Introduction
Low-grade myofibroblastic sarcoma (LGMS) is a
neoplasm of the soft tissue characterized by myofibroblastic
differentiation, which may occur anywhere in the body but
frequently occurs in the head and neck region (1,2). Of those,
the most common was the tongue (1).
Owing to a previous lack of clear diagnostic criteria, the tumor is
considered to more frequently occur than the number of times
reflected in the literature (3).
Furthermore, reports describing the clinical details such as tumor
size, method of treatment, presence or absence of recurrence (local
recurrence, regional recurrence and distant metastasis) and patient
survival are sparse. Currently, despite being distinctly classified
by the World Health Organization (WHO) (3), obtaining a differential diagnosis of
this tumor from a benign lesion remains challenging (4). Owing to the scarcity of reported cases,
the complete clinical picture of LGMS, including mortality rates,
incidence rates, methods of treatment and risk factors, is unclear.
Regarding recurrence, Yamada et al (4) reported that tumors larger than 3 cm
tended to recur. Metastasis is reportedly rare (3). Therefore, definitive treatment criteria
for LGMS remain unknown, and the requirement for postoperative
radiotherapy or chemotherapy also remains undetermined. The tumor
typically presents as a slow-growing painless mass that is often
mistaken for a benign lesion due to its indolent growth; however,
it is a malignant neoplasm that is able to recur or metastasize
following an extended period of time (1). The present study details a case of a
patient with LGMS of the buccal subcutaneous tissues on the
buccinator muscle, and reviews 55 relevant cases of head and neck
LGMS.
Case report
A 43-year-old Japanese female was referred to
University Hospital of the Ryukyus (Okinawa, Japan) in May 2013 by
her dentist due to the presence of a mass in the left buccal area
that had developed over a 2-month period. The patient had noticed
the lesion 2 months earlier, but did not present to the hospital
for 2 months. There was no associated pain or paresthesia, and a
systematic examination revealed that the patient was otherwise fit
and healthy and reported no tobacco or alcohol use (4). The patient's family history indicated
that her father had previously been treated for rectal cancer.
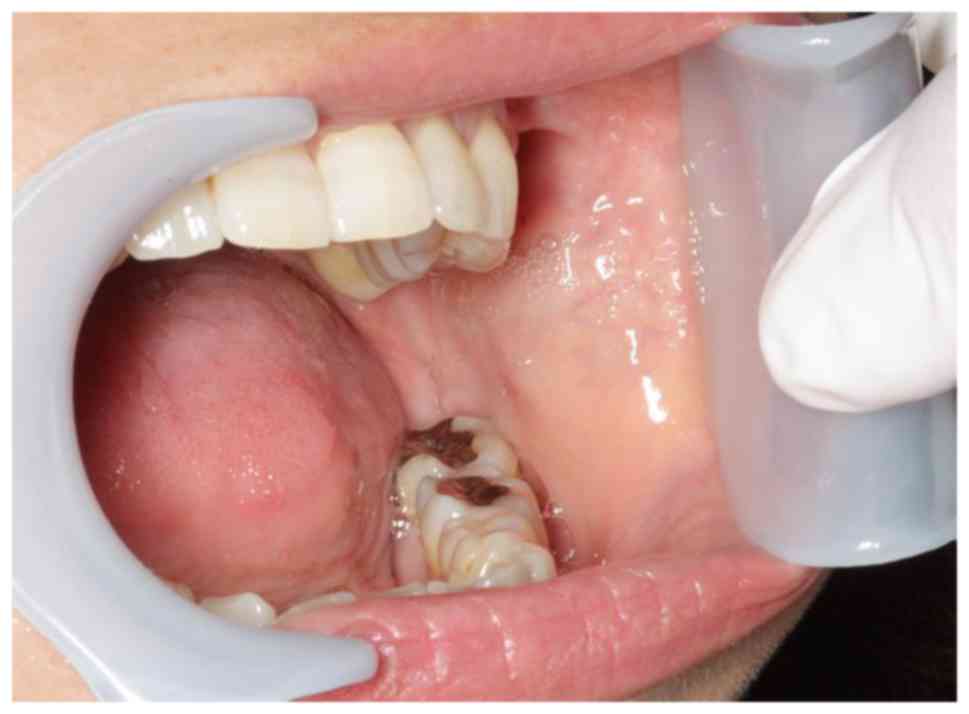
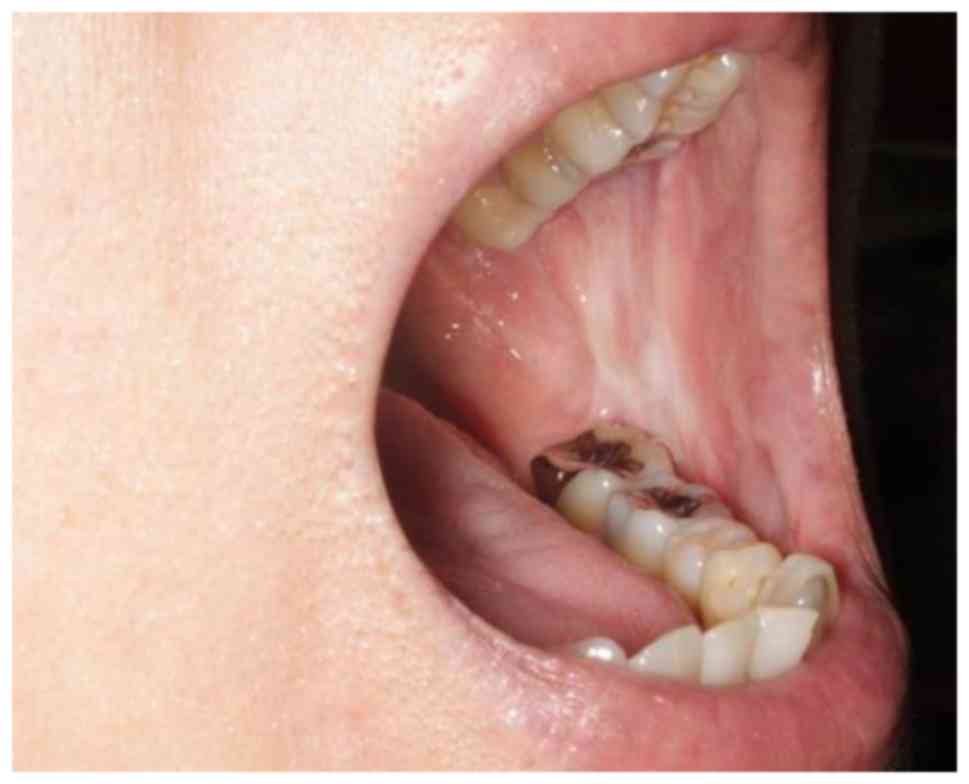
A physical examination revealed an elastic hard
13×10 mm mass of the left buccal tissue with distinct margins. The
overlying mucosa was normal in color and texture (Fig. 1) and no other causal factors
underlying the presence of the mass were observed. The mucosa
appeared healthy, no other lesions were observed and no palpable
lymphadenopathy was detected in the neck of the patient.
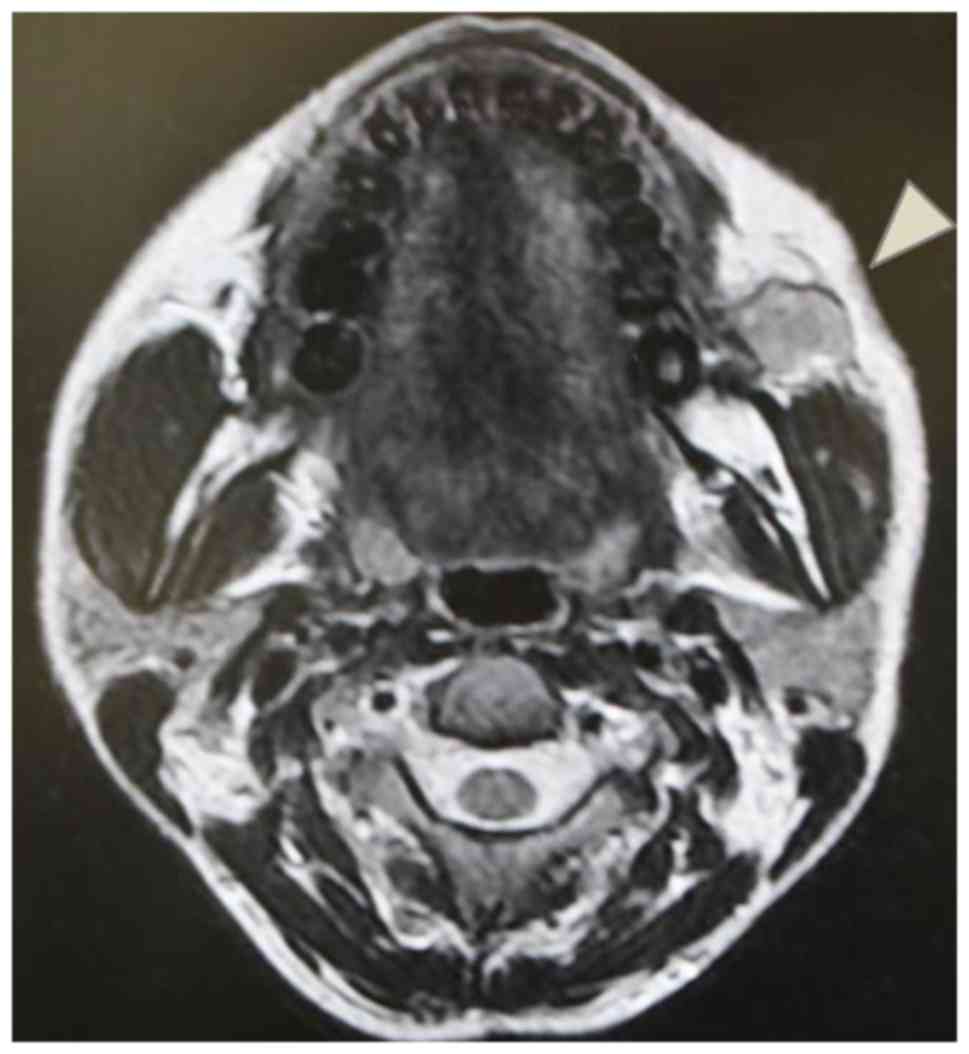
Contrast-enhanced computed tomography (CT) and
magnetic resonance imaging (MRI) revealed a 1.4-cm, well-defined
mass in the left subcutaneous tissue of the cheek. The lesion was
located on the buccinator muscle (Fig.
2, indicated by an arrow), and no other primary or metastatic
lesions were detected in all examinations prior to surgery.
A biopsy revealed spindle cell proliferation.
Therefore, an excisional biopsy was performed in order to remove
the malignant neoplasm. Sections (3 µm) were cut and stained with
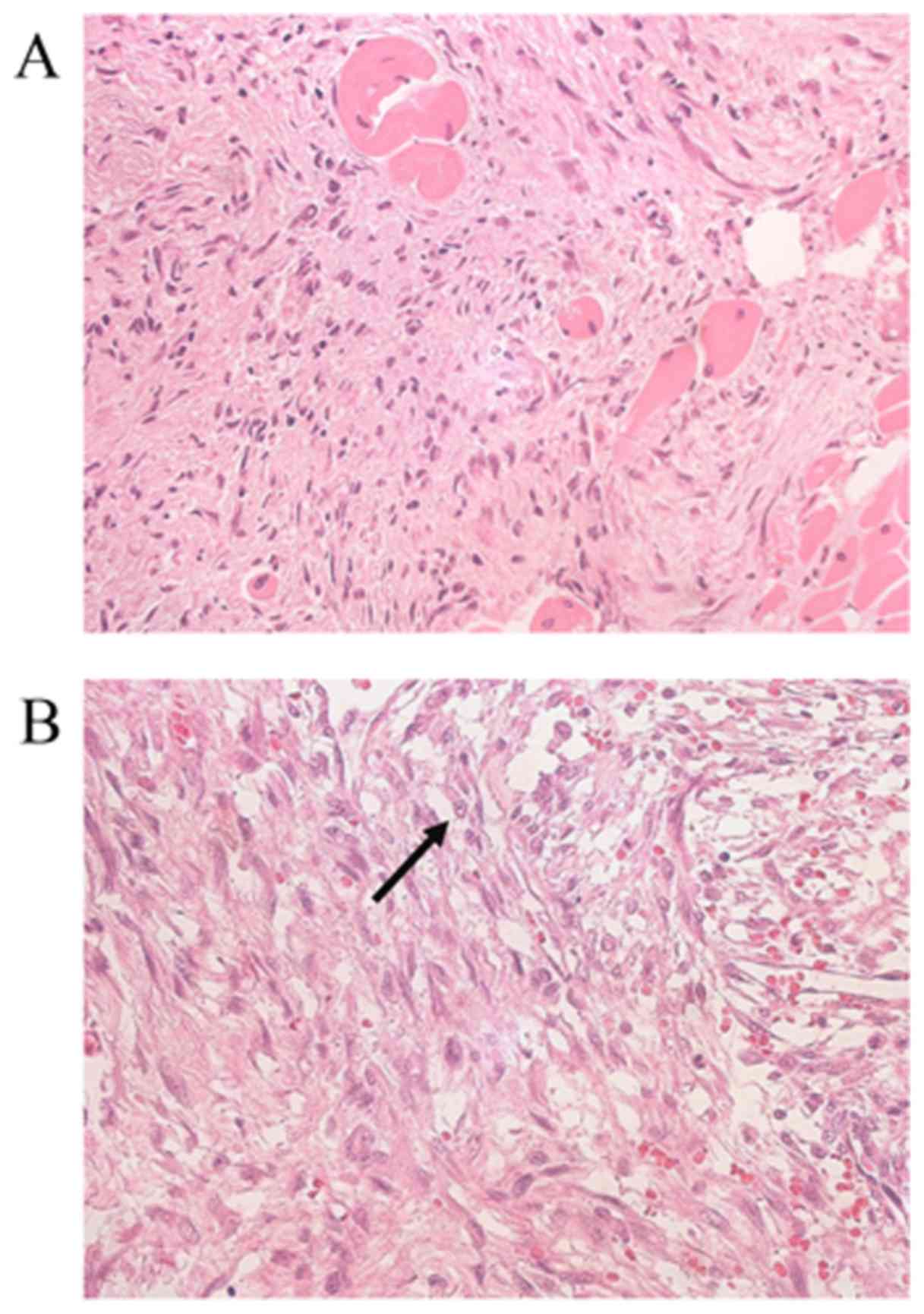
hematoxylin and eosin. The histopathological results revealed that
the tumor was primarily composed of spindle-shaped cells presenting
as diffusely infiltrative growth into the surrounding muscle
tissues on a myxoid background. The tumor cells were predominately
arranged in fascicles, whereas storiform patterns were focally
observed. The tumor cells were atypical, with large round or
spindle-like nuclei and ill-defined palely eosinophilic cytoplasm.
The infiltration of cells around skeletal muscle fibers was also
observed (Fig. 3A). Few mitotic cells
(<2 mitosis/10 high-power fields; Fig.
3B, indicated by an arrow) were observed and no tumor necrosis
was observed (Fig. 3B). The lesions
were low grade (5).
Immunohistochemical analysis was performed with the
following antibodies: α-smooth muscle actin (1:160; cat. no. M0851;
Dako; Agilent Technologies, Inc., Santa Clara, CA, USA); mindbomb
E3 ubiquitin protein ligase 1 (MIB-1; 1:100; cat. no. M7240; Dako;
Agilent Technologies, Inc.); cluster of differentiation 34 (1:200;
cat. no. QB-END/10; Novocastra Laboratories, Newcastle, UK); desmin
(1:320; cat. no. M0760; Dako; Agilent Technologies, Inc.);
caldesmon (1:100; cat. no. M3557; Dako; Agilent Technologies,
Inc.); nuclear β-catenin (1:1,000; cat. no. 610,154; BD
Biosciences, San Diego, CA, USA); anaplastic lymphoma kinase (1:50;
cat. no. M7195; Dako; Agilent Technologies, Inc.); S-100 protein
(1:2,000; cat. no. Z0311; Dako; Agilent Technologies, Inc.);
anti-pan cytokeratin antibody 1/3 (1:800; cat. no. M3515; Dako;
Agilent Technologies, Inc.); and anti-cytokeratin CAM 5.2 (1:16;
cat. no. 349205; BD Biosciences, San Jose, CA, USA). Sections (3
µm) were cut and stained. The results identified that the majority
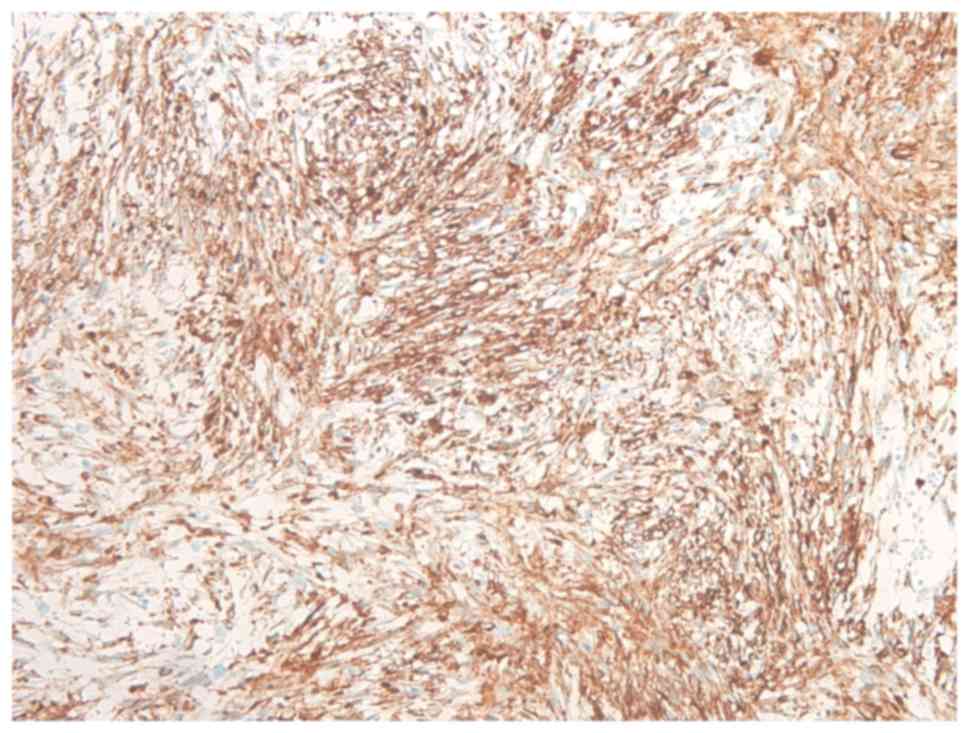
of the spindle cells were focally immunoreactive for α-smooth
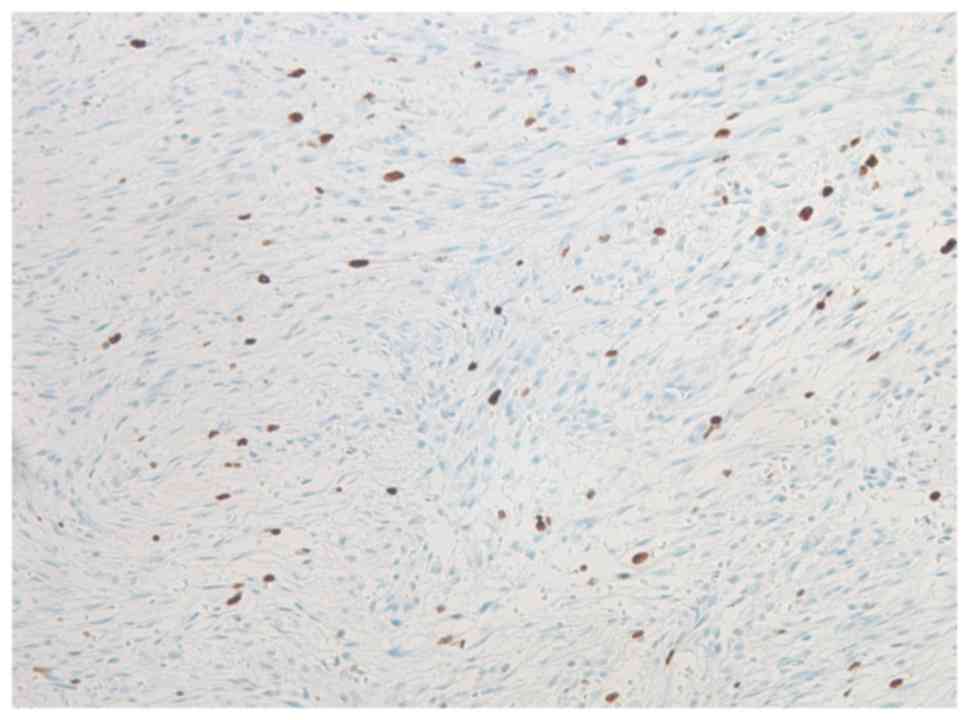
muscle actin (Fig. 4), and <10% of
the lesional cells stained positive for MIB-1 (Fig. 5). By contrast, immunostaining was
negative for other markers, including cluster of differentiation
34, desmin, caldesmon, nuclear β-catenin, anaplastic lymphoma
kinase, S-100 protein and 2 markers of cytokeratin: anti-pan
cytokeratin antibody 1/3 and anti-cytokeratin CAM 5.2. Therefore,
the histological and immunohistochemical features of LGMS were
diagnosed.
Subsequently, re-excision was performed to obtain
clear margins. No residual tumor tissue was observed in the
re-excised specimen and no chemotherapy or radiotherapy was
administered. At the end of the 2-year follow-up period, the
patient was alive and healthy with no clinical or radiological
signs of recurrence or metastasis (Fig.
6).
Written informed consent was obtained from the
patient for the publication of this case report and all
accompanying images. This case report was submitted for ethical
review to the Ethics Committee of the University of the Ryukyus
(Okinawa, Japan), who waived the requirement for review per
institutional protocol, as the study does not contain content that
required ethical approval. The Ethics Committee approved the
submission and publication of the manuscript.
The first case of LGMS in the head and neck region
was described in 1991 (2,6). Literature reports published between 1991
and 2015 were identified using the search terms in PubMed and
Google Scholar, excluding non-English language reports. A total of
55 cases were statistically analyzed. Gaussian distribution was
confirmed by the Shapiro-Wilk test. Age was evaluated by one-factor
analysis of variance (mean ± standard deviation). The size of the
tumor was evaluated by the Kruskal-Wallis test (median,
minimum-maximum). Recurrence was evaluated by the Fisher's exact
test (two-sided). P<0.05 was considered to indicate a
statistically significant difference. Analyses were conducted with
the use of SPSS for Windows version 22 (IBM SPSS, Armonk, NY,
USA).
Discussion
The present study observed 2 important clinical
issues. First, to the best of our knowledge, this case is the first
report of LGMS in the buccal subcutaneous tissues of the buccinator
muscle. Secondly, a literature review of 55 cases of this tumor in
the head and neck region revealed that the indolent growth of these
lesions may contribute to a delay in the diagnosis of LGMS: the
studies reviewed indicated that the average time between the onset
of clinical symptoms and hospital admission is 3.9 months.
LGMS has been detected in various sites in the body,
including the skin, extremities, trunk, breast, abdominal/pelvic
cavity, kidney, vulva, pulmonary artery and bone (2,7–13), but frequently occurs in the head and
neck region (2,14,15). As
presented in Table I, LGMS has been
reported to occur in a number of locations in the head and neck
region (1,2,4,6,13,15–40). Of
these, three cases of LGMS in the cheek have reported, including
the present case, one case located at the nasolabial fold and one
from the buccal mucosa (16,23). The most common site was the tongue,
followed by the mandible, neck, larynx, palate, maxilla and lips.
LGMS was also observed in the gingiva, nasal/paranasal cavity,
face, skull, external acoustic meatus, and deep tissue spaces,
including the parapharyngeal space, as well as anywhere in the head
and neck region (Table I).
 | Table I.Cases of LGMS in the head and neck
region. |
Table I.
Cases of LGMS in the head and neck
region.
| Case | Year | Gender | Age, years | Site | Clinical symptoms
to admission to the hospital, months | Tumor size, cm | Treatment | Outcome | Follow-up,
months | (Refs.) |
|---|
| 1 | 1991 | M | 75 | Parietal | NA | NA | NA | LR | 20 | (6) |
| 2 | 1991 | M | 85 | Face | NA | NA | NA | NED | 11 | (6) |
| 3 | 1992 | F | 43 | Nasolabial | 2 | 1.5 | Full excision | NA | NA | (16) |
| 4 | 1998 | F | 51 | Tongue | NA | 2.5 | Local excision | NA | NA | (2) |
| 5 | 1998 | M | 70 | Tongue | NA | 1.4 | Local excision | NED | 42 | (2) |
| 6 | 1998 | M | 24 | Tongue | NA | 1.5 | Local excision | NED | 40 | (2) |
| 7 | 1998 | M | 66 | Tongue | NA | 1.8 | Wide excision | NED | 28 | (2) |
| 8 | 1998 | M | 19 | Mandible | NA | 3.5 | Local excision, RT,
CHT | NED | 30 | (2) |
| 9 | 1999 | F | 71 | Parotid gland | 4 | 1.7 | Local excision | NED | 24 | (17) |
| 10 | 2001 | M | 41 | Hard palate | NA | 3.5 | Wide excision | NED | 18 | (15) |
| 11 | 2001 | M | 35 | Palate | NA | 1.6 | Local excision | NED | 12 | (15) |
| 12 | 2001 | M | 63 | Neck | NA | 4.0 | NA | NA | NA | (15) |
| 13 | 2001 | F | 54 | Gingiva | NA | 1.5 | Local excision | LR after 4
months | 4 | (15) |
| 14 | 2001 | M | 24 | Maxillary
sinus | 12 | 4.0 | Maxillectomy | NED | 40 | (18) |
| 15 | 2001 | F | 77 | Nasal cavity | NA | 3.0 | Excision | NED | 14 | (19) |
| 16 | 2001 | F | 28 | Nostril | NA | 1.5 | Local excision | NA | NA | (20) |
| 17 | 2004 | F | 8 | Pterygoid
region | NA | 6.0 | Wide excision,
CHT | NED | 96 | (21) |
| 18 | 2004 | F | 1 | Temporal fossa | 5 weeks | 2.9 | Local excision | NED | 48 | (21) |
| 19 | 2006 | M | 42 | Parapharyngeal
space | 6 | NA | RT, CHT | NED (dead) | 6 | (22) |
| 20 | 2006 | F | 37 | Buccal mucosa | 2 | 2.0 | Wide excision | NED | 6 | (23) |
| 21 | 2006 | F | 24 | Tongue | 6 weeks | 2.0 | Local excision | NED | 12 | (24) |
| 22 | 2007 | M | 41 | Tongue | 2 days | 1.7 | Local excision | LR after 6
years | 108 | (1) |
| 23 | 2007 | F | 7 | Neck | NA | 3.0 | Surgical
resection | NED | 30 | (25) |
| 24 | 2007 | M | 30 | Skull | NA | 3.0 | Surgical
resection | LR | 20 | (25) |
| 25 | 2007 | M | 32 | Skull | NA | 4.0 | Surgical resection,
CHT | LR | 46 | (25) |
| 26 | 2007 | F | 19 | External acoustic
Meatus | NA | 2.0 | Surgical
resection | NA | NA | (25) |
| 27 | 2007 | M | 53 | Tongue | NA | 2.0 | Surgical resection,
RT | LR | 28 | (25) |
| 28 | 2007 | M | 37 | Neck | NA | 2.0 | Surgical resection,
RT | NED | 25 | (25) |
| 29 | 2007 | M | 44 | Piriform fossa | NA | 3.0 | Local excision | LR after 4
years | 132 | (26) |
| 30 | 2007 | M | 74 | Nasal cavity | NA | 3.0 | Local resection,
RT | LR after 9
months | 27 | (27) |
| 31 | 2007 | M | 14 | Nasal cavity | NA | 5.0 | Local resection,
RT | LR after 8
months | 18 | (27) |
| 32 | 2007 | M | 14 | Ethmoid sinus | NA | 4.5 | Local resection,
RT | LR after 9
months | 32 | (27) |
| 33 | 2007 | M | 28 | Lower lip | 4 or 5 | 2.0 | Wide local
excision | NA | NA | (28) |
| 34 | 2009 | F | 28 | Maxillary
sinus | NA | 3.0 | NA | LR | 20 | (13) |
| 35 | 2009 | M | 52 | Ethmoid sinus | NA | 6.0 | NA | NED | 13 | (13) |
| 36 | 2009 | F | 6.5 | Maxillary
sinus | NA | 3.0 | NA | NED | 24 | (13) |
| 37 | 2009 | M | 54 | Mandible | 12 | 5.9 | Local excision | NED | NA | (29) |
| 38 | 2009 | M | 51 | Mandible | 2 | 3.0 | Local excision | NED | 24 | (30) |
| 39 | 2009 | F | 61 | Tongue | 3 | 2.0 | Local excision | NED | 12 | (30) |
| 40 | 2010 | M | 37 | Mandible
gingival | 3 | 2.0 | Wide excision | NED | 18 | (31) |
| 41 | 2010 | M | 56 | Tongue base | NA | 4.2 | Local excision | NED | 36 | (32) |
| 42 | 2011 | F | 69 | Larynx | 6 | 16.0 | Complete
excision | NED | 12 | (33) |
| 43 | 2011 | F | 41 | Laryngeal | 6 | 3.0 | Wide resection | NED | 14 | (34) |
| 44 | 2012 | M | 73 | Palate | 2 | 1.0 | Local excision | NED | 24 | (4) |
| 45 | 2012 | M | 9 | Mandible | 1 | 3.0 | Mass excision +
alveolectomy | NED | 18 | (35) |
| 46 | 2013 | M | 17 | Upper lip | 2 | 3.4 | Gross total
resection | NED | 12 | (36) |
| 47 | 2013 | M | 44 | Tongue | NA | 2.2 | Gross total
resection | NED | 2 | (36) |
| 48 | 2013 | F | 70 | Neck | NA | 3.0 | Unresectable, RT,
CHT | NED | 36 | (36) |
| 49 | 2013 | M | 55 | Larynx | 6 | NA | Wide excision,
RT | NED | 12 | (37) |
| 50 | 2014 | M | 40 | Larynx | 3 | NA | Wide resection | NED | 24 | (38) |
| 51 | 2015 | F | 75 | Maxillary
sinus | 1 | NA | Total maxillectomy,
RT | DM (humerusbone
metastasis after 1 year) | 12 | (39) |
| 52 | 2015 | M | 74 | Tongue base | NA | 4.1 | Total
laryngectomy | LR after 6 months,
DT | 6 | (39) |
| 53 | 2015 | M | 45 | Maxilla | 6 | 5.2 | Maxillectomy | NED | 30 | (40) |
| 54 | 2015 | F | 29 | Maxilla | NA | 2.5 | Wide local
resection | LR after 6
months | 12 | (40) |
| 55 | 2015 | F | 43 | Cheek | 2 | 1.4 | Wide resection | NED | 18 | Present case |
In addition, the present study demonstrated that the
indolent growth of LGMS may contribute to a delay in diagnosis. In
the present literature review (Table
I), the average time between the onset of clinical symptoms and
hospital admission was 3.9 months (the average of this time is
presented, with the exception of ‘not applicable’ (NA) cases, in
Table I. LGMS is reported as a
painless slow growing tumor with a relatively indolent course that
mimics a benign lesion (2,4). The majority of patients present with a
painless swelling or an enlarged mass (3). These neoplasms arise predominantly in
the subcutaneous and deeper soft tissue (3). The present study identified 55 cases of
LGMS in the head and neck region published in the English language,
including the present case (Table I),
with a mean patient age of 42.9 years (median patient age of 42.3
years; range, 1–85) and a male/female ratio of 3:2. Furthermore,
the median age at the time of the diagnosis of LGMS in the head and
neck region was determined to be younger, compared with that for
all head and neck cancer (42.3 years and 60 years, respectively)
(41). LGMS lesions are local,
aggressive and characterized by frequent recurrence and metastasis,
but exhibit a relatively indolent course, and tend to recur locally
rather than metastasizing (4,29). However, LGMS is able to metastasize to
distant sites, including the left humerus and the cardiac region
(39,42). Therefore, disease management via wide
excision of the tumor and long-term follow-up is suggested
(25). In the present literature
review, the rate of local recurrence and distant metastases were
29% (14/49) and 2% (1/49), respectively. In total, 6/55 cases did
not provide information regarding the incidence of recurrence or
metastasis. None of the 55 studies reviewed reported regional
recurrence.
Myofibroblasts were initially identified in 1971 as
modified fibroblasts, and are considered to function in the
contraction of granulation tissue (43). Myofibroblasts are morphologically and
functionally varied, compared with fibroblasts (14), and form the principal component of a
number of reactive and benign soft tissue lesions (44). In the past few decades, myofibroblasts
have also been identified in malignant soft tissue tumors (6,18). A
number of types of sarcoma with predominant myofibroblastic
differentiation have been identified and may be categorized into
several well-defined clinicopathological entities (26), including LGMS and inflammatory
fibroblastic tumor (44). Conversely,
non-malignant myofibroblastic lesions include nodular fasciitis and
myofibroma (44).
Various studies have provided a detailed analysis of
LGMS to date (2,4,13,15,25). The
criteria for the classification of LGMS lesions have historically
been disputed (45); however, such
masses have been distinctly reclassified in the 2013 WHO
classification of Soft Tissue and Bone (Fourth Edition) (3). LGMS is sometimes misdiagnosed as a
benign lesion (4,31), and a fine-needle aspiration biopsy may
be inappropriate because it can obscure the tumor (22,29).
Therefore, for the diagnosis of LGMS, incisional biopsy is
appropriate (30). An incisional
biopsy must be undertaken with caution (27), as if the correct areas are not sampled
by the biopsy, including in small or superficial biopsy samples,
misdiagnosis may occur owing to the diverse histological appearance
of LGMS cells in tissues from the same tumor (27). Until the year 2007, 18 cases of LGMS
in the head and neck had been reported (26), whereas by the end of 2015, a total of
55 cases had been identified (Table
I), and the case reports of LGMS may continue to increase.
Regarding LGMS treatment, the value of
post-operative radiotherapy or chemotherapy remains to be
established. Ni et al (34)
recommended that the treatment of LGMS include wide excision, with
tumor-free margins and post-operative radiotherapy or chemotherapy
if required. By contrast, certain reports have described that
radiotherapy and chemotherapy are of uncertain clinical value
because the tumor was unresponsive. These authors recommended
surgical treatment, including excision along the free margin
(21,40). Table II
presents the recurrence rate (includes metastasis) of the
resectable LGMS cases listed in Table
I (excluding those with treatments or outcomes indicated as NA)
following surgical resection. The recurrence rate following surgery
only, as well as following surgery and radiotherapy, was 18.8%
(6/32) and 71.4% (5/7), respectively. The statistical analysis
revealed that the treatment of surgery and radiotherapy
significantly increased the rate of recurrence (P=0.016; Table III). By contrast, the age and the
size of tumor showed no bias between the treatments (P=0.312 and
0.162, respectively). According to the aforementioned results, the
present study suggests that radiotherapy must be avoided following
resection of LGMS, as this treatment may induce the recurrence of
LGMS.
 | Table II.Recurrence rate and treatment of the
LGMS instances reported in Table
I. |
Table II.
Recurrence rate and treatment of the
LGMS instances reported in Table
I.
| Treatment | Number of patients
(male) | Mean age,
years | Mean size of tumor,
cm | Number of patients
with recurrence (rate, %) |
|---|
| Surgery only | 32 (20) | 43.4±20.5 | 3.1 | 6 (18.8) |
| Surgery + RT | 7 (6) | 46±25.4 | 3.3 | 5 (71.4) |
| Surgery + CHT | 2 (1) | 20±17 | 5 | 1 (50) |
| Surgery + RT +
CHT | 1 (1) | 19 | 3.5 | 0 (0) |
| Total | 42 (28) | 42.1±21.5 | 3.2 | 12 (28.6) |
 | Table III.Comparison of age, tumor size and
recurrence between the treatment groups. |
Table III.
Comparison of age, tumor size and
recurrence between the treatment groups.
|
| Surgery only
(n=32) | Surgery + RT
(n=7) | Surgery + CHT
(n=2) | Surgery + RT + CHT
(n=1) | P-value |
|---|
| Age (years) | 43.4±20.5 | 46.0±25.4 | 20.0±17.0 | 19.0 | 0.312 |
| Size of tumor
(cm) | 2.9 (1.0–16.0) | 3.0 (2.0–5.0) | 5.0 (4.0–6.0) | 3.5 (3.5–3.5) | 0.162 |
| Recurrence | 6 (18.8%) | 5
(71.4%) | 1
(50.0%) | 0 (0.0%) |
0.016a |
After a 2-year follow-up period, the patient was
alive and healthy with no clinical or radiological evidence of
recurrence or metastasis; however, the patient required further
observation. In conclusion, to the best of our knowledge, the
present case report is the first report of LGMS in the buccal
subcutaneous tissue of the buccinator muscle. It is suggested that
incisional biopsy be performed to eliminate LGMS when clinicians
encounter patients with the aforementioned indolent lesions
anywhere in the body. It is also suggested that radiotherapy is
avoided following resection of LGMS, as radiotherapy may induce the
recurrence of LGMS. Further reports, including long-term follow-up
data and adequate clinical information, are required to develop
novel treatment protocols for LGMS, and to prevent
radiation-induced LGMS, which may be more common than previously
considered.
Acknowledgements
The authors would like to thank the staff of the
Department of Pathology, University Hospital of the Ryukyus, who
contributed to patient diagnosis, and all medical staff at
University Hospital of Ryukyus who contributed to the treatment and
care of the present patient. In addition, the authors would like to
thank Enago (www.enago.jp) for the English language
review of the present study.
Glossary
Abbreviations
Abbreviations:
|
LGMS
|
low-grade myofibroblastic sarcoma
|
|
WHO
|
World Health Organization
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
NA
|
not applicable
|
|
RT
|
radiotherapy
|
|
CHT
|
chemotherapy
|
References
|
1
|
Jay A, Piper K, Farthing PM, Carter J and
Diwakar A: Low-grade myofibroblastic sarcoma of the tongue. Oral
Surg Oral Med Oral Pathol Oral Radiol Endod. 104:e52–e58. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mentzel T, Dry S, Katenkamp D and Fletcher
CD: Low-grade myofibroblastic sarcoma: Analysis of 18 cases in the
spectrum of myofibroblastic tumors. Am J Surg Pathol. 22:1228–1238.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mentzel T: Low grade myofibroblastic
sarcomaWorld Health Organization classification of Soft Tissue and
Bone. Fletcher CDM, Bridge JA, Hogendoorn P and Mertens F: 4th.
IARC Press; Lyon: pp. 85–86. 2013
|
|
4
|
Yamada T, Yoshimura T, Kitamura N, Sasabe
E, Ohno S and Yamamoto T: Low-grade myofibroblastic sarcoma of the
palate. Int J Oral Sci. 4:170–173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guillou L, Coindre JM, Bonichon F, Nguyen
BB, Terrier P, Collin F, Vilain MO, Mandard AM, Le Doussal V,
Leroux A, et al: Comparative study of the national cancer institute
and french federation of cancer centers sarcoma group grading
systems in a population of 410 adult patients with soft tissue
sarcoma. J Clin Oncol. 15:350–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eyden BP, Banerjee SS, Harris M and Mene
A: A study of spindle cell sarcomas showing myofibroblastic
differentiation. Ultrastruct Pathol. 15:367–378. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diaz-Cascajo C, Borghi S, Weyers W and
Metze D: Fibroblastic/myofibroblastic sarcoma of the skin: A report
of five cases. J Cutan Pathol. 30:128–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roth TM, Fratkin J, Woodring TC and
McGehee RP: Low-grade myofibroblastic sarcoma of the vulva. Gynecol
Oncol. 92:361–364. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe K, Ogura G, Tajino T, Hoshi N and
Suzuki T: Myofibrosarcoma of the bone: A clinicopathologic study.
Am J Surg Pathol. 25:1501–1507. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Humphries WE III, Satyan KB, Relyea K, Kim
ES, Adesina AM, Chintagumpala M and Jea A: Low-grade
myofibroblastic sarcoma of the sacrum. J Neurosurg Pediatr.
6:286–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morgan PB, Chundru S, Hatch SS, Hawkins
HK, Adegboyega PA and Eltorky MA: Uncommon malignancies: Case 1.
Low-grade myofibroblastic sarcoma of the breast. J Clin Oncol.
23:6249–6251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tavora F, Miettinen M, Fanburg-Smith J,
Franks TJ and Burke A: Pulmonary artery sarcoma: A histologic and
follow-up study with emphasis on a subset of low-grade
myofibroblastic sarcomas with a good long-term follow-up. Am J Surg
Pathol. 32:1751–1761. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng GZ, Zhang HY, Zhang Z, Wei B and Bu
H: Myofibroblastic sarcoma vs nodular fasciitis: A comparative
study of chromosomal imbalances. Am J Clin Pathol. 131:701–709.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation ofconnective
tissue remodeling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montgomery E, Goldblum JR and Fisher C:
Myofibrosarcoma: A clinicopathologic study. Am J Surg Pathol.
25:219–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eyden BP, Christensen L, Tagore V and
Harris M: Myofibrosarcoma of subcutaneous soft tissue of the cheek.
J Submicrosc Cytol Pathol. 24:307–313. 1992.PubMed/NCBI
|
|
17
|
Bisceglia M and Magro G: Low-grade
myofibroblastic sarcoma of the salivary gland. Am J Surg Pathol.
23:1435–1436. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bisceglia M, Tricarico N, Minenna P, Magro
G and Pasquinelli G: Myofibrosarcoma of the upper jawbones: A
clinicopathologic and ultrastructural study of two cases.
Ultrastruct Pathol. 25:385–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kondo S, Yoshizaki T, Minato H, Horikawa
I, Tatsumi A and Furukawa M: Myofibrosarcoma of the nasal cavity
and paranasal sinus. Histopathology. 39:216–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang SE, Choi JH, Sung KJ, Moon KC, Koh
JK, Lee TJ, Ro JY and Silverman JS: A case of cutaneous low-grade
myofibroblastic sarcoma. J Dermatol. 28:383–387. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Keller C, Gibbs CN, Kelly SM, Haller JR,
White KS, Coffin CM and Lemons RS: Low-grade myofibrosarcoma of the
head and neck: Importance of surgical therapy. J Pediatr Hematol
Oncol. 26:119–120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahama A Jr, Nascimento AG, Brum MC,
Vargas PA and Lopes MA: Low-grade myofibroblastic sarcoma of the
parapharyngeal space. Int J Oral Maxillofac Surg. 35:965–968. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Artopoulou II, Lemon JC, Clayman GL and
Chambers MS: Stent fabrication for graft immobilization following
wide surgical excision of myofibroblastic sarcoma of the buccal
mucosa: A clinical report. J Prosthet Dent. 95:280–285. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laco J, Simáková E, Slezák R, Tucek L,
Mottl R, Spacek J and Ryska A: Low grade myofibroblastic sarcoma of
tongue: A case report. Cesk Patol. 42:150–153. 2006.PubMed/NCBI
|
|
25
|
Meng GZ, Zhang HY, Bu H, Zhang XL, Pang
ZG, Ke Q, Liu X and Yang G: Myofibroblastic sarcomas: A
clinicopathological study of 20 cases. Chin Med J (Engl).
120:363–369. 2007.PubMed/NCBI
|
|
26
|
Coyne JD: Low-grade myofibroblastic
sarcoma of the piriform fossa: A case report with a literature
review of a tumor with a predilection for the head and neck. Br J
Oral Maxillofac Surg. 45:335–337. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng GZ, Zhang HY, Bu H, Yang GH, Zhang XL
and Yang G: Myofibroblastic sarcoma of the nasal cavity and
paranasal sinus: A clinicopathologic study of 6 cases and review of
the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
104:530–539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Imanguli MM, Karai LJ, Shanti RM, Stewart
DM and Brahim JS: Myofibroblastic tumor of the lower lip in a
patient with X-linked hypogammaglobulinemia and isolated growth
hormone deficiency: A case report. J Oral Maxillofac Surg.
65:1219–1222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niedzielska I, Janic T and Mrowiec B:
Low-grade myofibroblastic sarcoma of the mandible: A case report. J
Med Case Reports. 10:84582009. View Article : Google Scholar
|
|
30
|
Demarosi F, Bay A, Moneghini L and
Carrassi A: Low-grade myofibroblastic sarcoma of the oral cavity.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 108:248–254.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Montebugnoli L, Venturi M, Gissi DB,
Flamminio F and Foschini MP: Low-grade myofibroblastic sarcoma of
the gingiva. BMJ Case Rep. 2010:bcr0720103166. 2010. View Article : Google Scholar
|
|
32
|
Mori T, Shimane T, Hayashi T, Uzuki A,
Ikenoya Y, Akiyama R, Egawa S and Sanbe T: Low-grade
myofibroblastic sarcoma at the base of the tongue. Showa Univ J Med
Sci. 22:239–243. 2010. View Article : Google Scholar
|
|
33
|
Covello R, Licci S, Pichi B, Spriano G,
Vidiri A, Morelli L and Rosenberg AE: Low-grade myofibroblastic
sarcoma of the larynx. Int J Surg Pathol. 19:822–826. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ni C, Xu YY, Zhou SH and Wang SQ:
Differential diagnosis of inflammatory myofibroblastic tumor and
low-grade myofibroblastic sarcoma: Two case reports with a
literature review. J Int Med Res. 39:311–320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park KR, Jang HW, Won JH, Kim HS, Cha IH
and Kim HJ: Myofibroblastic sarcoma of the mandible: a case report.
J Korean Assoc Oral Maxillofac Surg. 38:240–244. 2012. View Article : Google Scholar
|
|
36
|
Cai C, Dehner LP and El-Mofty SK: In
myofibroblastic sarcomas of the head and neck, mitotic activity and
necrosis define grade: A case study and literature review. Virchows
Arch. 463:827–836. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khosla D, Yadav BS, Kumar R, Ghoshal S,
Vaiphei K, Verma R and Sharma SC: Low-grade myofibroblastic sarcoma
of the larynx: A rare entity with review of literature. J Cancer
Res Ther. 9:284–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kordač P, Nikolov DH, Smatanová K and
Kalfeřt D: Low-grade myofibroblastic Sarcoma of the larynx: Case
report and review of literature. Acta Medica (Hradec Kralove).
57:162–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guillermo GO, Ignacio AG, Adriana SAB,
Rocío SB, Fátima MP and Modesto AF: Low-grade myofibroblastic
sarcoma. Two rare tumors in two rare locations. Rev Esp Cir Oral
Maxillofac. 37:108–112. 2015. View Article : Google Scholar
|
|
40
|
Qiu JY, Liu P, Shi C and Han B: Low-grade
myofibroblastic sarcomas of the maxilla. Oncol Lett. 9:619–625.
2015.PubMed/NCBI
|
|
41
|
Baxi SS, Pinheiro LC, Patil SM, Pfister
DG, Oeffinger KC and Elkin EB: Causes of death in long-term
survivors of head and neck cancer. Cancer. 120:1507–1513. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oylumlu M, Yildiz A, Ercan S, Oylumlu M
and Davutoglu V: Cardiac metastasis of a low-grade myofibroblastic
sarcoma. Echocardiography. 31:E1–E4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gabbiani G, Ryan GB and Majne G: Presence
of modified fibroblasts in granulation tissue and their possible
role in wound contraction. Experientia. 27:549–550. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fisher C: Myofibroblastic malignancies.
Adv Anat Pathol. 11:190–201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lagace R, Semir TA, Gabianni G and Schurch
W: Myofibroblastic sarcoma. Am J Surg Pathol. 23:1432–1438. 1999.
View Article : Google Scholar : PubMed/NCBI
|