apoptosis of hepatoma cells. However, miR-21 was reported
to target the suppressor gene phosphatase and tensin homolog
(PTEN), thereby negatively regulating programmed cell death factor
4 (PDCD4) (34). In the present
study, with an increase in the expression of anti-miR-21, the
expression of miR-21 decreased. As a result, there was upregulation
in the expression of PTEN and PDCD4, and the apoptosis of HepG2
cells was induced. Previous studies revealed that miR-221 affects
several tumorigenic pathways in the early stages (35–38). An
overexpression of miR-221 was associated with the invasion
phenotype in patients with HCC, while miR-21 inhibited apoptosis by
targeting B-cell lymphoma-2 modifying factor (BMF) (35,36). A
number of studies indicated that miR-221 was negatively associated
with cyclin dependent kinase inhibitors (CDKN1B/p27 and DKN1C/p57)
in patients diagnosed with HCC (37,38).
miR-221 initiates tumorigenesis by targeting p27 and p57. Thus,
cell proliferation is promoted to target BMF and inhibit apoptosis.
The present data also revealed that anti-miR-221 inhibits cell
proliferation and promotes cell apoptosis. With the expression of
anti-miR-21, the expression of miR-21 decreased. Consequently,
there was upregulation in the expression of CDKN1B/p27 and
DKN1C/p57, while the expression of BMF was downregulated. Together,
these events led to the apoptosis of HepG2 cells.
In the present study, miR-199a was downregulated in
patients diagnosed with HCC, leading to poor prognosis of patients
diagnosed with HCC (39). In the
present study, miR-199a inhibited cell proliferation to the
greatest extent. It was reported that miR-199a inhibits the
proliferation of hepatoma cells by targeting hypoxia-inducible
factor-1a and cluster of differentiation 44, as well as by
regulating the cell cycle. In addition, miR-199a upregulates
CDKNlB/p27 and CDKN1A/p21 to inhibit the progression of the cell
cycle, thereby inducing apoptosis (40).
In conclusion, the parameters for ultrasound SF6
microbubble-mediated gene delivery were optimized. The conditions
for the transfection of recombinant plasmids were then optimized,
which contained anti-miR-21, anti-miR-221 and miR-199a. Finally, it
was identified that anti-miR-21/miR-221 and miR-199a inhibited cell
proliferation and induced cell apoptosis in HepG2 cells. This
indicated that these three miRNAs may be novel gene therapy
targets.
References
|
1
|
Stewart BW and Wild CP: World Cancer
Report 2014. World Health Organization. (In Press). 2014.
|
|
2
|
Sun J, Lu H, Wang X and Jin H: MicroRNAs
in hepatocellular carcinoma: Regulation, function, and clinical
implications. ScientificWorldJournal. 2013:9242062013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang N, Ekanem NR, Sakyi CA and Ray SD:
Hepatocellular carcinoma and microRNA: New perspectives on
therapeutics and diagnostics. Adv Drug Deliv Rev. 81:62–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hung CH, Chiu YC, Chen CH and Hu TH:
MicroRNAs in hepatocellular carcinoma: Carcinogenesis, progression,
and therapeutic target. Biomed Res Int. 2014:4864072014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bellissimo F, Pinzone MR, Cacopardo B and
Nunnari G: Diagnostic and therapeutic management of hepatocellular
carcinoma. World J Gastroenterol. 21:12003–12021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gomaa AI and Waked I: Recent advances in
multidisciplinary management of hepatocellular carcinoma. World J
Hepatol. 7:673–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang XD, Pan LH, Wang L, Ke Y, Cao J, Yang
C, Zhong JH, Luo W, Guo J and Li LQ: Systematic review of single
large and/or multinodular hepatocellular carcinoma: Surgical
resection improves survival. Asian Pac J Cancer Prev. 16:5541–5547.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kudo M: Surveillance, diagnosis,
treatment, and outcome of liver cancer in Japan. Liver Cancer.
4:39–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim KC, Chow PK, Allen JC, Siddiqui FJ,
Chan ES and Tan SB: Systematic review of outcomes of liver
resection for early hepatocellular carcinoma within the Milan
criteria. Br J Surg. 99:1622–1629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiggins JF, Ruffino L, Kelnar K, Omotola
M, Patrawala L, Brown D and Bader AG: Development of a lung cancer
therapeutic based on the tumor suppressor microRNA-34. Cancer Res.
70:5923–5930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raisch J, Darfeuille-Michaud A and Nguyen
HT: Role of microRNAs in the immune system, inflammation and
cancer. World J Gastroenterol. 19:2985–2996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vettori S, Gay S and Distler O: Role of
MicroRNAs in Fibrosis. Open Rheumatol J. 6:130–139. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murakami Y, Yasuda T, Saigo K, Urashima T,
Toyoda H, Okanoue T and Shimotohno K: Comprehensive analysis of
microRNA expression patterns in hepatocellular carcinoma and
non-tumorous tissues. Oncogene. 25:2537–2545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang J, Gusev Y, Aderca I, Mettler TA,
Nagorney DM, Brackett DJ, Roberts LR and Schmittgen TD: Association
of MicroRNA expression in hepatocellular carcinomas with hepatitis
infection, cirrhosis, and patient survival. Clin Cancer Res.
14:419–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang XH, Wang Q, Chen JS, Fu XH, Chen XL,
Chen LZ, Li W, Bi J, Zhang LJ, Fu Q, et al: Bead-based microarray
analysis of microRNA expression in hepatocellular carcinoma:
miR-338 is downregulated. Hepatol Res. 39:786–794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Connolly E, Melegari M, Landgraf P,
Tchaikovskaya T, Tennant BC, Slagle BL, Rogler LE, Zavolan M,
Tuschl T and Rogler CE: Elevated expression of the miR-17-92
polycistron and miR-21 in hepadnavirus-associated hepatocellular
carcinoma contributes to the malignant phenotype. Am J Pathol.
173:856–864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ladeiro Y, Couchy G, Balabaud C,
Bioulac-Sage P, Pelletier L, Rebouissou S and Zucman-Rossi J:
MicroRNA profiling in hepatocellular tumors is associated with
clinical features and oncogene/tumor suppressor gene mutations.
Hepatology. 47:1955–1963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki R, Oda Y, Utoguchi N and Maruyama
K: Progress in the development of ultrasound-mediated gene delivery
systems utilizing nano- and microbubbles. J Control Release.
149:36–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan C, Zhu D, Huang D and Xia G: Role of
ultrasound and microbubble-mediated heat shock protein 72 siRNA on
ischemia-reperfusion liver injury in rat. Int J Clin Exp Med.
8:5746–5752. 2015.PubMed/NCBI
|
|
22
|
Chen Z, Liang K, Xie M, Wang X, Lu Q and
Zhang J: Novel ultrasound-targeted microbubble destruction mediated
short hairpin RNA plasmid transfection targeting survivin inhibits
gene expression and induces apoptosis of HeLa cells. Mol Biol Rep.
36:2059–2067. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kimura S, Egashira K, Chen L, Nakano K,
Iwata E, Miyagawa M, Tsujimoto H, Hara K, Morishita R, Sueishi K,
et al: Nanoparticle-mediated delivery of nuclear factor kappaB
decoy into lungs ameliorates monocrotaline-induced pulmonary
arterial hypertension. Hypertension. 53:877–883. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fattal E and Barratt G: Nanotechnologies
and controlled release systems for the delivery of antisense
oligonucleotides and small interfering RNA. Br J Pharmacol.
157:179–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen ZP, Brayman AA, Chen L and Miao CH:
Ultrasound with microbubbles enhances gene expression of plasmid
DNA in the liver via intraportal delivery. Gene Ther. 15:1147–1155.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia XQ, Cheng HQ, Qian X, Bian CX, Shi ZM,
Zhang JP, Jiang BH and Feng ZQ: Lentivirus-mediated overexpression
of microRNA-199a inhibits cell proliferation of human
hepatocellular carcinoma. Cell Biochem Biophys. 62:237–244. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verma IM and Somia N: Gene
therapy-promises, problems and prospects. Nature. 389:239–242.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomanin R and Scarpa M: Why do we need new
gene therapy viral vectors? Characteristics, limitations and future
perspectives of viral vector transduction. Curr Gene Ther.
4:357–372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sonoda S, Tachibana K, Uchino E, Okubo A,
Yamamoto M, Sakoda K, Hisatomi T, Sonoda KH, Negishi Y, Izumi Y, et
al: Gene transfer to corneal epithelium and keratocytes mediated by
ultrasound with microbubbles. Invest Ophthalmol Vis Sci.
47:558–564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bekeredjian R, Bohris C, Hansen A, Katus
HA, Kuecherer HF and Hardt SE: Impact of microbubbles on shock
wave-mediated DNA uptake in cells in vitro. Ultrasound Med Biol.
33:743–750. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang Y, Yang YB, Zhang XH, Yu XL, Wang ZB
and Cheng XC: MicroRNA-21 gene and cancer. Med Oncol. 30:3762013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gramantieri L, Fornari F, Ferracin M,
Veronese A, Sabbioni S, Calin GA, Grazi GL, Croce CM, Bolondi L and
Negrini M: MicroRNA-221 targets Bmf in hepatocellular carcinoma and
correlates with tumor multifocality. Clin Cancer Res. 15:5073–5081.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Turato C, Simonato D, Quarta S, Gatta A
and Pontisso P: MicroRNAs and SerpinB3 in hepatocellular carcinoma.
Life Sci. 100:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fu X, Wang Q, Chen J, Huang X, Chen X, Cao
L, Tan H, Li W, Zhang L, Bi J, et al: Clinical significance of
miR-221 and its inverse correlation with p27Kip¹ in hepatocellular
carcinoma. Mol Biol Rep. 38:3029–3035. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fornari F, Gramantieri L, Ferracin M,
Veronese A, Sabbioni S, Calin GA, Grazi GL, Giovannini C, Croce CM,
Bolondi L and Negrini M: MiR-221 controls CDKN1C/p57 and CDKN1B/p27
expression in human hepatocellular carcinoma. Oncogene.
27:5651–5661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong
Q, Qin L, Wu X, Zheng Y, Yang Y, et al: Identification of miRNomes
in human liver and hepatocellular carcinoma reveals miR-199a/b-3p
as therapeutic target for hepatocellular carcinoma. Cancer Cell.
19:232–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fornari F, Milazzo M, Chieco P, Negrini M,
Calin GA, Grazi GL, Pollutri D, Croce CM, Bolondi L and Gramantieri
L: MiR-199a-3p regulates mTOR and c-Met to influence the
doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res.
70:5184–5193. 2010. View Article : Google Scholar : PubMed/NCBI
|