Introduction
The major role of vitamin D in vertebrate animals
and humans is to increase calcium and phosphate absorption for the
mineralization of the skeleton. The most important source of this
vitamin for individuals living at mid and low latitudes is vitamin
D synthesis induced by solar ultraviolet-B (UVB) radiation
(1). The recommended daily allowance
(RDA) for vitamin D in the United States is 200 IU (5.0 µg), which
is reasonable for adults who receive some sun exposure; however, in
the absence of sun exposure, this RDA may be 2 to 3 times lower
than that actually required to satisfy what the body requires
(2). Vitamin D3 is efficiently
produced via pre-vitamin D3 from the precursor 7-dehydrocholesterol
when human skin is exposed to solar UVB (3,4).
The total volume of atmospheric ozone and cloudiness
are also important factors that control UVB radiation, and the area
of exposed skin and skin type is relevant to the amount of vitamin
D synthesis (5). At subtropical
latitudes, there is no alteration of vitamin D production during
winter. At low latitudes, vitamin D synthesis continues throughout
the winter at reduced capacity, and at high latitudes, there is no
production for 4–6 months of the year (6). Vitamin D synthesis originates from two
sources, firstly from solar or artificial UVB exposure, and
secondly from dietary products and supplements in individuals who
live at higher latitudes where solar UVB radiation is weak in
winter (3,4).
Vitamin D deficiency is commonly found in risk
groups such as postmenopausal women and individuals suffering from
rickets, osteomalacia and osteoporosis, since vitamin D is vital
for normal calcium metabolism and the maintenance of bone density
(7). Vitamin D deficiency is also
associated with an increased cardiovascular risk and arterial
stiffness (8). Furthermore, it is
associated with epidemic influenza, as solar radiation produces a
seasonal variation that profoundly affects the pathogenesis of the
disease (9,10).
Non-melanoma skin cancer (NMSC) is the most commonly
occurring cancer type in the Caucasian population. The non-melanoma
tumors may originate from squamous or basal cells. These cells in
the skin occasionally change and do not behave normally, resulting
in precancerous conditions. As a consequence, there is a high
chance that these abnormal cells will become cancerous (11). MSC is a result of the transformation
of skin melanocytes. At present, MSC is the most common tumor
diagnosed in the United States and numerous other countries, and
NMSC and MSC cases have each markedly increased in number over the
last few decades. Melanomas represent <10% of all skin cancers,
but they are responsible for the majority of skin cancer-related
mortalities due to a high metastatic potential and therapeutic
resistance (9,12,13).
Previous studies demonstrated that risk factors for cutaneous
melanoma depended upon the anatomical site and the
clinicopathological variant (14),
and that there was an inverse association between vitamin D blood
levels and cutaneous thickness melanoma at diagnosis (15).
The associations among skin type IV, the time to
induce solar erythema (TEry) and the time required to
produce 1,000 IU vitamin D (TVitD) have previously been
studied in Arica, a city located in the northern region of Chile,
close to the equator and surrounded by desert and ocean (16,17). The
association between skin cancer rates per 100,000 inhabitants and
the solar UV index (UVI) was previously studied between 2001 and
2006 in this city (18), and between
2007 and 2011 with latitude variation (19). The most common skin types in Chile
have been found to be type III for women and type IV for men
(20). The skin type is expressed as
the number of standard erythema doses (SED) according to the
Fitzpatrick skin type classification; skin type III for women (3–5
SED) induced erythema type III in Caucasian skin, whereas skin type
IV for men (4.5–6 SED) induced type IV in Mediterranean skin
(21). Vitamin D is formed mainly in
the skin upon exposure to UVB and it is taken orally with food or
through supplements (14,22–24). Since
sun exposure is a known factor for skin cancer development, the aim
of the present study was to analyze the association between NMSC
and MSC rates, and solar UVI data obtained from 6 different cities
in Chile ranging from latitude 18 to 53°S.
Materials and methods
Measurements
Solar UVI measurements were obtained using a
YES-UVB-1 UV biometer (Yankee Environmental Systems, Inc., Turners
Falls, MA, USA), according to the manufacturer's instructions
(25), in the Solar Ultraviolet
Laboratory of Tarapaca University in Arica, Chile. The data
provided by this instrument included the latitude, longitude and
altitude, as well as the ozone layer and cloudiness for each
location. This laboratory is run in agreement with and as part of
the Chilean Meteorological Organization network. This instrument is
calibrated in accordance with their recommendations. It is accepted
that solar UVI fluctuates from 1 to 11 according to the World
Meteorological Organization. An algorithm developed by McKenzie
et al (26) was used to
calculate the TVitD from experimental measurements of
solar UVI in different cities. The minimal erythema dose (MED) is
the minimum amount of UV that produces redness 24 h after exposure,
where one MED is equal to 240 J/m2 of UV doses required
to induce erythema (27), according
to the Fitzpatrick skin type classification (21). The Tery in unprotected skin
(only hands and face, i.e., 10% of the body) was calculated using
the following equation:
Tery=MED(skintype)UVery.
The amount of UV solar radiation to induce erythema
(UVery) was directly obtained from the following
equation [where the unit for UVery is milliwatt (mW)
divided by square meters (m2)]:
UVery=UVI40(mWm2).
Population data
The NMSC ratio was calculated in males and females
for each city in comparison to Punta Arenas. NMSC and MSC rates
were considered for each location and the data for skin cancer rate
normalized by patient age was obtained between 2005 and 2007 from
the Ministerio de Salud (Ministry of Health) of Chile, where open
access to cancer population data has been established by Resolution
number 23.01.2002, therefore Ethics/guidelines must be considered
in each hospital of Chile.
Statistical analysis
Statistical analysis was performed using standard
statistical criteria, and correlations were assessed by evaluating
the quotient between covariance and standard deviation for each
variable. Pearson's linear correlation was used for statistical
purposes. Statistical analysis performed using StatDisk software
(version 12.0.2; Pearson plc., London, UK). P<0.05 was
considered to indicate a statistically significant difference.
Results
The present study considered solar UVI measurements,
NMSC and MSC rates, mortality rates per 100,000 inhabitants and the
association with the time required to synthesize adequate levels of
vitamin D in different geographical locations of Chile.
TVitD was calculated to explain the association between
NMSC and MSC rates in 6 cities in Chile between latitude 18 and
53°S. The monthly averages of the TVitD were calculated
from the maximum UVI daily values obtained from Arica, Antofagasta,
Santiago, Concepción, Valdivia and Punta Arenas, with latitudes of
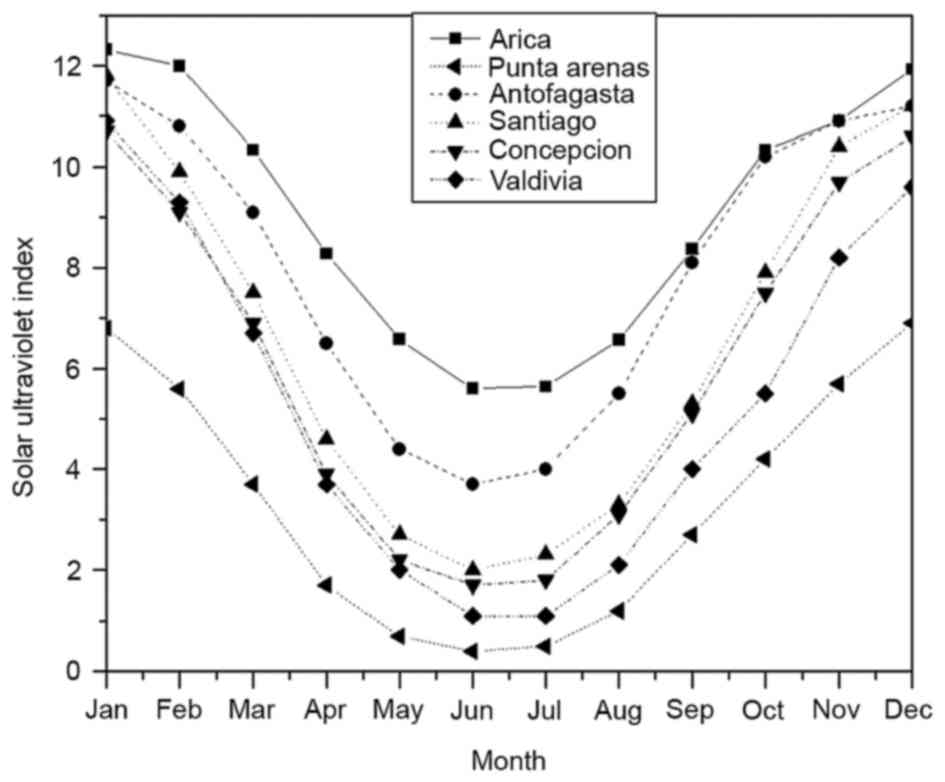
18, 23, 33, 36, 39 and 53°S, respectively. Fig. 1 shows the mean solar UVI per month
considering the maximum value for each day (at noon) and the
latitude of these cities between January and December. Solar UVI
values fluctuated from a minimum in winter (June) to a maximum in
summer (January), recorded as 5.6–12.3 in Arica, 3.7–11.7 in
Antofagasta, 2.0–11.8 in Santiago, 1.7–10.7 in Concepción, 1.1–10.9
in Valdivia and 0.4–6.9 in Punta Arenas.
Tables I–III show solar UVI data, and NMSC and MSC
rates from different geographical locations in Chile. Table I presents latitude, longitude and
altitude, and solar UVI data per year, from the following
geographical locations in Chile: Arica, Antofagasta, Santiago,
Concepción, Valdivia and Punta Arenas. Table II presents NMSC and MSC mortality
rates per 100,000 inhabitants in the same locations as
aforementioned between 1990 and 2005, while Table III presents NMSC and MSC rates in
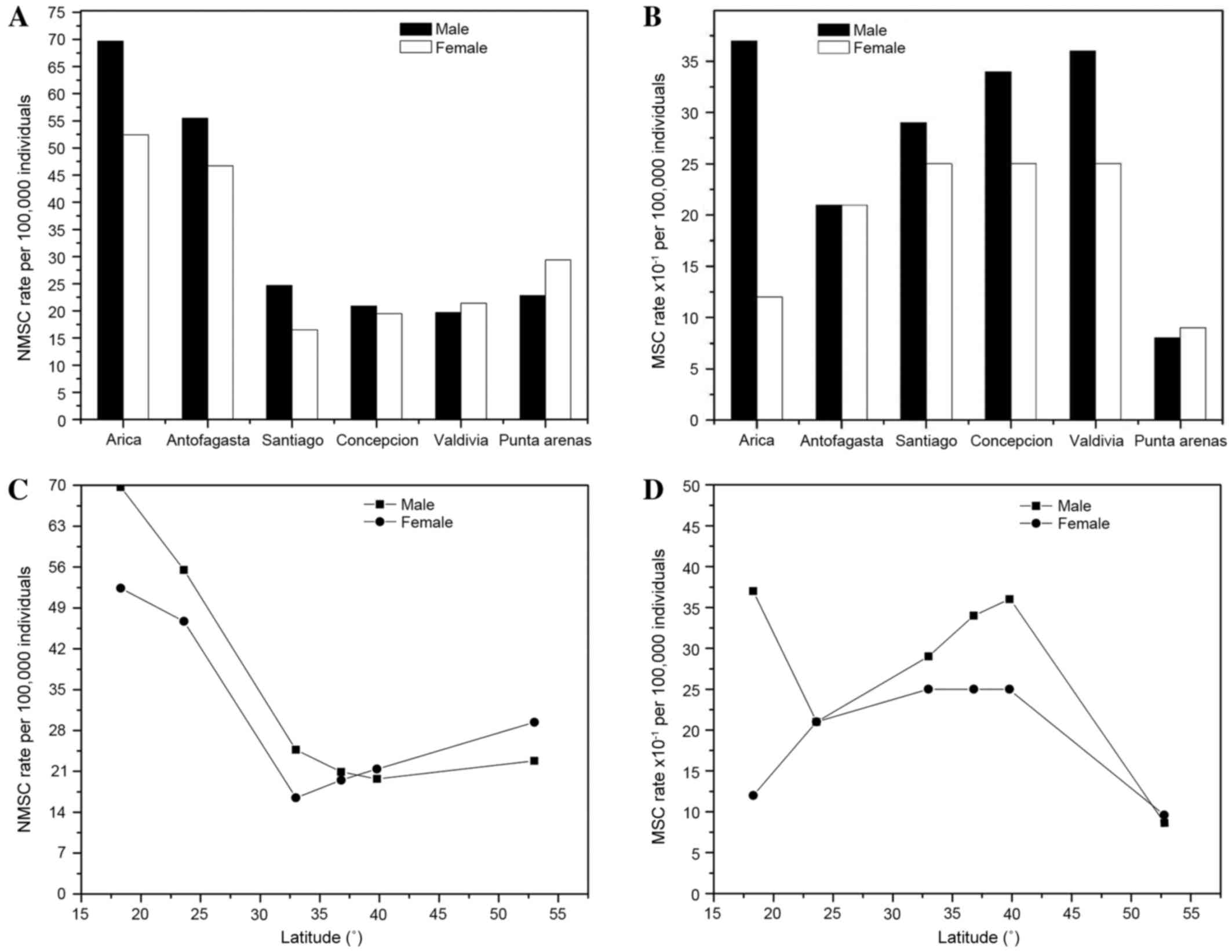
males and females in these cities between 2003 and 2007 (28). Data from Table III is also shown in Fig. 2A and B. The results indicated that the
NMSC rates in males and females were greater in the northern cities
of Chile, such as Arica and Antofagasta, compared with the rate in
cities located farther south, such as Concepción and Valdivia
(Fig. 2A). Fig. 2B shows MSC rates in males and females
from Arica to Punta Arenas; the data indicated that the rate of MSC
was lower in females than males in Arica and in cities located
farther south, such as Santiago, Concepción and Valdivia. An
inverse association was observed between NMSC and latitude in males
and females, as observed at 18°S in Arica and 36°S in Concepción
(Fig. 2C); thus rates decreased as
latitude increased. Fig. 2D shows
that the MSC rates in males and females increased as latitude
increased (23 and 40°S), with the exception of Punta Arenas (53°S),
in which the MSC rates in males and females decreased.
 | Table I.Latitude, longitude, altitude and
solar UVI data per year in different geographical locations of
Chile. |
Table I.
Latitude, longitude, altitude and
solar UVI data per year in different geographical locations of
Chile.
| City | Latitude | Longitude | Altitude, m | Solar UVI data,
years |
|---|
| Arica | 18°18′S | 70°18′W | 25 | 2006–2013 |
| Antofagasta | 23°38′S | 70°24′W | 40 | 1997–2005 |
| Santiago | 33°23′S | 70°47′W | 475 | 2004–2009 |
| Concepción | 36°46′S | 73°03′W | 8 | 2003–2007 |
| Valdivia | 39°48′S | 73°14′W | 9 | 2001–2004 |
| Punta Arenas | 53°00′S | 70°51′W | 37 | 2001–2005 |
 | Table III.NMSC and MSC rates per 100,000
individuals in males and females between 2003 and 2007 (28). |
Table III.
NMSC and MSC rates per 100,000
individuals in males and females between 2003 and 2007 (28).
|
| NMSC rate | MSC rate |
|---|
|
|
|
|
|---|
| City | M | F | M | F |
|---|
| Arica | 69.7 | 52.4 | 3.7 | 1.2 |
| Antofagasta | 55.5 | 46.7 | 2.1 | 2.1 |
| Santiago | 24.7 | 16.5 | 2.9 | 2.5 |
| Concepción | 20.9 | 19.5 | 3.4 | 2.5 |
| Valdivia | 19.7 | 21.4 | 3.6 | 2.5 |
| Punta Arenas | 22.8 | 29.4 | 0.8 | 0.9 |
 | Table II.NMSC and MSC mortality rates per
100,000 individuals between 1990 and 2005 (28). |
Table II.
NMSC and MSC mortality rates per
100,000 individuals between 1990 and 2005 (28).
| City | NMSC | MSC |
|---|
| Arica | 1.07 | 0.54 |
| Antofagasta | 1.89 | 0.75 |
| Santiago | 0.75 | 1.00 |
| Concepción | 0.84 | 1.07 |
| Valdivia | 0.57 | 0.82 |
| Punta Arenas | 0.89 | 1.21 |
In Chile, the most common skin types are type III
for women that tan moderately and uniformly, with an MED of
0.30–0.50 kJ/m2, and skin type IV for men with
corresponding light brown skin that tans minimally, moderately and
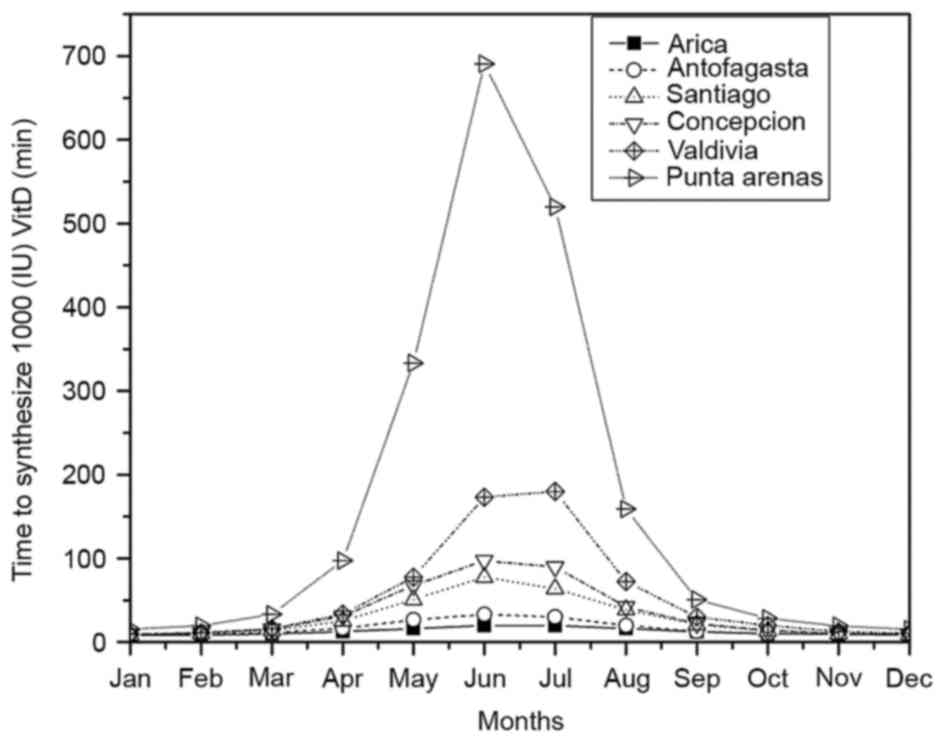
easily with an MED of 0.40–0.60 kJ/m2 (27). Fig. 3
shows the TVitD in 6 cities of Chile every month. The
maximum values were observed in June (winter) and varied from
18–700 min in Arica and Punta Arenas, respectively.
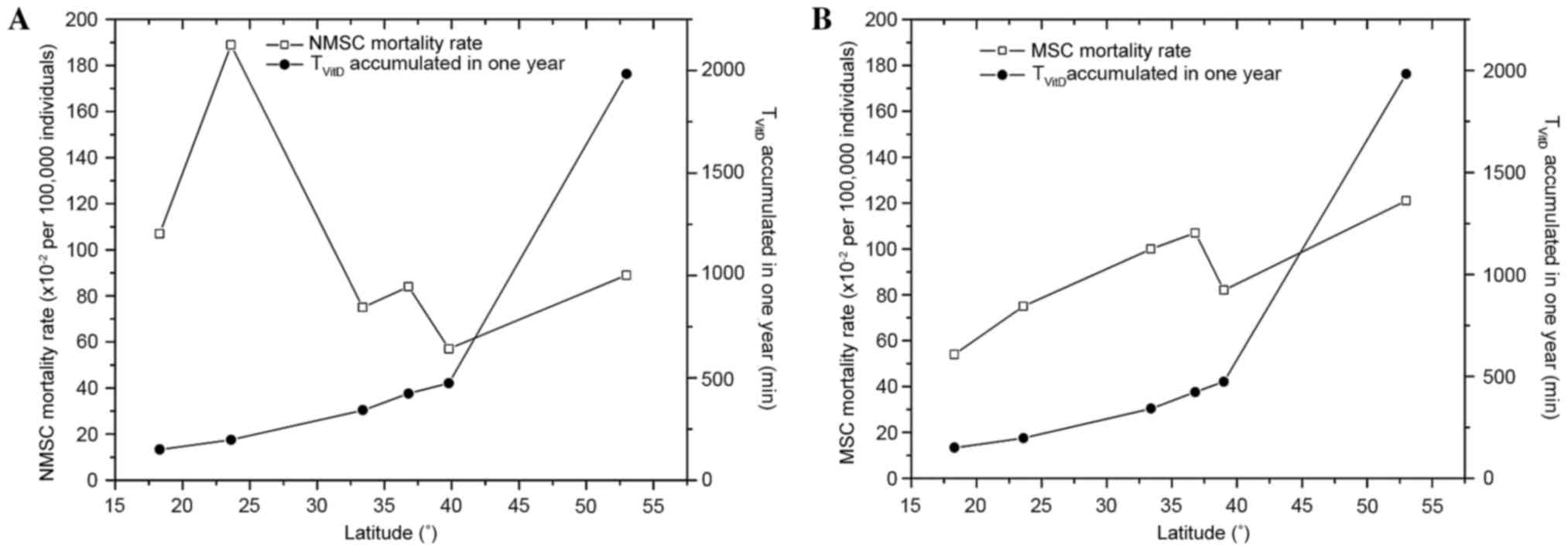
Fig. 4A-B shows NMSC
and MSC mortality rates with regard to latitude and
TVitD accumulated over 1 year. Fig. 4A shows that there was a negative
correlation between NMSC mortality rates and latitude (r=−0.53),
and no correlation between NMSC mortality rate and TVitD
accumulated in 1 year (r=−0.25). A strong correlation was also
found between MSC mortality rates and latitude (r=0.88), and
between MSC and TVitD accumulated over 1 year (r=0.72),
as shown in Fig. 4B. The NMSC ratio
was calculated in males and females for each city in comparison to
Punta Arenas. This was found to be 3.1 and 1.8 in Arica, 2.4 and
1.6 in Antofagasta, 1.1 and 0.6 in Santiago, 0.9 and 0.7 in
Concepción, and 0.9 and 0.7 in Valdivia, respectively. This was
probably due to the low solar UVB radiation levels in the southern
cities of Chile in winter (June 21 to September 20). The MSC ratio
was calculated for each city in comparison to Punta Arenas in males
and females. This was found to be 4.6 and 1.3 in Arica, 2.6 and 2.3
in Antofagasta, 3.6 and 2.8 in Santiago, 4.3 and 2.8 in Concepción,
and 4.5 and 2.8 in Valdivia, respectively.
Discussion
The dose-response associations between vitamin D and
cancer risk reduction have been previously estimated, and data has
indicated that it takes 1,500 IU vitamin D3 per day to obtain a 29%
cancer risk reduction for male cancer mortality rates in the United
States (29). The present study
provides an analysis of solar UVI levels reported in 6 cities from
the north to the south of Chile in different months. The results
showed that the solar UVI ratio of monthly mean values was 1.8
times higher in Arica than in Punta Arenas in January (summer in
Chile), whereas it was 14 times higher in June (winter in Chile).
This factor is an important consideration since vitamin D synthesis
is directly associated with the exposure of individuals to solar
UVB radiation. A similar trend was observed in Antofagasta,
Santiago, Concepcion, Valdivia and Punta Arenas.
Therefore, high solar UVB radiation levels imply a
great capability to synthesize vitamin D in the northern regions of
Chile. A recent analysis determined that the diagnosis of NMSC was
associated with a reduced risk of several vitamin D-sensitive
cancers (30,31). This was in accordance with low solar
UVB radiation levels in the southern cities of Chile in winter.
Therefore, high solar UVB radiation levels imply a lower capability
to synthesize vitamin D in the southern regions of Chile than in
the northern regions. Melanoma is another cancer that has been
linked to solar UV irradiance. However, it has been associated with
sun burning, limited solar UV irradiance (32), solar UVA irradiance (24,33,34), low
dietary vitamin D intake (35) and a
lack of chronic solar UVB irradiation at higher latitudes (36,37).
An inverse correlation was found between NMSC rates
in males and females, and different latitudes of 18°S in Arica and
36°S in Concepción. NMSC rates decreased as latitude increased,
which was in accordance with the low UVI values measured in those
cities. There was a strong inverse correlation coefficient between
NMSC rate and latitude (r=−0.84) among males, whereas there was a
weak inverse correlation in females (r=−0.65). The NMSC rate in
males in Punta Arenas was similar to the rates in Santiago and
Concepcion, however, this rate was higher in females. NMSC appears
to be directly associated with the Caucasian type of skin, that is,
types II and III. It is notable that the population from Punta
Arenas has a Croatian origin, with skin types II and III (50% of
the population according to the Republic of Croatia State Office
for Croats Abroad) (38). NMSC is the
most common malignant neoplasm in fair-skinned people, and in a
number of sunny countries this frequency exceeds the total number
of all other neoplasms. The incidence of basal cell carcinoma is
always greater than that of squamous cell carcinoma, varying by
latitude from 10:1 to 2.5:1 (39).
Studies have also indicated that NMSC has been increasing by 2–3%
per year, at least in the United States, which is most likely
caused by greater outdoor exposure for leisure and social
reasons.
The results of the present study indicated that MSC
decreased between latitude 18°S (Arica) and 23°S (Antofagasta) in
males. MSC rates increased between latitude 23°S (Antofagasta) and
40°S (Valdivia) in males and females. However, MSC rates decreased
at higher latitudes in each gender; this trend is consistent with
the reported annual doses of UVA radiation, which decreased with
increasing latitude (24).
There was a weak inverse coefficient correlation
between MSC rate and latitude (r=−0.56) among males, and none
(r=−0.12) in females. TVitD was similar in Arica,
Antofagasta, Santiago and Concepción, with maximum values that
varied from 175 to 700 min in Valdivia and Punta Arenas in winter,
and where TvitD was 39 times higher in Punta Arenas than
in Arica.
There was a weak inverse correlation coefficient
between NMSC mortality rates and latitude (r=−0.53). However, there
was a strong correlation between MSC mortality rates and latitude
(r=0.88). Mortality rate and TVitD accumulated in 1 year
were analyzed. Results showed that there was no correlation between
NMSC and TVitD accumulated in 1 year. However, there was
a strong correlation between MSC mortality rates and
TVitD accumulated over the same period (r=0.72). In
Punta Arenas, the MSC mortality rate was higher despite a lower MSC
incidence.
When explaining the lack of correlation between
different skin cancers and sunny cities, such as Arica, and less
sunny cities, such as Punta Arenas, there are several aspects to
take into consideration; for example, less body exposure to solar
UVB, which thereby reduces the amount of vitamin D production
(23,40), and lower UVB doses. The low risk for
skin cancers in dark-skinned individuals is partly attributable to
the photo-protection provided by the epidermal melanin barrier,
which halves the penetration of UVB through the epidermis in black
people compared with that in those of individuals of white European
ethnicity (Caucasians, corresponding to type I and type II skin
classification) (41). One study
previously reported a comprehensive bibliographic search of the
literature that identified studies on cutaneous malignant melanomas
and non-melanomas, vitamin D receptor polymorphisms, vitamin D
intake and 25(OH) D serum levels. An association was found for two
types of polymorphisms and melanoma (42). It can be concluded from these studies
that there is a direct correlation between NMSC rates and mortality
with UVB radiation, meaning that this type of cancer would not
depend on vitamin D synthesis and therefore on calcium uptake; by
contrast, MSC rates increased with decreased levels of vitamin D
and thus calcium uptake in all cities, with the only exception
being Punta Arenas.
Acknowledgements
This study was supported by grants from Tarapacá
University, Arica, Chile (Grant UTA-Mayor-4728-2016) (MR and ER)
and Convenio de Desempeño UTA1117 (GMC). The authors would like to
thank Mr. Leodán A. Crispin for his suggestions and the Chilean
Metereological Organization (Santiago, Chile).
References
|
1
|
Engelsen O: The relationship between
ultraviolet radiation exposure and vitamin D status. Nutrients.
2:482–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holick MF: The use and interpretation of
assays for vitamin D and its metabolites. J Nutr. 120:(Suppl 11).
S1464–S1469. 1990.
|
|
3
|
Matsuoka LY, Wortsman J, Haddad JG, Kolm P
and Hollis BW: Racial pigmentation and the cutaneous synthesis of
vitamin D. Arch Dermatol. 127:536–538. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Porojnicu AC, Lagunova Z, Robsahm TE, Berg
JP, Dahlback A and Moan J: Changes in risk of death from breast
cancer with season and latitude: Sun exposure and breast cancer
survival in Norway. Breast Cancer Res Treat. 102:323–328. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsuoka LY, Wortsman J, Dannenberg MJ,
Hollis BW, Lu Z and Holick MF: Clothing prevents ultraviolet-B
radiation-dependent photosynthesis of vitamin D3. J Clin Endocrinol
Metab. 75:1099–1103. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McKenna MJ: Differences in vitamin D
status between countries in young adults and the elderly. Am J Med.
93:69–77. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edvardsen K, Brustad M, Engelsen O and
Aksnes L: The solar UV radiation level needed for cutaneous
production of vitamin D3 in the face. A study conducted among
subjects living at high latitude (68 degrees N). Photochem
Photobiol Sci. 6:57–62. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Casado J, Parra P, Vega L and Suárez C:
Relación entre hormona paratiroidea y riesgo cardiovascular en
pacientes con insuficiencia de vitamina D. Med Clin. 141:292–294.
2013. View Article : Google Scholar
|
|
9
|
Miller DL and Weinstock MA: Non-melanoma
skin cancer in the United States: Incidence. J Am Acad Dermatol.
30:774–778. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cannell JJ, Vieth R, Umhau JC, Holick MF,
Grant WB, Madronich S, Garland CF and Giovannucci E: Epidemic
influenza and vitamin D. Epidemiol Infect. 134:1129–1140. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lucena SR, Salazar N, Gracia-Cazaña T,
Zamarrón A, González S, Juarranz A and Gilaberte Y: Combined
treatments with photodynamic therapy for non-melanoma skin cancer.
Int J Mol Sci. 16:25912–25933. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Houghton AN and Polsky D: Focus on
melanoma. Cancer Cell. 2:275–278. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Donaldson MR and Coldiron BM: No end in
sight: The skin cancer epidemic continues. Semin Cutan Med Surg.
30:3–5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caini S, Gandini S, Sera F, Raimondi S,
Fargnoli MC, Boniol M and Amstrong BK: Meta-analysis of risk
factors for cutaneous melanoma according to anatomical site and
clinic-pathological variant. Eur J Cancer. 45:3054–3063. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caini S, Boniol M, Bazolli E, Tosti G,
Bazolli B, Russel-Edu W, Giusti F, Testori A and Gandini S: The
risk of developing a secondary primary cancer in melanoma patients:
A comprehensive review of the literature and meta-analysis. J
Dermatol Sci. 75:3–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rivas M, Rojas E and Calaf GM: Prediction
of skin cancer occurrence by ultraviolet solar index. Oncol Lett.
3:893–896. 2012.PubMed/NCBI
|
|
17
|
Rivas M, Rojas E, Araya MC and Calaf GM:
Ultraviolet light exposure, skin cancer risk and vitamin D
production. Oncol Lett. 10:2259–2264. 2015.PubMed/NCBI
|
|
18
|
Rivas M, Araya MC, Durán V, Rojas E,
Cortes J and Calaf GM: Ultraviolet light exposure and skin cancer
in the city of Arica, Chile. Mol Med Rep. 2:567–572.
2009.PubMed/NCBI
|
|
19
|
Rivas M, Araya MC, Caba F, Rojas E and
Calaf GM: Ultraviolet light exposure influences skin cancer in
association with latitude. Oncol Rep. 25:1153–1159. 2011.PubMed/NCBI
|
|
20
|
Zemelman V, Alvarado O, Von Beck P and
Valenzuela C: Assessment of skin type, eye and hair color, freckles
tendency in Chilean adolescents. J Eur Acad Dermatol Venereol.
12:3211990.
|
|
21
|
Fitzpatrick TB: The validity and
practicality of sun-reactive skin types I through VI. Arch
Dermatol. 124:869–871. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grant WB: An ecologic study of cancer
mortality rates in Spain with respect to indices of solar UVB
irradiance and smoking. Int J Cancer. 120:1123–1128. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grant WB: The effect of solar UVB doses
and vitamin D production, skin cancer action spectra, and smoking
in explaining links between skin cancers and solid tumours. Eur J
Cancer. 44:12–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grigalavicius M, Moan J, Dahlback A and
Juzeniene A: Daily, seasonal, and latitudinal variations in solar
ultraviolet A and B radiation in relation to vitamin D production
and risk for skin cancer. Int J Dermatol. 55:e23–e28. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Systems YES: Operation manual calibrated
high range YES-Biometer. Yankee Environmental Systems, Inc; Turners
Falls, OK, USA: 2006 http://www.yesinc.com/Accessed. September
5–2016
|
|
26
|
McKenzie RL, Liley JB and Björn LO: UV
radiation: Balancing risks and benefits. Photochem Photobiol.
85:88–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Holick MF: Biologic effects of light:
Historical and new perspectivesHolick MF and Jung EG: Biologic
effects of light 1998. Proceedings of a symposium Basel,
Switzerland November 1–3, 1998. Boston: Kluwer Academic Publishers;
pp. 11–32. 1999, View Article : Google Scholar
|
|
28
|
Vallebuono C: Primer informe de registros
poblacionales de cáncer de Chile, quinquenio 2003–2007, unidad de
vigilancia de enfermedades no transmisibles y estudios. Ministry of
Health of Chile. Epidemiology Section. 2012.
|
|
29
|
Giovannucci E, Liu Y, Rimm EB, Hollis BW,
Fuchs CS, Stampfer MJ and Willett WC: Prospective study of
predictors of vitamin D status and cancer incidence and mortality
in men. J Natl Cancer Inst. 98:451–459. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
De Hertog SA, Wensveen CA, Bastiaens MT,
Kielich CJ, Berkhout MJ, Westendorp RG, Vermeer BJ and Bavinck
Bouwes JN: Leiden Skin Cancer Study: Relation between smoking and
skin cancer. J Clin Oncol. 19:231–238. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Milán T, Verkasalo PK, Kaprio J and
Koskenvuo M: Lifestyle differences in twin pairs discordant for
basal cell carcinoma of the skin. Br J Dermatol. 149:115–123. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garland FC, White MR, Garland CF, Shaw E
and Gorham ED: Occupational sunlight exposure and melanoma in the
U.S. Navy. Arch Environ Health. 45:261–267. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moan J, Dahlback A and Setlow RB:
Epidemiological support for an hypothesis for melanoma induction
indicating a role for UVA radiation. Photochem Photobiol.
70:243–247. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garland CF, Garland FC and Gorham ED:
Epidemiologic evidence for different roles of ultraviolet A and B
radiation in melanoma mortality rates. Ann Epidemiol. 13:395–404.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Millen AE, Tucker MA, Hartge P, Halpern A,
Elder DE, Guerry D VI, Holly EA, Sagebiel RW and Potischman N: Diet
and melanoma in a case-control study. Cancer Epidemiol Biomarkers
Prev. 13:1042–1051. 2004.PubMed/NCBI
|
|
36
|
Berwick M, Armstrong BK, Ben-Porat L, Fine
J, Kricker A, Eberle C and Barnhill R: Sun exposure and mortality
from melanoma. J Natl Cancer Inst. 97:195–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gandini S, Sera F, Cattaruzza MS, Pasquini
P, Picconi O, Boyle P and Melchi CF: Meta-analysis of risk factors
for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 41:45–60.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Republic of Croatia State Office for
Croats Abroad. 2013–2016. http://www.hrvatiizvanrh.hr/en/hmiu/croatian-diaspora-in-chile/22Accessed.
September 5–2016.
|
|
39
|
Urbach F: Incidence of nonmelanoma skin
cancer. Dermatol Clin. 9:751–755. 1991.PubMed/NCBI
|
|
40
|
Webb AR and Engelsen O: Calculated
ultraviolet exposure levels for a healthy vitamin D status.
Photochem Photobiol. 82:1697–1703. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brenner M and Hearing VJ: The protective
role of melanin against UV damage in human skin. Photochem
Photobiol. 84:539–549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gandini S, Raimondi S, Gnagnarella P, Doré
JF, Maisonneuve P and Testori A: Vitamin D and skin cancer: A
meta-analysis. Eur J Cancer. 45:634–641. 2009. View Article : Google Scholar : PubMed/NCBI
|